Analytical Tools for Electrolytic Cell Reaction Pathways
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolytic Analysis Background and Objectives
Electrolytic cell reaction pathways have been a subject of intense research and development in the field of electrochemistry for decades. The analysis of these pathways is crucial for understanding and optimizing various electrochemical processes, including energy storage, chemical synthesis, and environmental remediation. The evolution of analytical tools for studying these reaction mechanisms has significantly advanced our ability to probe complex electrochemical systems at increasingly finer scales and with greater precision.
The primary objective of developing analytical tools for electrolytic cell reaction pathways is to elucidate the fundamental mechanisms governing electron transfer processes, ion transport, and chemical transformations at electrode-electrolyte interfaces. These tools aim to provide real-time, in-situ measurements of reaction intermediates, products, and kinetics, enabling researchers to construct detailed reaction models and optimize cell performance.
Over the years, the field has witnessed a shift from macroscopic measurements to microscopic and even nanoscopic probing techniques. Early analytical methods relied heavily on bulk electrochemical measurements, such as cyclic voltammetry and chronoamperometry. While these techniques remain valuable, they often provide limited insight into localized reactions and intermediate species.
The advent of spectroscopic techniques coupled with electrochemical cells marked a significant milestone in the field. Methods such as in-situ Raman spectroscopy, Fourier-transform infrared spectroscopy (FTIR), and X-ray absorption spectroscopy (XAS) have enabled researchers to identify and track reaction intermediates with unprecedented detail. These spectroelectrochemical approaches have been instrumental in unraveling complex reaction mechanisms in various systems, from fuel cells to batteries.
More recently, the integration of advanced microscopy techniques with electrochemical analysis has opened new avenues for studying reaction pathways at the nanoscale. Scanning electrochemical microscopy (SECM), atomic force microscopy (AFM), and transmission electron microscopy (TEM) adapted for electrochemical environments have provided valuable insights into local reactivity, surface morphology changes, and even single-molecule reactions at electrode surfaces.
The current technological trajectory is moving towards multi-modal analytical platforms that combine various techniques to provide a comprehensive view of electrolytic cell reactions. These integrated systems aim to correlate structural, chemical, and electrical information across multiple length and time scales, offering a holistic understanding of reaction pathways.
As we look to the future, the development of analytical tools for electrolytic cell reaction pathways is expected to focus on enhancing temporal and spatial resolution, improving sensitivity to detect trace intermediates, and expanding the range of operando conditions under which measurements can be made. The ultimate goal is to develop a suite of tools that can provide a complete, real-time picture of electrochemical processes, from the atomic scale to the full cell level, under realistic operating conditions.
The primary objective of developing analytical tools for electrolytic cell reaction pathways is to elucidate the fundamental mechanisms governing electron transfer processes, ion transport, and chemical transformations at electrode-electrolyte interfaces. These tools aim to provide real-time, in-situ measurements of reaction intermediates, products, and kinetics, enabling researchers to construct detailed reaction models and optimize cell performance.
Over the years, the field has witnessed a shift from macroscopic measurements to microscopic and even nanoscopic probing techniques. Early analytical methods relied heavily on bulk electrochemical measurements, such as cyclic voltammetry and chronoamperometry. While these techniques remain valuable, they often provide limited insight into localized reactions and intermediate species.
The advent of spectroscopic techniques coupled with electrochemical cells marked a significant milestone in the field. Methods such as in-situ Raman spectroscopy, Fourier-transform infrared spectroscopy (FTIR), and X-ray absorption spectroscopy (XAS) have enabled researchers to identify and track reaction intermediates with unprecedented detail. These spectroelectrochemical approaches have been instrumental in unraveling complex reaction mechanisms in various systems, from fuel cells to batteries.
More recently, the integration of advanced microscopy techniques with electrochemical analysis has opened new avenues for studying reaction pathways at the nanoscale. Scanning electrochemical microscopy (SECM), atomic force microscopy (AFM), and transmission electron microscopy (TEM) adapted for electrochemical environments have provided valuable insights into local reactivity, surface morphology changes, and even single-molecule reactions at electrode surfaces.
The current technological trajectory is moving towards multi-modal analytical platforms that combine various techniques to provide a comprehensive view of electrolytic cell reactions. These integrated systems aim to correlate structural, chemical, and electrical information across multiple length and time scales, offering a holistic understanding of reaction pathways.
As we look to the future, the development of analytical tools for electrolytic cell reaction pathways is expected to focus on enhancing temporal and spatial resolution, improving sensitivity to detect trace intermediates, and expanding the range of operando conditions under which measurements can be made. The ultimate goal is to develop a suite of tools that can provide a complete, real-time picture of electrochemical processes, from the atomic scale to the full cell level, under realistic operating conditions.
Market Demand for Reaction Pathway Tools
The market demand for analytical tools focused on electrolytic cell reaction pathways has been steadily growing in recent years, driven by the increasing complexity of electrochemical processes and the need for more efficient and sustainable energy solutions. This demand spans across various industries, including renewable energy, battery technology, fuel cells, and industrial electrolysis.
In the renewable energy sector, there is a pressing need for tools that can optimize electrolytic processes for hydrogen production. As green hydrogen becomes a key player in the global energy transition, companies are seeking advanced analytical solutions to improve the efficiency and cost-effectiveness of water electrolysis. These tools are crucial for identifying and optimizing reaction pathways, ultimately leading to higher hydrogen yields and reduced energy consumption.
The battery industry is another significant driver of market demand for reaction pathway tools. With the rapid growth of electric vehicles and energy storage systems, manufacturers are constantly striving to develop more efficient and longer-lasting batteries. Analytical tools that can provide insights into the complex electrochemical reactions within battery cells are highly sought after. These tools enable researchers to understand degradation mechanisms, optimize electrode materials, and develop novel electrolyte formulations.
In the field of fuel cells, particularly proton exchange membrane (PEM) fuel cells, there is a growing demand for tools that can analyze and optimize reaction pathways. As fuel cell technology advances, companies are looking for ways to enhance performance, durability, and cost-effectiveness. Analytical tools play a crucial role in understanding the intricate processes occurring at the electrode-electrolyte interface, helping researchers develop more efficient catalysts and membrane materials.
The industrial electrolysis sector, which includes chlor-alkali production and metal refining, also contributes significantly to the market demand for reaction pathway tools. These industries require precise control and optimization of electrochemical processes to maximize product yield and minimize energy consumption. Analytical tools that can provide real-time insights into reaction kinetics and mass transport phenomena are highly valued in this sector.
Furthermore, there is an increasing demand for integrated software solutions that combine reaction pathway analysis with machine learning and artificial intelligence capabilities. These advanced tools can process large datasets, identify patterns, and predict optimal reaction conditions, enabling faster and more efficient process development and optimization.
As environmental regulations become more stringent, there is also a growing market for analytical tools that can help industries reduce their carbon footprint and improve the sustainability of their electrochemical processes. This includes tools for analyzing and optimizing CO2 reduction reactions, which are becoming increasingly important in the context of carbon capture and utilization technologies.
In the renewable energy sector, there is a pressing need for tools that can optimize electrolytic processes for hydrogen production. As green hydrogen becomes a key player in the global energy transition, companies are seeking advanced analytical solutions to improve the efficiency and cost-effectiveness of water electrolysis. These tools are crucial for identifying and optimizing reaction pathways, ultimately leading to higher hydrogen yields and reduced energy consumption.
The battery industry is another significant driver of market demand for reaction pathway tools. With the rapid growth of electric vehicles and energy storage systems, manufacturers are constantly striving to develop more efficient and longer-lasting batteries. Analytical tools that can provide insights into the complex electrochemical reactions within battery cells are highly sought after. These tools enable researchers to understand degradation mechanisms, optimize electrode materials, and develop novel electrolyte formulations.
In the field of fuel cells, particularly proton exchange membrane (PEM) fuel cells, there is a growing demand for tools that can analyze and optimize reaction pathways. As fuel cell technology advances, companies are looking for ways to enhance performance, durability, and cost-effectiveness. Analytical tools play a crucial role in understanding the intricate processes occurring at the electrode-electrolyte interface, helping researchers develop more efficient catalysts and membrane materials.
The industrial electrolysis sector, which includes chlor-alkali production and metal refining, also contributes significantly to the market demand for reaction pathway tools. These industries require precise control and optimization of electrochemical processes to maximize product yield and minimize energy consumption. Analytical tools that can provide real-time insights into reaction kinetics and mass transport phenomena are highly valued in this sector.
Furthermore, there is an increasing demand for integrated software solutions that combine reaction pathway analysis with machine learning and artificial intelligence capabilities. These advanced tools can process large datasets, identify patterns, and predict optimal reaction conditions, enabling faster and more efficient process development and optimization.
As environmental regulations become more stringent, there is also a growing market for analytical tools that can help industries reduce their carbon footprint and improve the sustainability of their electrochemical processes. This includes tools for analyzing and optimizing CO2 reduction reactions, which are becoming increasingly important in the context of carbon capture and utilization technologies.
Current Challenges in Electrolytic Cell Analysis
The analysis of electrolytic cell reaction pathways faces several significant challenges in the current scientific and industrial landscape. One of the primary obstacles is the complexity of electrochemical systems, which involve multiple interacting species, varying reaction rates, and complex mass transport phenomena. This complexity makes it difficult to accurately model and predict reaction pathways, especially in large-scale industrial applications.
Another major challenge lies in the limitations of existing analytical tools. While techniques such as cyclic voltammetry and electrochemical impedance spectroscopy provide valuable insights, they often lack the spatial and temporal resolution necessary to fully elucidate reaction mechanisms at the electrode-electrolyte interface. This gap in analytical capabilities hinders the development of more efficient and selective electrocatalysts.
The dynamic nature of electrolytic cells poses additional challenges for analysis. Electrode surfaces can undergo significant changes during operation, including restructuring, poisoning, and the formation of passivation layers. These dynamic processes can dramatically alter reaction pathways and cell performance over time, making long-term predictions and stability assessments particularly challenging.
Furthermore, the multiscale nature of electrolytic processes presents a significant hurdle. Reactions occur at the nanoscale on electrode surfaces, but their effects propagate to the macroscale of industrial reactors. Bridging this scale gap in analytical tools and models remains a formidable challenge, often requiring the integration of multiple analytical techniques and computational approaches.
The heterogeneity of electrode surfaces and electrolyte compositions adds another layer of complexity to the analysis. Local variations in surface structure, composition, and electrolyte concentration can lead to spatially varying reaction rates and pathways. Capturing this heterogeneity in analytical tools and models is crucial for accurate predictions but remains technically challenging.
Lastly, the development of in situ and operando analytical techniques for electrolytic cells is an ongoing challenge. While these techniques offer the promise of real-time, high-resolution insights into reaction pathways under realistic operating conditions, their implementation often requires overcoming significant engineering and design hurdles. The harsh environments typical of many electrolytic processes further complicate the development of robust analytical tools capable of withstanding these conditions while providing accurate, high-resolution data.
Another major challenge lies in the limitations of existing analytical tools. While techniques such as cyclic voltammetry and electrochemical impedance spectroscopy provide valuable insights, they often lack the spatial and temporal resolution necessary to fully elucidate reaction mechanisms at the electrode-electrolyte interface. This gap in analytical capabilities hinders the development of more efficient and selective electrocatalysts.
The dynamic nature of electrolytic cells poses additional challenges for analysis. Electrode surfaces can undergo significant changes during operation, including restructuring, poisoning, and the formation of passivation layers. These dynamic processes can dramatically alter reaction pathways and cell performance over time, making long-term predictions and stability assessments particularly challenging.
Furthermore, the multiscale nature of electrolytic processes presents a significant hurdle. Reactions occur at the nanoscale on electrode surfaces, but their effects propagate to the macroscale of industrial reactors. Bridging this scale gap in analytical tools and models remains a formidable challenge, often requiring the integration of multiple analytical techniques and computational approaches.
The heterogeneity of electrode surfaces and electrolyte compositions adds another layer of complexity to the analysis. Local variations in surface structure, composition, and electrolyte concentration can lead to spatially varying reaction rates and pathways. Capturing this heterogeneity in analytical tools and models is crucial for accurate predictions but remains technically challenging.
Lastly, the development of in situ and operando analytical techniques for electrolytic cells is an ongoing challenge. While these techniques offer the promise of real-time, high-resolution insights into reaction pathways under realistic operating conditions, their implementation often requires overcoming significant engineering and design hurdles. The harsh environments typical of many electrolytic processes further complicate the development of robust analytical tools capable of withstanding these conditions while providing accurate, high-resolution data.
Existing Analytical Solutions
01 Computational modeling of reaction pathways
Advanced computational tools are used to model and predict reaction pathways in complex chemical systems. These tools employ algorithms and simulations to analyze potential reaction routes, transition states, and energy profiles, providing insights into reaction mechanisms and kinetics.- Computational methods for analyzing reaction pathways: Advanced computational tools are used to analyze and predict reaction pathways in chemical and biological systems. These methods involve algorithms and models that simulate molecular interactions, energy landscapes, and transition states. They help researchers understand complex reaction mechanisms and optimize processes in various fields, including drug discovery and materials science.
- Mass spectrometry-based analysis of reaction pathways: Mass spectrometry techniques are employed to study reaction pathways by identifying and quantifying intermediates and products. These analytical tools provide high-resolution data on molecular structures and can track the progression of reactions in real-time. They are particularly useful in metabolomics, proteomics, and the study of complex biological systems.
- Microfluidic devices for reaction pathway analysis: Microfluidic platforms are developed to study reaction pathways at microscale levels. These devices allow for precise control of reaction conditions, rapid mixing, and real-time monitoring of chemical processes. They are particularly useful for high-throughput screening and optimization of reaction conditions in pharmaceutical and chemical industries.
- Biosensors and electrochemical tools for pathway analysis: Biosensors and electrochemical analytical tools are used to monitor reaction pathways in biological systems. These devices can detect specific molecules or metabolites involved in biochemical reactions, providing insights into cellular processes and metabolic pathways. They are valuable in studying enzyme kinetics, signal transduction, and drug metabolism.
- Machine learning approaches for reaction pathway prediction: Machine learning algorithms are increasingly used to predict and analyze reaction pathways. These tools can process large datasets of chemical reactions to identify patterns, predict outcomes, and suggest optimal reaction conditions. They are particularly useful in retrosynthesis planning, drug discovery, and materials design, offering new insights into complex chemical systems.
02 Mass spectrometry-based analysis of reaction intermediates
Mass spectrometry techniques are utilized to identify and characterize reaction intermediates and products. This analytical approach allows for real-time monitoring of reaction progress, detection of transient species, and elucidation of complex reaction networks.Expand Specific Solutions03 Microfluidic devices for reaction pathway analysis
Microfluidic platforms are developed for studying reaction pathways at microscale. These devices enable precise control over reaction conditions, rapid screening of multiple parameters, and integration with various analytical techniques for high-throughput analysis of reaction kinetics and mechanisms.Expand Specific Solutions04 Machine learning approaches for reaction prediction
Machine learning algorithms are applied to predict reaction outcomes and optimize reaction conditions. These tools analyze large datasets of known reactions to identify patterns and correlations, enabling the prediction of new reaction pathways and the design of more efficient synthetic routes.Expand Specific Solutions05 In situ spectroscopic techniques for reaction monitoring
Various spectroscopic methods, such as IR, Raman, and NMR spectroscopy, are employed for in situ monitoring of reaction progress. These techniques provide real-time information on the formation and consumption of reactants, intermediates, and products, allowing for detailed analysis of reaction pathways and kinetics.Expand Specific Solutions
Key Players in Electrochemical Analysis
The analytical tools for electrolytic cell reaction pathways are in a rapidly evolving phase, with the market showing significant growth potential. The technology is advancing from early-stage research to more practical applications, driven by increasing demand for efficient energy storage and conversion solutions. Key players like Centre National de la Recherche Scientifique, Massachusetts Institute of Technology, and Xiamen University are at the forefront of research, while companies such as Bio-Rad Laboratories and Agilent Technologies are developing commercial applications. The market is characterized by a mix of academic institutions, established industry leaders, and emerging startups, indicating a dynamic and competitive landscape with opportunities for innovation and collaboration across sectors.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced analytical tools for studying electrolytic cell reaction pathways, including in-situ spectroscopic techniques and machine learning algorithms. Their approach combines operando X-ray absorption spectroscopy (XAS) with density functional theory (DFT) calculations to elucidate reaction mechanisms in real-time[1]. They have also pioneered the use of surface-enhanced Raman spectroscopy (SERS) for monitoring electrochemical reactions at the electrode-electrolyte interface[2]. Additionally, MIT researchers have implemented artificial intelligence methods to predict reaction pathways and optimize electrocatalysts, significantly accelerating the discovery of new materials for energy conversion and storage applications[3].
Strengths: Cutting-edge spectroscopic techniques, integration of experimental and computational methods, AI-driven catalyst discovery. Weaknesses: High equipment costs, complexity in data interpretation, potential limitations in scalability to industrial processes.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has developed a comprehensive suite of analytical tools for investigating electrolytic cell reaction pathways. Their approach integrates high-performance liquid chromatography (HPLC) with mass spectrometry (MS) to identify and quantify reaction intermediates and products with high sensitivity and selectivity[4]. They have also introduced innovative electrochemical detection systems that can be coupled with chromatographic techniques for real-time monitoring of redox processes[5]. Agilent's latest advancements include the development of miniaturized electrochemical cells compatible with their analytical instruments, allowing for in-situ characterization of electrode materials and electrolyte compositions under realistic operating conditions[6].
Strengths: High-precision analytical instruments, integrated hardware and software solutions, wide range of compatible accessories. Weaknesses: High initial investment costs, need for specialized training, potential limitations in analyzing highly complex or unstable intermediates.
Innovative Approaches in Reaction Pathway Analysis
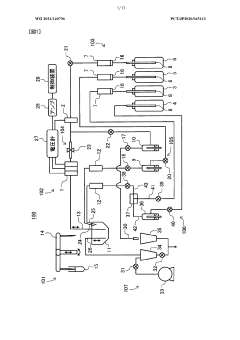
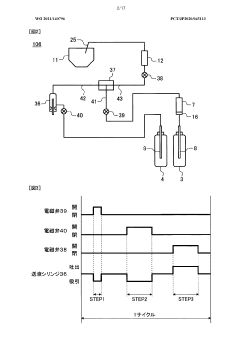
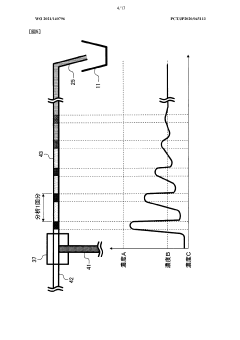
Analytical tool for for detecting sample supply condition
PatentActiveEP2916126A1
Innovation
- A method involving a working electrode and counter electrode with divided active portions within the flow path, where the detection current is analyzed for threshold exceedance, peak detection, and monotonic decrease to determine sufficient sample supply, without the need for additional detection electrodes or complex structures.
Electrolyte analysis apparatus
PatentWO2021140796A1
Innovation
- A simplified device configuration that includes a high concentration reagent bottle, a reagent diluent bottle, and a liquid feeding mechanism to merge and mix the reagents at a predetermined ratio, ensuring accurate dilution and preparation of reagents within the dilution tank, reducing the need for frequent reagent replacements and calibration.
Electrochemical Industry Standards
The electrochemical industry relies heavily on standardized practices and guidelines to ensure safety, efficiency, and consistency in operations. These standards play a crucial role in the development and implementation of analytical tools for electrolytic cell reaction pathways. Organizations such as the International Electrotechnical Commission (IEC) and the American Society for Testing and Materials (ASTM) have established comprehensive standards that govern various aspects of electrochemical processes and equipment.
One of the key areas addressed by these standards is the design and operation of electrolytic cells. The IEC 60050-831 standard, for instance, provides a standardized vocabulary for electrochemistry and electrochemical applications, ensuring clear communication among industry professionals. This common language is essential when developing and utilizing analytical tools for studying reaction pathways in electrolytic cells.
Safety standards are paramount in the electrochemical industry. The IEC 62282 series of standards focuses on fuel cell technologies, including safety requirements for various types of fuel cells. These standards are particularly relevant when analyzing reaction pathways in fuel cell electrolytic processes, as they provide guidelines for safe experimental setups and data collection methods.
Performance evaluation standards, such as ASTM E1641, outline procedures for decomposition kinetics by thermogravimetry. This standard is applicable to the analysis of reaction pathways in electrolytic cells, providing a framework for interpreting thermal analysis data and understanding reaction mechanisms.
The ASTM G5 standard practice for making potentiostatic and potentiodynamic anodic polarization measurements is another crucial guideline. It offers a standardized approach to electrochemical measurements, which is essential for developing and validating analytical tools used in studying electrolytic cell reaction pathways.
Calibration and measurement standards, like ISO/IEC 17025, ensure the accuracy and reliability of analytical instruments used in electrochemical research. This standard provides general requirements for the competence of testing and calibration laboratories, which is critical when developing and implementing analytical tools for reaction pathway studies.
Environmental considerations are also addressed in industry standards. The ISO 14001 standard for environmental management systems can be applied to electrochemical processes, ensuring that analytical methods and tools are developed and used in an environmentally responsible manner.
As the field of electrochemistry continues to evolve, these standards are regularly updated to incorporate new technologies and methodologies. Researchers and industry professionals working on analytical tools for electrolytic cell reaction pathways must stay informed about the latest revisions to ensure compliance and maintain best practices in their work.
One of the key areas addressed by these standards is the design and operation of electrolytic cells. The IEC 60050-831 standard, for instance, provides a standardized vocabulary for electrochemistry and electrochemical applications, ensuring clear communication among industry professionals. This common language is essential when developing and utilizing analytical tools for studying reaction pathways in electrolytic cells.
Safety standards are paramount in the electrochemical industry. The IEC 62282 series of standards focuses on fuel cell technologies, including safety requirements for various types of fuel cells. These standards are particularly relevant when analyzing reaction pathways in fuel cell electrolytic processes, as they provide guidelines for safe experimental setups and data collection methods.
Performance evaluation standards, such as ASTM E1641, outline procedures for decomposition kinetics by thermogravimetry. This standard is applicable to the analysis of reaction pathways in electrolytic cells, providing a framework for interpreting thermal analysis data and understanding reaction mechanisms.
The ASTM G5 standard practice for making potentiostatic and potentiodynamic anodic polarization measurements is another crucial guideline. It offers a standardized approach to electrochemical measurements, which is essential for developing and validating analytical tools used in studying electrolytic cell reaction pathways.
Calibration and measurement standards, like ISO/IEC 17025, ensure the accuracy and reliability of analytical instruments used in electrochemical research. This standard provides general requirements for the competence of testing and calibration laboratories, which is critical when developing and implementing analytical tools for reaction pathway studies.
Environmental considerations are also addressed in industry standards. The ISO 14001 standard for environmental management systems can be applied to electrochemical processes, ensuring that analytical methods and tools are developed and used in an environmentally responsible manner.
As the field of electrochemistry continues to evolve, these standards are regularly updated to incorporate new technologies and methodologies. Researchers and industry professionals working on analytical tools for electrolytic cell reaction pathways must stay informed about the latest revisions to ensure compliance and maintain best practices in their work.
Environmental Impact of Electrolytic Processes
Electrolytic processes, while essential for various industrial applications, can have significant environmental impacts that require careful consideration and management. The environmental footprint of these processes extends beyond the immediate production site, affecting air, water, and soil quality, as well as contributing to broader ecological concerns.
One of the primary environmental concerns associated with electrolytic processes is the emission of greenhouse gases, particularly in cases where the electricity used is generated from fossil fuel sources. The carbon footprint of electrolytic operations can be substantial, contributing to global climate change. However, the increasing adoption of renewable energy sources for powering electrolytic cells is gradually mitigating this impact.
Water pollution is another critical environmental issue stemming from electrolytic processes. The discharge of electrolytes, metal ions, and other chemical byproducts can contaminate local water bodies if not properly treated. This pollution can have far-reaching effects on aquatic ecosystems, potentially disrupting food chains and biodiversity. Implementing advanced wastewater treatment systems and closed-loop water recycling processes is crucial for minimizing these impacts.
The production and disposal of electrodes used in electrolytic cells also present environmental challenges. Many electrodes contain heavy metals or other toxic materials that can leach into the environment if not handled and disposed of properly. Developing more environmentally friendly electrode materials and improving recycling techniques for spent electrodes are active areas of research aimed at reducing this environmental burden.
Soil contamination is another potential consequence of electrolytic processes, particularly in cases of accidental spills or improper waste management. Heavy metals and other pollutants can accumulate in soil, affecting its fertility and posing risks to plant and animal life. Implementing robust containment measures and adhering to strict waste management protocols are essential for preventing soil contamination.
The energy-intensive nature of many electrolytic processes also contributes to their environmental impact. Efforts to improve energy efficiency through process optimization, advanced cell designs, and the use of more efficient catalysts can significantly reduce the overall environmental footprint of these operations.
As environmental regulations become more stringent globally, industries relying on electrolytic processes are increasingly focusing on developing cleaner technologies and implementing more sustainable practices. This includes exploring alternative reaction pathways that minimize harmful byproducts, adopting circular economy principles to reduce waste, and investing in environmental monitoring and remediation technologies.
One of the primary environmental concerns associated with electrolytic processes is the emission of greenhouse gases, particularly in cases where the electricity used is generated from fossil fuel sources. The carbon footprint of electrolytic operations can be substantial, contributing to global climate change. However, the increasing adoption of renewable energy sources for powering electrolytic cells is gradually mitigating this impact.
Water pollution is another critical environmental issue stemming from electrolytic processes. The discharge of electrolytes, metal ions, and other chemical byproducts can contaminate local water bodies if not properly treated. This pollution can have far-reaching effects on aquatic ecosystems, potentially disrupting food chains and biodiversity. Implementing advanced wastewater treatment systems and closed-loop water recycling processes is crucial for minimizing these impacts.
The production and disposal of electrodes used in electrolytic cells also present environmental challenges. Many electrodes contain heavy metals or other toxic materials that can leach into the environment if not handled and disposed of properly. Developing more environmentally friendly electrode materials and improving recycling techniques for spent electrodes are active areas of research aimed at reducing this environmental burden.
Soil contamination is another potential consequence of electrolytic processes, particularly in cases of accidental spills or improper waste management. Heavy metals and other pollutants can accumulate in soil, affecting its fertility and posing risks to plant and animal life. Implementing robust containment measures and adhering to strict waste management protocols are essential for preventing soil contamination.
The energy-intensive nature of many electrolytic processes also contributes to their environmental impact. Efforts to improve energy efficiency through process optimization, advanced cell designs, and the use of more efficient catalysts can significantly reduce the overall environmental footprint of these operations.
As environmental regulations become more stringent globally, industries relying on electrolytic processes are increasingly focusing on developing cleaner technologies and implementing more sustainable practices. This includes exploring alternative reaction pathways that minimize harmful byproducts, adopting circular economy principles to reduce waste, and investing in environmental monitoring and remediation technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!