Metal-Organic Frameworks in Electrolytic Cell Research
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MOF Electrolytic Research Background
Metal-Organic Frameworks (MOFs) have emerged as a promising class of materials in the field of electrolytic cell research, offering unique properties that could revolutionize energy storage and conversion technologies. The integration of MOFs into electrolytic cells represents a convergence of materials science, electrochemistry, and sustainable energy research, addressing critical challenges in the quest for more efficient and environmentally friendly energy solutions.
The exploration of MOFs in electrolytic cell applications began in the early 2000s, driven by the need for advanced materials with high surface areas, tunable pore structures, and diverse chemical functionalities. As global energy demands continue to rise and the urgency to transition to renewable energy sources intensifies, the potential of MOFs to enhance electrolytic processes has garnered significant attention from both academia and industry.
MOFs offer several advantages in electrolytic cell research, including their exceptional porosity, which facilitates ion transport and electrolyte diffusion. Their modular nature allows for precise control over pore size and chemical composition, enabling tailored designs for specific electrochemical reactions. Furthermore, the ability to incorporate catalytically active sites within MOF structures presents opportunities for improving reaction kinetics and selectivity in electrolytic processes.
The evolution of MOF research in electrolytic cells has been marked by several key milestones. Initial studies focused on utilizing MOFs as electrode materials, leveraging their high surface area to enhance charge storage capacity. Subsequent research expanded to explore MOFs as electrocatalysts for water splitting, CO2 reduction, and other electrochemical reactions of environmental significance.
Recent advancements have seen the development of MOF-based composite materials, combining the benefits of MOFs with other functional components such as conductive polymers or nanoparticles. These hybrid systems aim to address limitations in electrical conductivity and stability that have historically challenged the widespread adoption of MOFs in electrochemical applications.
The current trajectory of MOF research in electrolytic cells is directed towards overcoming practical challenges and scaling up for commercial viability. Efforts are underway to enhance the stability of MOFs under harsh electrochemical conditions, improve their conductivity, and develop cost-effective synthesis methods for large-scale production.
As the field progresses, interdisciplinary collaboration between materials scientists, electrochemists, and engineers is becoming increasingly crucial. The integration of computational modeling and advanced characterization techniques is accelerating the discovery and optimization of MOF-based electrolytic systems, paving the way for next-generation energy technologies.
The exploration of MOFs in electrolytic cell applications began in the early 2000s, driven by the need for advanced materials with high surface areas, tunable pore structures, and diverse chemical functionalities. As global energy demands continue to rise and the urgency to transition to renewable energy sources intensifies, the potential of MOFs to enhance electrolytic processes has garnered significant attention from both academia and industry.
MOFs offer several advantages in electrolytic cell research, including their exceptional porosity, which facilitates ion transport and electrolyte diffusion. Their modular nature allows for precise control over pore size and chemical composition, enabling tailored designs for specific electrochemical reactions. Furthermore, the ability to incorporate catalytically active sites within MOF structures presents opportunities for improving reaction kinetics and selectivity in electrolytic processes.
The evolution of MOF research in electrolytic cells has been marked by several key milestones. Initial studies focused on utilizing MOFs as electrode materials, leveraging their high surface area to enhance charge storage capacity. Subsequent research expanded to explore MOFs as electrocatalysts for water splitting, CO2 reduction, and other electrochemical reactions of environmental significance.
Recent advancements have seen the development of MOF-based composite materials, combining the benefits of MOFs with other functional components such as conductive polymers or nanoparticles. These hybrid systems aim to address limitations in electrical conductivity and stability that have historically challenged the widespread adoption of MOFs in electrochemical applications.
The current trajectory of MOF research in electrolytic cells is directed towards overcoming practical challenges and scaling up for commercial viability. Efforts are underway to enhance the stability of MOFs under harsh electrochemical conditions, improve their conductivity, and develop cost-effective synthesis methods for large-scale production.
As the field progresses, interdisciplinary collaboration between materials scientists, electrochemists, and engineers is becoming increasingly crucial. The integration of computational modeling and advanced characterization techniques is accelerating the discovery and optimization of MOF-based electrolytic systems, paving the way for next-generation energy technologies.
Market Analysis for MOF-based Electrolytic Cells
The market for Metal-Organic Frameworks (MOFs) in electrolytic cell applications is experiencing significant growth, driven by the increasing demand for efficient and sustainable energy storage and conversion technologies. The global MOF market, valued at approximately $70 million in 2020, is projected to reach $291 million by 2025, with a compound annual growth rate (CAGR) of 33.1%. Within this broader market, the application of MOFs in electrolytic cells represents a promising segment with substantial growth potential.
The electrolytic cell market, particularly in the context of water electrolysis for hydrogen production, is witnessing rapid expansion due to the global push for clean energy solutions. The water electrolysis market is expected to grow from $0.3 billion in 2020 to $2.5 billion by 2025, with a CAGR of 52.7%. MOF-based electrolytic cells are poised to capture a significant portion of this market due to their unique properties and potential advantages over traditional materials.
Key drivers for the adoption of MOF-based electrolytic cells include the increasing focus on renewable energy integration, the growing hydrogen economy, and the need for more efficient and cost-effective electrolysis technologies. MOFs offer several advantages in this context, such as high surface area, tunable pore size, and the ability to incorporate catalytic sites, which can enhance the performance and efficiency of electrolytic cells.
The automotive industry, particularly the fuel cell electric vehicle (FCEV) sector, represents a major potential market for MOF-based electrolytic cells. As the FCEV market is projected to grow from 20,000 units in 2020 to over 1 million units by 2030, the demand for efficient hydrogen production technologies, including advanced electrolytic cells, is expected to surge.
Industrial sectors such as chemical manufacturing, steel production, and ammonia synthesis are also showing increased interest in MOF-based electrolytic cells for on-site hydrogen production. These industries are exploring ways to reduce their carbon footprint and transition to cleaner production processes, creating additional market opportunities for MOF-based technologies.
Geographically, Asia-Pacific is expected to be the fastest-growing market for MOF-based electrolytic cells, driven by significant investments in hydrogen infrastructure in countries like Japan, South Korea, and China. North America and Europe are also key markets, with strong government support for clean energy technologies and established research and development ecosystems.
However, challenges such as high production costs, scalability issues, and competition from established technologies may impact market growth. Overcoming these barriers through continued research and development efforts will be crucial for the widespread adoption of MOF-based electrolytic cells in the coming years.
The electrolytic cell market, particularly in the context of water electrolysis for hydrogen production, is witnessing rapid expansion due to the global push for clean energy solutions. The water electrolysis market is expected to grow from $0.3 billion in 2020 to $2.5 billion by 2025, with a CAGR of 52.7%. MOF-based electrolytic cells are poised to capture a significant portion of this market due to their unique properties and potential advantages over traditional materials.
Key drivers for the adoption of MOF-based electrolytic cells include the increasing focus on renewable energy integration, the growing hydrogen economy, and the need for more efficient and cost-effective electrolysis technologies. MOFs offer several advantages in this context, such as high surface area, tunable pore size, and the ability to incorporate catalytic sites, which can enhance the performance and efficiency of electrolytic cells.
The automotive industry, particularly the fuel cell electric vehicle (FCEV) sector, represents a major potential market for MOF-based electrolytic cells. As the FCEV market is projected to grow from 20,000 units in 2020 to over 1 million units by 2030, the demand for efficient hydrogen production technologies, including advanced electrolytic cells, is expected to surge.
Industrial sectors such as chemical manufacturing, steel production, and ammonia synthesis are also showing increased interest in MOF-based electrolytic cells for on-site hydrogen production. These industries are exploring ways to reduce their carbon footprint and transition to cleaner production processes, creating additional market opportunities for MOF-based technologies.
Geographically, Asia-Pacific is expected to be the fastest-growing market for MOF-based electrolytic cells, driven by significant investments in hydrogen infrastructure in countries like Japan, South Korea, and China. North America and Europe are also key markets, with strong government support for clean energy technologies and established research and development ecosystems.
However, challenges such as high production costs, scalability issues, and competition from established technologies may impact market growth. Overcoming these barriers through continued research and development efforts will be crucial for the widespread adoption of MOF-based electrolytic cells in the coming years.
Current MOF Challenges in Electrolytic Applications
Metal-Organic Frameworks (MOFs) have shown great potential in electrolytic cell research, but their application faces several significant challenges. One of the primary obstacles is the stability of MOFs in electrolytic environments. Many MOFs degrade or dissolve when exposed to aqueous electrolytes, limiting their long-term performance and reliability in electrolytic cells.
Another critical challenge is the conductivity of MOFs. While these materials offer high surface areas and tunable pore structures, most MOFs are inherently poor electrical conductors. This limitation hinders their effectiveness in electrocatalysis and charge transfer processes, which are crucial for efficient electrolytic cell operation.
The synthesis and scalability of MOFs for electrolytic applications also present significant hurdles. Producing MOFs with consistent quality and desired properties at large scales remains challenging, impacting their commercial viability in electrolytic cell technologies.
Furthermore, the integration of MOFs into electrolytic cell architectures poses design and engineering challenges. Optimizing the interface between MOFs and other cell components, such as electrodes and membranes, is crucial for maximizing performance but often complex to achieve.
The selectivity of MOFs in electrolytic processes is another area of concern. While MOFs can be designed for specific molecular interactions, achieving high selectivity for target reactions or ion transport in complex electrolytic environments remains challenging.
Durability under operational conditions is a significant issue for MOFs in electrolytic cells. The harsh chemical and electrochemical environments, including pH extremes and potential cycling, can lead to structural degradation and loss of functionality over time.
Additionally, the cost-effectiveness of MOFs compared to traditional materials used in electrolytic cells is a practical challenge. The complex synthesis processes and sometimes expensive precursors for high-performance MOFs can make them economically uncompetitive for large-scale applications.
Lastly, the fundamental understanding of MOF behavior in electrolytic systems is still evolving. There is a need for more comprehensive studies on the mechanisms of ion and electron transport within MOFs under electrolytic conditions, as well as their interactions with electrolytes and electrode surfaces.
Another critical challenge is the conductivity of MOFs. While these materials offer high surface areas and tunable pore structures, most MOFs are inherently poor electrical conductors. This limitation hinders their effectiveness in electrocatalysis and charge transfer processes, which are crucial for efficient electrolytic cell operation.
The synthesis and scalability of MOFs for electrolytic applications also present significant hurdles. Producing MOFs with consistent quality and desired properties at large scales remains challenging, impacting their commercial viability in electrolytic cell technologies.
Furthermore, the integration of MOFs into electrolytic cell architectures poses design and engineering challenges. Optimizing the interface between MOFs and other cell components, such as electrodes and membranes, is crucial for maximizing performance but often complex to achieve.
The selectivity of MOFs in electrolytic processes is another area of concern. While MOFs can be designed for specific molecular interactions, achieving high selectivity for target reactions or ion transport in complex electrolytic environments remains challenging.
Durability under operational conditions is a significant issue for MOFs in electrolytic cells. The harsh chemical and electrochemical environments, including pH extremes and potential cycling, can lead to structural degradation and loss of functionality over time.
Additionally, the cost-effectiveness of MOFs compared to traditional materials used in electrolytic cells is a practical challenge. The complex synthesis processes and sometimes expensive precursors for high-performance MOFs can make them economically uncompetitive for large-scale applications.
Lastly, the fundamental understanding of MOF behavior in electrolytic systems is still evolving. There is a need for more comprehensive studies on the mechanisms of ion and electron transport within MOFs under electrolytic conditions, as well as their interactions with electrolytes and electrode surfaces.
Existing MOF Electrolytic Cell Solutions
01 Synthesis and structure of Metal-Organic Frameworks
Metal-Organic Frameworks (MOFs) are crystalline materials composed of metal ions or clusters coordinated to organic ligands. The synthesis of MOFs involves various methods such as solvothermal, microwave-assisted, and mechanochemical techniques. The structure of MOFs can be tailored by selecting appropriate metal centers and organic linkers, resulting in diverse pore sizes, shapes, and functionalities.- Synthesis and structure of Metal-Organic Frameworks: Metal-Organic Frameworks (MOFs) are crystalline materials composed of metal ions or clusters coordinated to organic ligands. The synthesis of MOFs involves various methods such as solvothermal, microwave-assisted, and mechanochemical techniques. The structure of MOFs can be tailored by selecting different metal nodes and organic linkers, resulting in diverse pore sizes, shapes, and functionalities.
- Applications of Metal-Organic Frameworks: MOFs have a wide range of applications due to their unique properties. They are used in gas storage and separation, catalysis, drug delivery, sensing, and environmental remediation. Their high surface area and tunable pore structure make them particularly suitable for adsorption-based applications, such as carbon capture and hydrogen storage.
- Functionalization and modification of Metal-Organic Frameworks: MOFs can be functionalized or modified to enhance their properties or introduce new functionalities. This can be achieved through post-synthetic modification, incorporation of functional groups in the organic linkers, or by creating composite materials. Such modifications can improve the stability, selectivity, or catalytic activity of MOFs.
- Characterization techniques for Metal-Organic Frameworks: Various analytical techniques are used to characterize MOFs, including X-ray diffraction, gas sorption analysis, spectroscopic methods, and microscopy. These techniques provide information about the crystal structure, surface area, pore size distribution, and chemical composition of MOFs, which is crucial for understanding their properties and potential applications.
- Scale-up and industrial production of Metal-Organic Frameworks: The scale-up and industrial production of MOFs is an important area of research and development. This includes optimizing synthesis conditions, developing continuous flow processes, and addressing challenges related to cost, reproducibility, and large-scale manufacturing. Efforts are also focused on improving the stability and durability of MOFs for practical applications.
02 Applications of Metal-Organic Frameworks
MOFs have a wide range of applications due to their unique properties. They are used in gas storage and separation, catalysis, drug delivery, sensing, and environmental remediation. Their high surface area and tunable pore structure make them particularly suitable for adsorption-based applications, such as carbon capture and hydrogen storage.Expand Specific Solutions03 Functionalization and modification of Metal-Organic Frameworks
MOFs can be functionalized or modified to enhance their properties or introduce new functionalities. This can be achieved through post-synthetic modification, incorporation of functional groups in the organic linkers, or by creating composite materials. Such modifications can improve the stability, selectivity, or catalytic activity of MOFs.Expand Specific Solutions04 Characterization techniques for Metal-Organic Frameworks
Various analytical techniques are used to characterize MOFs, including X-ray diffraction, gas sorption analysis, spectroscopic methods, and microscopy. These techniques provide information about the crystal structure, porosity, surface area, and chemical composition of MOFs, which is crucial for understanding their properties and potential applications.Expand Specific Solutions05 Scale-up and industrial production of Metal-Organic Frameworks
The scale-up and industrial production of MOFs present challenges in maintaining product quality, reducing costs, and ensuring environmental sustainability. Continuous flow synthesis, spray-drying, and other innovative manufacturing processes are being developed to address these challenges and enable large-scale production of MOFs for commercial applications.Expand Specific Solutions
Key Players in MOF Electrolytic Research
The research on Metal-Organic Frameworks (MOFs) in electrolytic cell applications is in a rapidly evolving phase, with significant market potential due to the growing demand for advanced energy storage and conversion technologies. The field is characterized by intense competition among academic institutions and industry players, with varying levels of technological maturity. Key players like Northwestern University, BASF Corp., and Massachusetts Institute of Technology are at the forefront, leveraging their research capabilities to develop innovative MOF-based solutions. The market is witnessing increased collaboration between academia and industry, as exemplified by partnerships involving universities such as The University of Chicago and King Abdullah University of Science & Technology, aiming to accelerate commercialization of MOF technologies in electrolytic applications.
Northwestern University
Technical Solution: Northwestern University has developed innovative Metal-Organic Frameworks (MOFs) for electrolytic cell applications. Their research focuses on creating highly porous MOFs with tailored structures for enhanced electrocatalytic performance. They have synthesized MOFs with atomically dispersed metal sites, achieving high catalytic activity for oxygen evolution reaction (OER) and oxygen reduction reaction (ORR) [1]. The university has also pioneered the use of MOFs as precursors for derived carbon materials with improved conductivity and stability in electrolytic cells [2]. Their recent work includes the development of bimetallic MOFs that exhibit synergistic effects, leading to superior electrocatalytic activity compared to single-metal counterparts [3].
Strengths: Cutting-edge research in MOF design, high catalytic activity, and innovative precursor approaches. Weaknesses: Potential scalability issues and cost considerations for large-scale production.
BASF Corp.
Technical Solution: BASF Corp. has made significant strides in the application of Metal-Organic Frameworks (MOFs) for electrolytic cell research. They have developed a range of MOF-based materials optimized for various electrochemical processes. BASF's approach involves the creation of hierarchical porous structures within MOFs to facilitate mass transport and enhance electrocatalytic performance [4]. They have also focused on improving the stability of MOFs in aqueous electrolytes, a crucial factor for their application in electrolytic cells. BASF has successfully integrated MOFs into electrode materials, demonstrating improved efficiency in water splitting and CO2 reduction reactions [5]. Their research extends to the development of MOF-derived catalysts with high surface areas and controlled compositions for enhanced electrocatalytic activity.
Strengths: Strong industrial research capabilities, focus on practical applications, and potential for large-scale production. Weaknesses: Possible limitations in exploring more fundamental aspects of MOF chemistry compared to academic institutions.
Innovative MOF Structures for Electrolysis
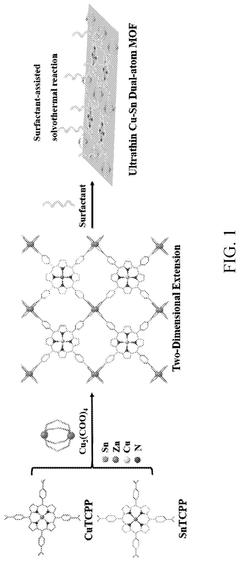
Method of preparing ultrathin metal-organic frameworks as dual-atom electrocatalysts for carbon dioxide reduction
PatentPendingUS20250019845A1
Innovation
- The development of dual-atom catalysts derived from ultrathin two-dimensional metal-organic frameworks using a surfactant-assistant solvothermal reaction process, incorporating copper and tin ions, which are supported by carboxylic acid modified carbon nanotubes to enhance CO2 reduction selectivity and prevent atom aggregation.
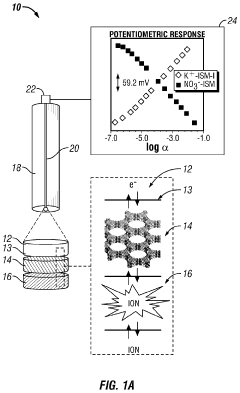
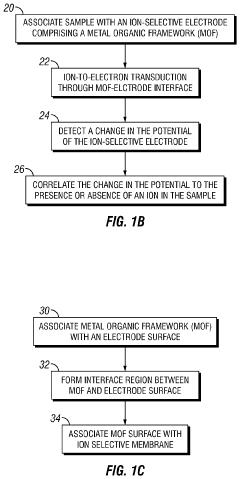
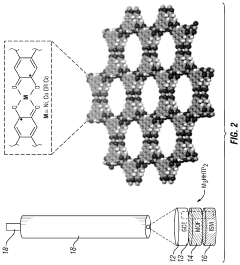
Metal-organic frameworks as ion-to-electron transducers and detectors
PatentActiveUS11486850B2
Innovation
- The development of ion-selective electrodes that incorporate a metal-organic framework associated with an electrode surface, forming an interface for ion-to-electron transduction, and optionally include a potentiostat, wiring, and an ion-selective membrane for enhanced ion detection.
Environmental Impact of MOF-based Electrolysis
The environmental impact of Metal-Organic Framework (MOF)-based electrolysis is a critical consideration in the development and implementation of this technology. MOFs have shown great promise in enhancing the efficiency and selectivity of electrocatalytic processes, particularly in water splitting and CO2 reduction. However, their widespread adoption necessitates a thorough assessment of their environmental implications.
One of the primary environmental benefits of MOF-based electrolysis is its potential to contribute to the production of clean energy carriers, such as hydrogen, through water splitting. This process, when powered by renewable energy sources, can significantly reduce greenhouse gas emissions compared to traditional fossil fuel-based hydrogen production methods. Additionally, MOF-based electrocatalysts have demonstrated improved efficiency in CO2 reduction, potentially aiding in carbon capture and utilization efforts.
However, the environmental impact of MOF synthesis and production must be carefully evaluated. Many MOFs require energy-intensive synthesis processes and may involve the use of toxic or environmentally harmful precursors. The scalability of MOF production while maintaining environmental sustainability remains a challenge that researchers are actively addressing through the development of green synthesis methods and the use of bio-based precursors.
The long-term stability and degradation of MOFs in electrolytic cells also warrant consideration. While many MOFs exhibit excellent stability under operating conditions, some may degrade over time, potentially releasing metal ions or organic linkers into the environment. This necessitates the development of robust MOF structures and effective containment strategies to prevent environmental contamination.
Recycling and end-of-life management of MOF-based electrocatalysts present both challenges and opportunities. The diverse composition of MOFs, often containing valuable metals, makes them potential candidates for recycling. However, effective separation and recovery processes need to be developed to ensure the economic viability and environmental benefits of MOF recycling.
The use of MOFs in electrolysis may also indirectly impact water resources. While water splitting for hydrogen production consumes water, the high efficiency of MOF-based electrocatalysts could potentially reduce overall water consumption compared to less efficient technologies. Nevertheless, the implementation of MOF-based electrolysis on a large scale would require careful water resource management, particularly in water-stressed regions.
In conclusion, while MOF-based electrolysis shows promise in advancing clean energy technologies, a comprehensive life cycle assessment is crucial to fully understand and mitigate its environmental impacts. Ongoing research focuses on developing more sustainable synthesis methods, improving MOF stability, and establishing effective recycling processes to ensure that the environmental benefits of this technology outweigh its potential drawbacks.
One of the primary environmental benefits of MOF-based electrolysis is its potential to contribute to the production of clean energy carriers, such as hydrogen, through water splitting. This process, when powered by renewable energy sources, can significantly reduce greenhouse gas emissions compared to traditional fossil fuel-based hydrogen production methods. Additionally, MOF-based electrocatalysts have demonstrated improved efficiency in CO2 reduction, potentially aiding in carbon capture and utilization efforts.
However, the environmental impact of MOF synthesis and production must be carefully evaluated. Many MOFs require energy-intensive synthesis processes and may involve the use of toxic or environmentally harmful precursors. The scalability of MOF production while maintaining environmental sustainability remains a challenge that researchers are actively addressing through the development of green synthesis methods and the use of bio-based precursors.
The long-term stability and degradation of MOFs in electrolytic cells also warrant consideration. While many MOFs exhibit excellent stability under operating conditions, some may degrade over time, potentially releasing metal ions or organic linkers into the environment. This necessitates the development of robust MOF structures and effective containment strategies to prevent environmental contamination.
Recycling and end-of-life management of MOF-based electrocatalysts present both challenges and opportunities. The diverse composition of MOFs, often containing valuable metals, makes them potential candidates for recycling. However, effective separation and recovery processes need to be developed to ensure the economic viability and environmental benefits of MOF recycling.
The use of MOFs in electrolysis may also indirectly impact water resources. While water splitting for hydrogen production consumes water, the high efficiency of MOF-based electrocatalysts could potentially reduce overall water consumption compared to less efficient technologies. Nevertheless, the implementation of MOF-based electrolysis on a large scale would require careful water resource management, particularly in water-stressed regions.
In conclusion, while MOF-based electrolysis shows promise in advancing clean energy technologies, a comprehensive life cycle assessment is crucial to fully understand and mitigate its environmental impacts. Ongoing research focuses on developing more sustainable synthesis methods, improving MOF stability, and establishing effective recycling processes to ensure that the environmental benefits of this technology outweigh its potential drawbacks.
Scalability of MOF Electrolytic Technologies
The scalability of Metal-Organic Framework (MOF) electrolytic technologies represents a critical factor in their potential for widespread industrial adoption. As research progresses from laboratory-scale experiments to larger pilot projects, several key challenges emerge that must be addressed to ensure successful scaling.
One primary concern is the synthesis of MOFs in large quantities while maintaining consistent quality and performance. Traditional batch processes may face limitations in terms of production capacity and uniformity. To overcome this, continuous flow synthesis methods are being explored, which offer the potential for increased throughput and better control over reaction conditions. However, these methods require careful optimization to ensure that the desired MOF structures are consistently produced at scale.
Another significant challenge lies in the fabrication of MOF-based electrodes for large-scale electrolytic cells. The integration of MOFs into electrode structures that can withstand industrial operating conditions while maintaining their catalytic activity is crucial. Researchers are investigating various approaches, including the direct growth of MOFs on conductive substrates and the development of composite materials that combine MOFs with more robust support structures.
The stability and longevity of MOF-based electrocatalysts under prolonged operation is also a key consideration for scalability. Industrial electrolytic processes often require continuous operation for extended periods, and the MOF structures must maintain their integrity and performance under these conditions. Efforts are being made to enhance the chemical and mechanical stability of MOFs through strategies such as post-synthetic modification and the incorporation of stabilizing agents.
Economic viability is another critical aspect of scaling MOF electrolytic technologies. The cost of MOF synthesis and electrode fabrication must be competitive with existing technologies to justify industrial adoption. This has led to research into more cost-effective precursors and synthesis methods, as well as efforts to optimize the utilization of precious metal components in MOF-based catalysts.
Environmental considerations also play a role in the scalability of MOF electrolytic technologies. The potential environmental impact of large-scale MOF production and use must be carefully evaluated. This includes assessing the sustainability of precursor materials, the energy efficiency of synthesis processes, and the recyclability or disposal of MOF-based electrodes at the end of their operational life.
As the field progresses, collaborative efforts between academic researchers and industrial partners are becoming increasingly important to address these scalability challenges. Pilot-scale demonstrations and techno-economic analyses are providing valuable insights into the practical aspects of scaling MOF electrolytic technologies, helping to bridge the gap between laboratory discoveries and industrial implementation.
One primary concern is the synthesis of MOFs in large quantities while maintaining consistent quality and performance. Traditional batch processes may face limitations in terms of production capacity and uniformity. To overcome this, continuous flow synthesis methods are being explored, which offer the potential for increased throughput and better control over reaction conditions. However, these methods require careful optimization to ensure that the desired MOF structures are consistently produced at scale.
Another significant challenge lies in the fabrication of MOF-based electrodes for large-scale electrolytic cells. The integration of MOFs into electrode structures that can withstand industrial operating conditions while maintaining their catalytic activity is crucial. Researchers are investigating various approaches, including the direct growth of MOFs on conductive substrates and the development of composite materials that combine MOFs with more robust support structures.
The stability and longevity of MOF-based electrocatalysts under prolonged operation is also a key consideration for scalability. Industrial electrolytic processes often require continuous operation for extended periods, and the MOF structures must maintain their integrity and performance under these conditions. Efforts are being made to enhance the chemical and mechanical stability of MOFs through strategies such as post-synthetic modification and the incorporation of stabilizing agents.
Economic viability is another critical aspect of scaling MOF electrolytic technologies. The cost of MOF synthesis and electrode fabrication must be competitive with existing technologies to justify industrial adoption. This has led to research into more cost-effective precursors and synthesis methods, as well as efforts to optimize the utilization of precious metal components in MOF-based catalysts.
Environmental considerations also play a role in the scalability of MOF electrolytic technologies. The potential environmental impact of large-scale MOF production and use must be carefully evaluated. This includes assessing the sustainability of precursor materials, the energy efficiency of synthesis processes, and the recyclability or disposal of MOF-based electrodes at the end of their operational life.
As the field progresses, collaborative efforts between academic researchers and industrial partners are becoming increasingly important to address these scalability challenges. Pilot-scale demonstrations and techno-economic analyses are providing valuable insights into the practical aspects of scaling MOF electrolytic technologies, helping to bridge the gap between laboratory discoveries and industrial implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!