Emerging Electrode Materials for Efficient Electrolytic Cells
AUG 1, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolytic Cell Electrode Evolution and Objectives
Electrolytic cells have been a cornerstone of industrial electrochemistry for over a century, with their applications ranging from metal production to water treatment. The evolution of electrode materials has been pivotal in enhancing the efficiency and sustainability of these systems. This technological progression has been driven by the need for improved energy efficiency, reduced environmental impact, and increased durability of electrolytic processes.
The primary objective in the research of emerging electrode materials for efficient electrolytic cells is to develop electrodes that can significantly improve the overall performance of these systems. This includes increasing the reaction rates, reducing overpotentials, and enhancing the selectivity of desired products. Additionally, there is a growing emphasis on developing materials that are more environmentally friendly and sustainable, aligning with global efforts to reduce carbon footprints and minimize the use of rare or toxic elements.
Historically, the development of electrode materials has seen several key phases. The initial use of simple metal electrodes gave way to more complex alloys and composite materials. In recent decades, there has been a shift towards nanostructured materials and advanced catalysts, which offer superior surface area and catalytic activity. The current trend is moving towards smart materials that can adapt to changing reaction conditions and self-regenerate to maintain performance over extended periods.
The technological trajectory in this field is now focusing on multi-functional electrode materials that can simultaneously address multiple challenges. These include materials that not only facilitate efficient electron transfer but also possess properties such as self-cleaning, resistance to fouling, and the ability to selectively catalyze desired reactions while suppressing unwanted side reactions.
Looking ahead, the objectives for future electrode materials research are multifaceted. There is a strong push towards developing electrodes that can operate efficiently at lower temperatures and pressures, thereby reducing the overall energy requirements of electrolytic processes. Another key goal is to create electrode materials that are highly selective, capable of producing specific high-value chemicals with minimal by-products.
Sustainability is becoming an increasingly important factor in electrode material development. This includes not only the environmental impact of the materials themselves but also their entire lifecycle, from production to disposal or recycling. As such, there is growing interest in bio-inspired materials and those derived from abundant, renewable resources.
In conclusion, the evolution of electrode materials for electrolytic cells is driven by the dual imperatives of enhancing performance and promoting sustainability. The objectives for future research are centered on developing materials that can revolutionize industrial electrochemistry, making it more efficient, selective, and environmentally benign. This field stands at the intersection of materials science, electrochemistry, and sustainable technology, promising significant advancements in the coming years.
The primary objective in the research of emerging electrode materials for efficient electrolytic cells is to develop electrodes that can significantly improve the overall performance of these systems. This includes increasing the reaction rates, reducing overpotentials, and enhancing the selectivity of desired products. Additionally, there is a growing emphasis on developing materials that are more environmentally friendly and sustainable, aligning with global efforts to reduce carbon footprints and minimize the use of rare or toxic elements.
Historically, the development of electrode materials has seen several key phases. The initial use of simple metal electrodes gave way to more complex alloys and composite materials. In recent decades, there has been a shift towards nanostructured materials and advanced catalysts, which offer superior surface area and catalytic activity. The current trend is moving towards smart materials that can adapt to changing reaction conditions and self-regenerate to maintain performance over extended periods.
The technological trajectory in this field is now focusing on multi-functional electrode materials that can simultaneously address multiple challenges. These include materials that not only facilitate efficient electron transfer but also possess properties such as self-cleaning, resistance to fouling, and the ability to selectively catalyze desired reactions while suppressing unwanted side reactions.
Looking ahead, the objectives for future electrode materials research are multifaceted. There is a strong push towards developing electrodes that can operate efficiently at lower temperatures and pressures, thereby reducing the overall energy requirements of electrolytic processes. Another key goal is to create electrode materials that are highly selective, capable of producing specific high-value chemicals with minimal by-products.
Sustainability is becoming an increasingly important factor in electrode material development. This includes not only the environmental impact of the materials themselves but also their entire lifecycle, from production to disposal or recycling. As such, there is growing interest in bio-inspired materials and those derived from abundant, renewable resources.
In conclusion, the evolution of electrode materials for electrolytic cells is driven by the dual imperatives of enhancing performance and promoting sustainability. The objectives for future research are centered on developing materials that can revolutionize industrial electrochemistry, making it more efficient, selective, and environmentally benign. This field stands at the intersection of materials science, electrochemistry, and sustainable technology, promising significant advancements in the coming years.
Market Analysis for Advanced Electrolytic Cells
The market for advanced electrolytic cells is experiencing significant growth, driven by the increasing demand for clean energy solutions and the push towards a more sustainable future. The global electrolytic cell market is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) that reflects the industry's robust expansion. This growth is primarily fueled by the rising adoption of hydrogen as a clean energy carrier and the expanding applications of electrolysis in various industrial processes.
Several key factors are contributing to the market's positive outlook. Firstly, the growing emphasis on renewable energy integration and the need for energy storage solutions have created a strong demand for electrolytic cells, particularly in the production of green hydrogen. Governments worldwide are implementing supportive policies and incentives to promote the adoption of clean energy technologies, further stimulating market growth.
The industrial sector represents a significant portion of the market demand, with applications ranging from chemical processing to metal refining. The chlor-alkali industry, in particular, continues to be a major consumer of electrolytic cells for the production of chlorine, caustic soda, and hydrogen. Additionally, the water treatment sector is emerging as a promising market segment, utilizing electrolytic cells for disinfection and purification processes.
Geographically, Asia-Pacific is expected to dominate the market, driven by rapid industrialization, increasing energy demand, and substantial investments in clean energy infrastructure. North America and Europe are also significant markets, with a focus on reducing carbon emissions and transitioning to sustainable energy systems.
The market landscape is characterized by intense competition among established players and new entrants. Key market participants are investing heavily in research and development to improve the efficiency and cost-effectiveness of electrolytic cells. Innovations in electrode materials, such as the development of advanced catalysts and nanostructured electrodes, are at the forefront of technological advancements in this field.
Despite the positive outlook, the market faces certain challenges. The high initial capital costs associated with electrolytic cell installations and the need for specialized infrastructure can be barriers to widespread adoption. Additionally, the availability of low-cost fossil fuels in some regions may slow the transition to electrolysis-based technologies.
Looking ahead, the market for advanced electrolytic cells is poised for continued growth. The increasing focus on decarbonization, coupled with ongoing technological advancements, is expected to drive innovation and expand the application scope of electrolytic cells across various industries. As economies of scale are achieved and production costs decrease, the market is likely to see accelerated adoption rates and new opportunities for growth in emerging applications.
Several key factors are contributing to the market's positive outlook. Firstly, the growing emphasis on renewable energy integration and the need for energy storage solutions have created a strong demand for electrolytic cells, particularly in the production of green hydrogen. Governments worldwide are implementing supportive policies and incentives to promote the adoption of clean energy technologies, further stimulating market growth.
The industrial sector represents a significant portion of the market demand, with applications ranging from chemical processing to metal refining. The chlor-alkali industry, in particular, continues to be a major consumer of electrolytic cells for the production of chlorine, caustic soda, and hydrogen. Additionally, the water treatment sector is emerging as a promising market segment, utilizing electrolytic cells for disinfection and purification processes.
Geographically, Asia-Pacific is expected to dominate the market, driven by rapid industrialization, increasing energy demand, and substantial investments in clean energy infrastructure. North America and Europe are also significant markets, with a focus on reducing carbon emissions and transitioning to sustainable energy systems.
The market landscape is characterized by intense competition among established players and new entrants. Key market participants are investing heavily in research and development to improve the efficiency and cost-effectiveness of electrolytic cells. Innovations in electrode materials, such as the development of advanced catalysts and nanostructured electrodes, are at the forefront of technological advancements in this field.
Despite the positive outlook, the market faces certain challenges. The high initial capital costs associated with electrolytic cell installations and the need for specialized infrastructure can be barriers to widespread adoption. Additionally, the availability of low-cost fossil fuels in some regions may slow the transition to electrolysis-based technologies.
Looking ahead, the market for advanced electrolytic cells is poised for continued growth. The increasing focus on decarbonization, coupled with ongoing technological advancements, is expected to drive innovation and expand the application scope of electrolytic cells across various industries. As economies of scale are achieved and production costs decrease, the market is likely to see accelerated adoption rates and new opportunities for growth in emerging applications.
Current Challenges in Electrode Material Development
The development of efficient electrolytic cells faces several critical challenges in electrode material research. One of the primary obstacles is the limited stability of electrode materials under harsh operating conditions. Electrolytic processes often involve corrosive environments, high temperatures, and intense electrical currents, which can lead to rapid degradation of electrode materials. This degradation not only reduces the efficiency of the electrolytic cell but also increases maintenance costs and downtime.
Another significant challenge is the trade-off between catalytic activity and long-term durability. While some materials exhibit excellent catalytic properties, they may lack the necessary stability for prolonged use in industrial applications. Conversely, more stable materials often demonstrate lower catalytic efficiency, necessitating a delicate balance between these two crucial factors.
The scalability of novel electrode materials presents a further hurdle. Many promising materials developed in laboratory settings face difficulties in scaling up for commercial production. Issues such as complex synthesis processes, high production costs, and inconsistent performance at larger scales hinder the widespread adoption of these innovative materials in industrial electrolytic cells.
Additionally, the environmental impact of electrode materials is becoming an increasingly important consideration. There is a growing need for sustainable and eco-friendly materials that can replace traditional electrodes containing rare or toxic elements. However, finding alternatives that match or exceed the performance of conventional materials while meeting environmental standards remains a significant challenge.
The optimization of electrode surface properties also poses a considerable challenge. The performance of electrolytic cells is heavily influenced by the electrode's surface area, porosity, and morphology. Developing methods to precisely control these properties at the nanoscale, while maintaining them during operation, is crucial for enhancing efficiency and selectivity in electrolytic processes.
Furthermore, the integration of advanced electrode materials with existing infrastructure and technologies presents its own set of challenges. Many industrial electrolytic processes are based on well-established systems, and the introduction of new electrode materials often requires significant modifications to equipment and operating procedures. This can be a barrier to adoption, especially in industries with high capital investment in existing technologies.
Lastly, there is an ongoing challenge in understanding and controlling the complex interfacial phenomena occurring at the electrode-electrolyte interface. These interactions play a critical role in determining the overall performance of the electrolytic cell, yet they are often poorly understood at the molecular level. Developing a deeper understanding of these processes and how they are affected by different electrode materials is essential for designing more efficient and durable electrolytic systems.
Another significant challenge is the trade-off between catalytic activity and long-term durability. While some materials exhibit excellent catalytic properties, they may lack the necessary stability for prolonged use in industrial applications. Conversely, more stable materials often demonstrate lower catalytic efficiency, necessitating a delicate balance between these two crucial factors.
The scalability of novel electrode materials presents a further hurdle. Many promising materials developed in laboratory settings face difficulties in scaling up for commercial production. Issues such as complex synthesis processes, high production costs, and inconsistent performance at larger scales hinder the widespread adoption of these innovative materials in industrial electrolytic cells.
Additionally, the environmental impact of electrode materials is becoming an increasingly important consideration. There is a growing need for sustainable and eco-friendly materials that can replace traditional electrodes containing rare or toxic elements. However, finding alternatives that match or exceed the performance of conventional materials while meeting environmental standards remains a significant challenge.
The optimization of electrode surface properties also poses a considerable challenge. The performance of electrolytic cells is heavily influenced by the electrode's surface area, porosity, and morphology. Developing methods to precisely control these properties at the nanoscale, while maintaining them during operation, is crucial for enhancing efficiency and selectivity in electrolytic processes.
Furthermore, the integration of advanced electrode materials with existing infrastructure and technologies presents its own set of challenges. Many industrial electrolytic processes are based on well-established systems, and the introduction of new electrode materials often requires significant modifications to equipment and operating procedures. This can be a barrier to adoption, especially in industries with high capital investment in existing technologies.
Lastly, there is an ongoing challenge in understanding and controlling the complex interfacial phenomena occurring at the electrode-electrolyte interface. These interactions play a critical role in determining the overall performance of the electrolytic cell, yet they are often poorly understood at the molecular level. Developing a deeper understanding of these processes and how they are affected by different electrode materials is essential for designing more efficient and durable electrolytic systems.
State-of-the-Art Electrode Material Solutions
01 Novel electrode materials for improved efficiency
Research focuses on developing new electrode materials to enhance the efficiency of energy storage and conversion devices. These materials often include advanced composites, nanostructures, or novel chemical compositions that offer superior performance characteristics such as increased conductivity, stability, or energy density.- Novel electrode materials for improved efficiency: Research focuses on developing new electrode materials to enhance the efficiency of energy storage and conversion devices. These materials often include advanced composites, nanostructures, or novel chemical compositions designed to improve conductivity, stability, and overall performance.
- Surface modification of electrode materials: Techniques for modifying the surface of electrode materials are explored to enhance their efficiency. This includes coating, doping, or functionalizing the surface to improve electron transfer, increase active surface area, or protect against degradation during operation.
- Nanostructured electrode materials: Nanostructured materials are investigated for their potential to significantly improve electrode efficiency. These materials, such as nanoparticles, nanowires, or nanotubes, offer increased surface area and unique properties that can enhance charge storage and transfer capabilities.
- Composite electrode materials: Composite electrode materials combining different components are developed to synergize their properties and improve overall efficiency. These composites often integrate conductive additives, active materials, and binders to optimize performance in various electrochemical applications.
- Optimization of electrode material processing: Research is conducted on optimizing the processing methods for electrode materials to enhance their efficiency. This includes refining synthesis techniques, improving material uniformity, and developing novel fabrication processes to achieve desired structural and electrochemical properties.
02 Optimization of electrode structure and morphology
Efforts are directed towards optimizing the structure and morphology of electrode materials to improve their efficiency. This includes designing porous structures, controlling particle size and distribution, and creating hierarchical architectures that facilitate ion transport and electron transfer within the electrode.Expand Specific Solutions03 Surface modification and functionalization of electrodes
Techniques for modifying and functionalizing electrode surfaces are explored to enhance their performance. This may involve coating with conductive materials, introducing dopants, or attaching functional groups to improve the electrode's reactivity, stability, and overall efficiency in electrochemical processes.Expand Specific Solutions04 Composite electrode materials for synergistic effects
Research is conducted on developing composite electrode materials that combine multiple components to achieve synergistic effects. These composites often integrate materials with complementary properties to overcome limitations of individual components and enhance overall electrode efficiency.Expand Specific Solutions05 Advanced manufacturing techniques for electrode fabrication
Innovative manufacturing techniques are being developed to fabricate high-performance electrodes. These methods aim to precisely control the electrode's composition, structure, and properties at various scales, from nano to macro, to optimize their efficiency in energy-related applications.Expand Specific Solutions
Key Players in Electrolytic Cell Industry
The research on emerging electrode materials for efficient electrolytic cells is in a dynamic phase, with significant market potential and ongoing technological advancements. The industry is transitioning from early-stage development to commercial applications, driven by the growing demand for clean energy solutions. Major players like BASF, Sinopec, and Toshiba are investing heavily in R&D, while innovative startups such as NanoGraf and EvolOH are introducing novel materials and processes. Academic institutions, including Monash University and Tongji University, are contributing fundamental research. The market is expected to expand rapidly, fueled by increasing adoption of electrolytic technologies in various sectors. However, the technology's maturity varies across different electrode materials and applications, indicating a competitive landscape with opportunities for both established companies and new entrants.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: The Dalian Institute of Chemical Physics (DICP) has been at the forefront of research on emerging electrode materials for efficient electrolytic cells. Their approach focuses on developing novel nanostructured materials for both cathodes and anodes. They have successfully synthesized high-performance nickel-iron layered double hydroxide (NiFe-LDH) nanosheet arrays as efficient oxygen evolution reaction (OER) electrocatalysts[1]. These materials demonstrate superior catalytic activity and stability compared to traditional noble metal-based catalysts. DICP has also made significant progress in developing carbon-based materials doped with transition metals for hydrogen evolution reaction (HER) catalysts[2]. Their research extends to the design of composite materials that combine the advantages of different components to enhance overall electrocatalytic performance[3].
Strengths: Cutting-edge research in nanostructured materials, expertise in both OER and HER catalysts, strong focus on non-noble metal catalysts for cost-effectiveness. Weaknesses: Potential challenges in scaling up laboratory-scale synthesis for industrial applications, need for further long-term stability testing in real-world conditions.
BASF Corp.
Technical Solution: BASF Corp. has been actively researching and developing advanced electrode materials for efficient electrolytic cells, with a focus on sustainable and scalable solutions. Their approach involves the development of novel metal-organic frameworks (MOFs) as precursors for high-performance electrocatalysts[1]. BASF has successfully synthesized iron-nitrogen-carbon (Fe-N-C) catalysts derived from MOFs, which show excellent activity for oxygen reduction reaction (ORR) in fuel cells and metal-air batteries[2]. Additionally, they have made significant progress in developing cobalt-based perovskite oxides for oxygen evolution reaction (OER) in water electrolysis[3]. BASF's research also extends to the development of advanced polymer electrolyte membranes to enhance the overall efficiency of electrolytic cells[4].
Strengths: Strong expertise in materials science, extensive research facilities, ability to scale up production for commercial applications. Weaknesses: Potential higher costs associated with advanced materials, need for further optimization of catalyst stability and durability in long-term operation.
Breakthrough Electrode Material Innovations
Electrode materials for electrical cells
PatentInactiveEP2719001A4
Innovation
- Novel electrode material comprising a polymer with polysulfide bridges and carbon with at least 60% sp2-hybridized carbon atoms, addressing polysulfide ion migration issues.
- Inclusion of tungsten carbide as an additional component in the polymer to potentially improve conductivity and stability.
- Development of a new cathode material production method that is simpler and avoids drawbacks of previous approaches.
Positive electrode for non-aqueous electrolytic secondary cell and non-aqueous electrolytic secondary cell
PatentInactiveUS20060222946A1
Innovation
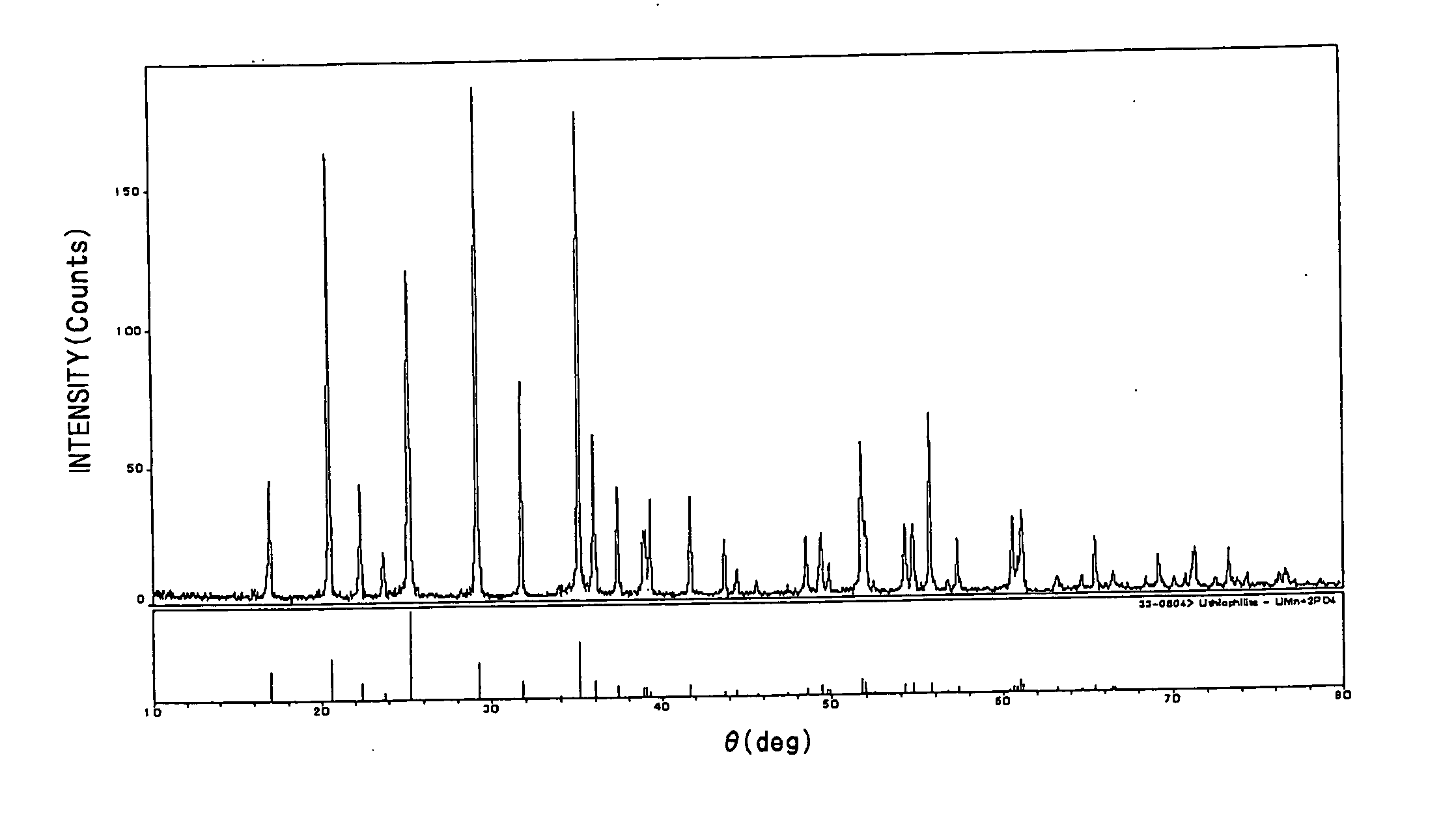
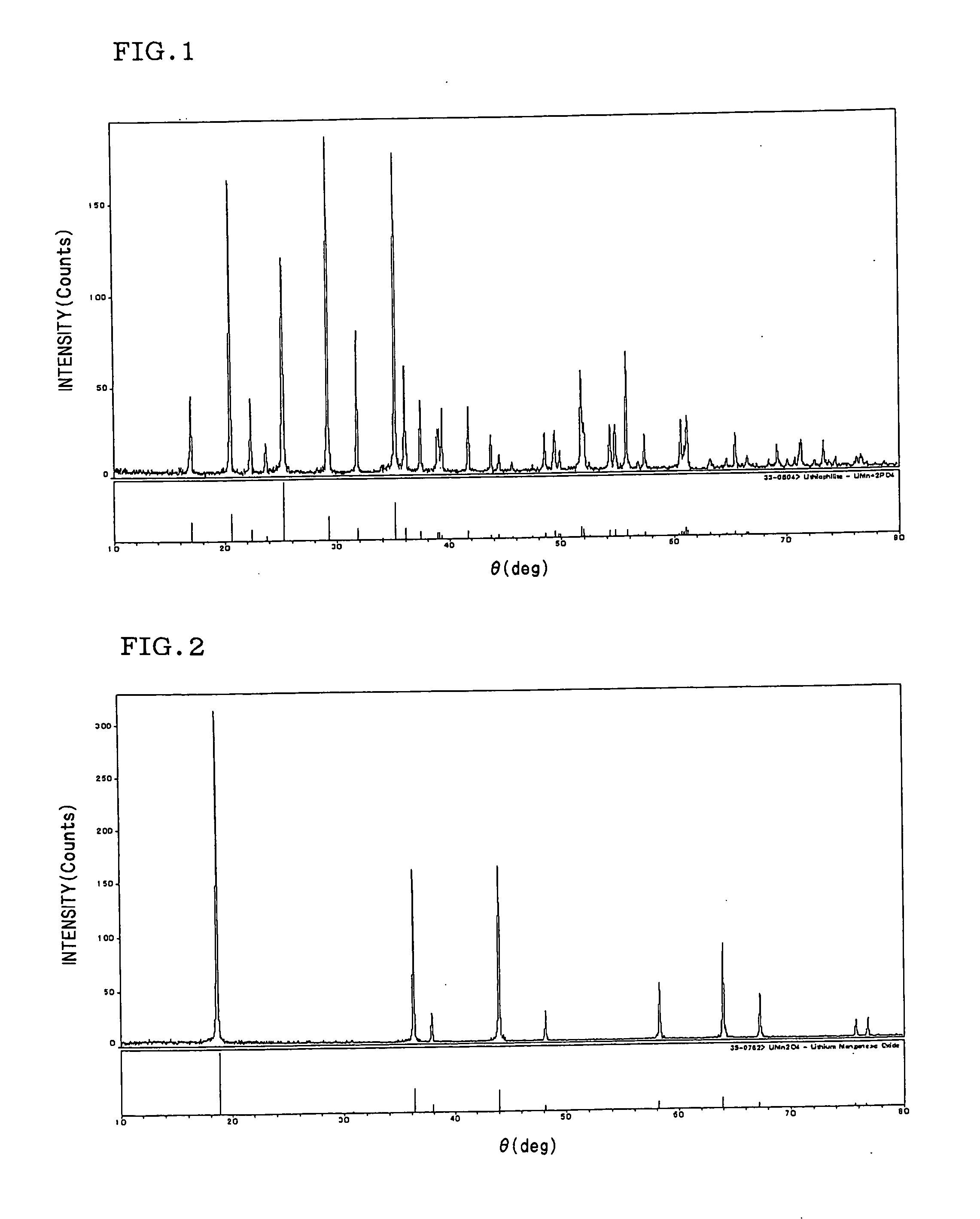
- A positive electrode for non-aqueous electrolytic secondary cells is developed by mixing olivine type lithium manganese phosphate with spinel type lithium manganate, allowing for a balanced performance in terms of capacity, safety, and high-temperature characteristics, with a mixed ratio of olivine type lithium manganese phosphate ranging from 10 to 90 mass % and substitution of manganese with other metal elements to enhance stability and conductivity.
Environmental Impact of Electrode Materials
The environmental impact of electrode materials in electrolytic cells is a critical consideration in the development of efficient and sustainable electrochemical technologies. As research on emerging electrode materials progresses, it is essential to evaluate their potential environmental consequences throughout their lifecycle, from production to disposal.
Traditional electrode materials, such as platinum and other noble metals, have long been associated with significant environmental concerns due to their scarcity and energy-intensive mining processes. The extraction and refining of these materials often result in habitat destruction, water pollution, and greenhouse gas emissions. Furthermore, the limited availability of these resources raises questions about the long-term sustainability of their use in large-scale applications.
In response to these challenges, researchers are exploring alternative electrode materials with reduced environmental footprints. Carbon-based electrodes, including graphene and carbon nanotubes, have shown promise in terms of performance and environmental sustainability. These materials can be synthesized from abundant carbon sources and often require less energy-intensive production processes compared to traditional metal electrodes.
Metal oxides and mixed metal oxides have also gained attention as potential electrode materials with lower environmental impact. These materials can be synthesized using more environmentally friendly methods and often exhibit improved stability and durability, potentially reducing the need for frequent replacement and disposal of electrodes.
However, the environmental benefits of these emerging materials must be carefully evaluated through comprehensive life cycle assessments. While they may offer advantages in terms of resource availability and production processes, potential issues such as nanomaterial toxicity and end-of-life disposal must be thoroughly investigated to ensure their overall environmental sustainability.
The development of bio-inspired and bio-derived electrode materials represents another promising avenue for reducing environmental impact. These materials, often based on naturally occurring compounds or structures, can potentially be produced using renewable resources and biodegradable components, minimizing their ecological footprint.
As research in this field progresses, it is crucial to consider not only the performance characteristics of emerging electrode materials but also their broader environmental implications. This includes assessing their potential for recycling and reuse, as well as their compatibility with existing waste management infrastructure.
Furthermore, the environmental impact of electrode materials extends beyond their direct production and disposal. The improved efficiency and durability of advanced electrode materials can lead to significant reductions in energy consumption and resource utilization in various electrochemical processes, indirectly contributing to environmental conservation efforts.
In conclusion, the environmental impact of electrode materials is a multifaceted issue that requires careful consideration in the development of efficient electrolytic cells. While emerging materials offer promising solutions to mitigate some of the environmental concerns associated with traditional electrodes, ongoing research and comprehensive assessments are necessary to ensure that these new technologies truly contribute to more sustainable electrochemical processes.
Traditional electrode materials, such as platinum and other noble metals, have long been associated with significant environmental concerns due to their scarcity and energy-intensive mining processes. The extraction and refining of these materials often result in habitat destruction, water pollution, and greenhouse gas emissions. Furthermore, the limited availability of these resources raises questions about the long-term sustainability of their use in large-scale applications.
In response to these challenges, researchers are exploring alternative electrode materials with reduced environmental footprints. Carbon-based electrodes, including graphene and carbon nanotubes, have shown promise in terms of performance and environmental sustainability. These materials can be synthesized from abundant carbon sources and often require less energy-intensive production processes compared to traditional metal electrodes.
Metal oxides and mixed metal oxides have also gained attention as potential electrode materials with lower environmental impact. These materials can be synthesized using more environmentally friendly methods and often exhibit improved stability and durability, potentially reducing the need for frequent replacement and disposal of electrodes.
However, the environmental benefits of these emerging materials must be carefully evaluated through comprehensive life cycle assessments. While they may offer advantages in terms of resource availability and production processes, potential issues such as nanomaterial toxicity and end-of-life disposal must be thoroughly investigated to ensure their overall environmental sustainability.
The development of bio-inspired and bio-derived electrode materials represents another promising avenue for reducing environmental impact. These materials, often based on naturally occurring compounds or structures, can potentially be produced using renewable resources and biodegradable components, minimizing their ecological footprint.
As research in this field progresses, it is crucial to consider not only the performance characteristics of emerging electrode materials but also their broader environmental implications. This includes assessing their potential for recycling and reuse, as well as their compatibility with existing waste management infrastructure.
Furthermore, the environmental impact of electrode materials extends beyond their direct production and disposal. The improved efficiency and durability of advanced electrode materials can lead to significant reductions in energy consumption and resource utilization in various electrochemical processes, indirectly contributing to environmental conservation efforts.
In conclusion, the environmental impact of electrode materials is a multifaceted issue that requires careful consideration in the development of efficient electrolytic cells. While emerging materials offer promising solutions to mitigate some of the environmental concerns associated with traditional electrodes, ongoing research and comprehensive assessments are necessary to ensure that these new technologies truly contribute to more sustainable electrochemical processes.
Scalability and Cost Analysis of New Electrode Materials
The scalability and cost analysis of new electrode materials for efficient electrolytic cells is crucial for their potential industrial application and commercialization. As emerging electrode materials show promise in laboratory settings, it is essential to evaluate their feasibility for large-scale production and economic viability.
Scalability of new electrode materials depends on several factors, including raw material availability, synthesis complexity, and manufacturing processes. Many novel electrode materials, such as nanostructured carbon-based materials or advanced metal oxides, often require sophisticated synthesis methods that may pose challenges for mass production. The ability to scale up these processes while maintaining the desired material properties is a key consideration.
Raw material sourcing is another critical aspect of scalability. Some emerging electrode materials rely on rare or expensive elements, which could limit their large-scale adoption. For instance, platinum-based catalysts, while highly efficient, face scalability issues due to the scarcity and high cost of platinum. Alternatives using more abundant elements, such as nickel or iron-based materials, may offer better scalability prospects.
Manufacturing processes for new electrode materials must be adaptable to existing production lines or require minimal modifications to be economically viable. Materials that can be produced using established techniques, such as roll-to-roll processing or chemical vapor deposition, have a significant advantage in terms of scalability.
Cost analysis of new electrode materials involves evaluating both production costs and long-term economic benefits. Initial production costs may be higher for novel materials due to specialized synthesis methods or expensive precursors. However, these costs must be weighed against potential improvements in electrolytic cell efficiency, durability, and overall performance.
Material durability and lifespan significantly impact the long-term cost-effectiveness of new electrode materials. Materials that exhibit enhanced stability and resistance to degradation can reduce maintenance and replacement costs, offsetting higher initial investments. Additionally, improved catalytic activity can lead to energy savings during operation, further enhancing the economic viability of these materials.
Environmental considerations also play a role in cost analysis. Materials that enable more environmentally friendly electrolytic processes or reduce the need for harmful chemicals can provide indirect cost benefits through reduced waste management and regulatory compliance expenses.
In conclusion, the scalability and cost analysis of new electrode materials is a multifaceted evaluation that requires balancing technological advancements with practical and economic considerations. Successful implementation of emerging electrode materials in industrial-scale electrolytic cells will depend on optimizing both scalability and cost-effectiveness to ensure their competitive edge in the market.
Scalability of new electrode materials depends on several factors, including raw material availability, synthesis complexity, and manufacturing processes. Many novel electrode materials, such as nanostructured carbon-based materials or advanced metal oxides, often require sophisticated synthesis methods that may pose challenges for mass production. The ability to scale up these processes while maintaining the desired material properties is a key consideration.
Raw material sourcing is another critical aspect of scalability. Some emerging electrode materials rely on rare or expensive elements, which could limit their large-scale adoption. For instance, platinum-based catalysts, while highly efficient, face scalability issues due to the scarcity and high cost of platinum. Alternatives using more abundant elements, such as nickel or iron-based materials, may offer better scalability prospects.
Manufacturing processes for new electrode materials must be adaptable to existing production lines or require minimal modifications to be economically viable. Materials that can be produced using established techniques, such as roll-to-roll processing or chemical vapor deposition, have a significant advantage in terms of scalability.
Cost analysis of new electrode materials involves evaluating both production costs and long-term economic benefits. Initial production costs may be higher for novel materials due to specialized synthesis methods or expensive precursors. However, these costs must be weighed against potential improvements in electrolytic cell efficiency, durability, and overall performance.
Material durability and lifespan significantly impact the long-term cost-effectiveness of new electrode materials. Materials that exhibit enhanced stability and resistance to degradation can reduce maintenance and replacement costs, offsetting higher initial investments. Additionally, improved catalytic activity can lead to energy savings during operation, further enhancing the economic viability of these materials.
Environmental considerations also play a role in cost analysis. Materials that enable more environmentally friendly electrolytic processes or reduce the need for harmful chemicals can provide indirect cost benefits through reduced waste management and regulatory compliance expenses.
In conclusion, the scalability and cost analysis of new electrode materials is a multifaceted evaluation that requires balancing technological advancements with practical and economic considerations. Successful implementation of emerging electrode materials in industrial-scale electrolytic cells will depend on optimizing both scalability and cost-effectiveness to ensure their competitive edge in the market.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!