Influence of Electric Field Fluctuations on Electrolytic Cell Performance
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolytic Cell Tech Background and Objectives
Electrolytic cells have been a cornerstone of industrial electrochemistry for over a century, playing a crucial role in various applications ranging from metal production to water treatment. The technology's evolution has been marked by continuous improvements in efficiency, scalability, and environmental sustainability. As we delve into the influence of electric field fluctuations on electrolytic cell performance, it is essential to understand the historical context and current technological landscape.
The primary objective of this research is to comprehensively analyze the impact of electric field fluctuations on the performance of electrolytic cells. This investigation aims to uncover the intricate relationships between electric field stability and key performance indicators such as energy efficiency, product quality, and process reliability. By gaining a deeper understanding of these dynamics, we seek to identify potential optimization strategies and innovative solutions to enhance electrolytic cell operations.
Electrolytic cells operate on the principle of electrolysis, where electrical energy drives non-spontaneous chemical reactions. The stability and uniformity of the electric field within these cells are critical factors that can significantly influence the overall process efficiency and product quality. Fluctuations in the electric field can arise from various sources, including power supply instabilities, electrode degradation, and changes in electrolyte composition.
Recent technological advancements have brought increased attention to the precise control and manipulation of electric fields within electrolytic cells. The integration of advanced power electronics, real-time monitoring systems, and sophisticated control algorithms has opened new avenues for optimizing cell performance. These developments have set the stage for a more nuanced understanding of how electric field fluctuations impact the intricate electrochemical processes occurring within the cell.
As we explore this topic, we will examine the current state of electrolytic cell technology, focusing on the latest innovations in electrode materials, cell design, and process control. We will also investigate emerging trends in the field, such as the application of pulsed electric fields and the use of advanced modeling techniques to predict and mitigate the effects of field fluctuations.
The outcomes of this research are expected to have far-reaching implications for industries relying on electrolytic processes. By elucidating the relationship between electric field stability and cell performance, we aim to provide valuable insights that can guide future technological developments and operational strategies. This knowledge will be instrumental in designing more efficient, reliable, and sustainable electrolytic systems, ultimately contributing to advancements in sectors such as metal production, chemical manufacturing, and renewable energy storage.
The primary objective of this research is to comprehensively analyze the impact of electric field fluctuations on the performance of electrolytic cells. This investigation aims to uncover the intricate relationships between electric field stability and key performance indicators such as energy efficiency, product quality, and process reliability. By gaining a deeper understanding of these dynamics, we seek to identify potential optimization strategies and innovative solutions to enhance electrolytic cell operations.
Electrolytic cells operate on the principle of electrolysis, where electrical energy drives non-spontaneous chemical reactions. The stability and uniformity of the electric field within these cells are critical factors that can significantly influence the overall process efficiency and product quality. Fluctuations in the electric field can arise from various sources, including power supply instabilities, electrode degradation, and changes in electrolyte composition.
Recent technological advancements have brought increased attention to the precise control and manipulation of electric fields within electrolytic cells. The integration of advanced power electronics, real-time monitoring systems, and sophisticated control algorithms has opened new avenues for optimizing cell performance. These developments have set the stage for a more nuanced understanding of how electric field fluctuations impact the intricate electrochemical processes occurring within the cell.
As we explore this topic, we will examine the current state of electrolytic cell technology, focusing on the latest innovations in electrode materials, cell design, and process control. We will also investigate emerging trends in the field, such as the application of pulsed electric fields and the use of advanced modeling techniques to predict and mitigate the effects of field fluctuations.
The outcomes of this research are expected to have far-reaching implications for industries relying on electrolytic processes. By elucidating the relationship between electric field stability and cell performance, we aim to provide valuable insights that can guide future technological developments and operational strategies. This knowledge will be instrumental in designing more efficient, reliable, and sustainable electrolytic systems, ultimately contributing to advancements in sectors such as metal production, chemical manufacturing, and renewable energy storage.
Market Analysis for Electrolytic Cell Applications
The electrolytic cell market has experienced significant growth in recent years, driven by increasing demand across various industries. The global market for electrolytic cells is projected to reach substantial value by 2025, with a compound annual growth rate (CAGR) exceeding industry averages. This growth is primarily attributed to the rising adoption of electrolytic processes in sectors such as chemical manufacturing, water treatment, and energy storage.
In the chemical industry, electrolytic cells play a crucial role in the production of chlorine, sodium hydroxide, and hydrogen. The demand for these chemicals continues to rise, particularly in developing economies, fueling the expansion of electrolytic cell applications. The water treatment sector also presents a lucrative market for electrolytic cells, as governments worldwide implement stricter regulations on water quality and wastewater management.
The energy storage sector has emerged as a key driver for electrolytic cell market growth. With the increasing focus on renewable energy sources and the need for efficient energy storage solutions, electrolytic cells are gaining traction in applications such as hydrogen production for fuel cells and grid-scale energy storage systems. This trend is expected to accelerate as countries strive to achieve their clean energy targets and reduce carbon emissions.
Geographically, Asia-Pacific dominates the electrolytic cell market, followed by North America and Europe. The rapid industrialization and urbanization in countries like China and India are driving the demand for electrolytic cells in various applications. In North America and Europe, the market is primarily driven by technological advancements and stringent environmental regulations.
The influence of electric field fluctuations on electrolytic cell performance is a critical factor affecting market dynamics. As industries seek to optimize their processes and improve efficiency, there is a growing demand for electrolytic cells that can maintain stable performance under varying electric field conditions. This has led to increased research and development efforts focused on enhancing cell designs and control systems to mitigate the impact of electric field fluctuations.
Market players are investing in innovative technologies to address this challenge, including advanced electrode materials, improved cell geometries, and sophisticated control algorithms. These developments are expected to drive market growth and create new opportunities for electrolytic cell manufacturers and suppliers.
In the chemical industry, electrolytic cells play a crucial role in the production of chlorine, sodium hydroxide, and hydrogen. The demand for these chemicals continues to rise, particularly in developing economies, fueling the expansion of electrolytic cell applications. The water treatment sector also presents a lucrative market for electrolytic cells, as governments worldwide implement stricter regulations on water quality and wastewater management.
The energy storage sector has emerged as a key driver for electrolytic cell market growth. With the increasing focus on renewable energy sources and the need for efficient energy storage solutions, electrolytic cells are gaining traction in applications such as hydrogen production for fuel cells and grid-scale energy storage systems. This trend is expected to accelerate as countries strive to achieve their clean energy targets and reduce carbon emissions.
Geographically, Asia-Pacific dominates the electrolytic cell market, followed by North America and Europe. The rapid industrialization and urbanization in countries like China and India are driving the demand for electrolytic cells in various applications. In North America and Europe, the market is primarily driven by technological advancements and stringent environmental regulations.
The influence of electric field fluctuations on electrolytic cell performance is a critical factor affecting market dynamics. As industries seek to optimize their processes and improve efficiency, there is a growing demand for electrolytic cells that can maintain stable performance under varying electric field conditions. This has led to increased research and development efforts focused on enhancing cell designs and control systems to mitigate the impact of electric field fluctuations.
Market players are investing in innovative technologies to address this challenge, including advanced electrode materials, improved cell geometries, and sophisticated control algorithms. These developments are expected to drive market growth and create new opportunities for electrolytic cell manufacturers and suppliers.
Electric Field Fluctuations: Current Challenges
Electric field fluctuations pose significant challenges in the operation and performance optimization of electrolytic cells. These fluctuations can arise from various sources, including power supply instabilities, electrode geometry irregularities, and changes in electrolyte composition. The primary concern is the impact on current distribution within the cell, which directly affects the efficiency and uniformity of the electrolytic process.
One of the most pressing challenges is the difficulty in maintaining a stable and uniform electric field across the entire electrode surface. Fluctuations can lead to localized areas of high or low current density, resulting in uneven material deposition or dissolution. This non-uniformity not only reduces overall process efficiency but can also lead to product quality issues and accelerated electrode degradation.
Another critical challenge is the complex interplay between electric field fluctuations and fluid dynamics within the electrolytic cell. Variations in the electric field can induce localized changes in electrolyte flow patterns, potentially leading to concentration gradients and mass transfer limitations. These effects can further exacerbate the non-uniformity of the electrolytic process and introduce additional variability in cell performance.
The accurate measurement and characterization of electric field fluctuations within operational electrolytic cells present a significant technical hurdle. Traditional sensing methods often struggle to provide real-time, high-resolution data without interfering with the electrolytic process itself. This limitation hampers the development of effective control strategies to mitigate the impact of fluctuations.
Furthermore, the dynamic nature of electric field fluctuations poses challenges for modeling and prediction. Current computational models often struggle to accurately capture the full complexity of these fluctuations, particularly in large-scale industrial applications. This gap in predictive capabilities hinders the optimization of cell design and operating parameters to minimize the impact of fluctuations.
The influence of electric field fluctuations on electrode kinetics and reaction mechanisms is another area of concern. Rapid changes in local electric field strength can affect the rate and selectivity of electrochemical reactions, potentially leading to unexpected byproduct formation or reduced product purity. Understanding and controlling these effects is crucial for maintaining consistent product quality and process efficiency.
Lastly, the development of robust control systems capable of rapidly responding to and mitigating electric field fluctuations remains a significant challenge. Such systems must integrate advanced sensing technologies, predictive algorithms, and adaptive control strategies to maintain optimal cell performance under varying conditions. The complexity of this task is compounded by the need for solutions that are both effective and economically viable for industrial-scale implementation.
One of the most pressing challenges is the difficulty in maintaining a stable and uniform electric field across the entire electrode surface. Fluctuations can lead to localized areas of high or low current density, resulting in uneven material deposition or dissolution. This non-uniformity not only reduces overall process efficiency but can also lead to product quality issues and accelerated electrode degradation.
Another critical challenge is the complex interplay between electric field fluctuations and fluid dynamics within the electrolytic cell. Variations in the electric field can induce localized changes in electrolyte flow patterns, potentially leading to concentration gradients and mass transfer limitations. These effects can further exacerbate the non-uniformity of the electrolytic process and introduce additional variability in cell performance.
The accurate measurement and characterization of electric field fluctuations within operational electrolytic cells present a significant technical hurdle. Traditional sensing methods often struggle to provide real-time, high-resolution data without interfering with the electrolytic process itself. This limitation hampers the development of effective control strategies to mitigate the impact of fluctuations.
Furthermore, the dynamic nature of electric field fluctuations poses challenges for modeling and prediction. Current computational models often struggle to accurately capture the full complexity of these fluctuations, particularly in large-scale industrial applications. This gap in predictive capabilities hinders the optimization of cell design and operating parameters to minimize the impact of fluctuations.
The influence of electric field fluctuations on electrode kinetics and reaction mechanisms is another area of concern. Rapid changes in local electric field strength can affect the rate and selectivity of electrochemical reactions, potentially leading to unexpected byproduct formation or reduced product purity. Understanding and controlling these effects is crucial for maintaining consistent product quality and process efficiency.
Lastly, the development of robust control systems capable of rapidly responding to and mitigating electric field fluctuations remains a significant challenge. Such systems must integrate advanced sensing technologies, predictive algorithms, and adaptive control strategies to maintain optimal cell performance under varying conditions. The complexity of this task is compounded by the need for solutions that are both effective and economically viable for industrial-scale implementation.
Current Solutions for Field Fluctuation Mitigation
01 Electrode materials and configurations
The performance of electrolytic cells can be significantly improved by optimizing electrode materials and configurations. This includes using advanced materials with high conductivity and catalytic activity, as well as designing electrode structures that maximize surface area and facilitate efficient mass transfer. Proper selection and arrangement of electrodes can enhance reaction kinetics, reduce overpotential, and increase overall cell efficiency.- Electrode materials and configurations: The performance of electrolytic cells can be significantly improved by optimizing electrode materials and configurations. This includes using advanced materials with high conductivity and catalytic activity, as well as designing electrode structures that maximize surface area and facilitate efficient mass transfer. Such improvements can lead to enhanced reaction rates, reduced energy consumption, and increased overall cell efficiency.
- Electrolyte composition and optimization: The composition of the electrolyte plays a crucial role in electrolytic cell performance. Optimizing the electrolyte by adjusting its concentration, pH, and additives can improve conductivity, reduce side reactions, and enhance the stability of the electrochemical process. This can result in higher current efficiency, better product quality, and extended cell lifespan.
- Temperature and pressure control: Maintaining optimal temperature and pressure conditions within the electrolytic cell is essential for maximizing performance. Proper control of these parameters can influence reaction kinetics, mass transfer rates, and the solubility of reactants and products. Advanced temperature and pressure management systems can lead to improved energy efficiency and product yield.
- Membrane technology and separators: The use of advanced membrane technology and separators in electrolytic cells can significantly enhance performance by improving ion selectivity, reducing crossover of unwanted species, and increasing overall efficiency. Innovations in this area include the development of novel polymer membranes, composite materials, and nanostructured separators that offer superior conductivity and durability.
- Process monitoring and control systems: Implementing sophisticated process monitoring and control systems can greatly improve electrolytic cell performance. These systems can include real-time sensors for measuring key parameters such as current density, voltage, and electrolyte composition. Advanced control algorithms and machine learning techniques can be used to optimize operating conditions dynamically, leading to improved efficiency, product quality, and cell longevity.
02 Electrolyte composition and management
The composition and management of the electrolyte play a crucial role in electrolytic cell performance. This involves optimizing the electrolyte's ionic conductivity, pH, and concentration to enhance reaction rates and selectivity. Proper electrolyte management includes techniques for maintaining electrolyte balance, preventing contamination, and ensuring uniform distribution within the cell. These factors contribute to improved current efficiency and product quality.Expand Specific Solutions03 Cell design and operating conditions
The design of the electrolytic cell and its operating conditions significantly impact performance. This includes optimizing cell geometry, membrane selection, and flow patterns to enhance mass transfer and reduce energy losses. Controlling parameters such as temperature, pressure, and current density can improve reaction kinetics and efficiency. Advanced cell designs may incorporate features like flow-through electrodes or divided cells to enhance specific processes.Expand Specific Solutions04 Membrane technology and ion exchange
Advancements in membrane technology and ion exchange processes can greatly enhance electrolytic cell performance. This includes developing selective membranes that improve product separation and purity while reducing energy consumption. Ion exchange materials can be used to control electrolyte composition and remove impurities, leading to more stable and efficient cell operation. These technologies are particularly important in applications such as chlor-alkali production and water electrolysis.Expand Specific Solutions05 Process monitoring and control systems
Implementing advanced monitoring and control systems can significantly improve electrolytic cell performance. This includes real-time monitoring of key parameters such as voltage, current, temperature, and electrolyte composition. Automated control systems can adjust operating conditions to maintain optimal performance and respond to changes in feed composition or product demand. Integration of data analytics and machine learning algorithms can further optimize cell operation and predict maintenance needs.Expand Specific Solutions
Key Players in Electrolytic Industry
The field of electric field fluctuations in electrolytic cell performance is in a developing stage, with growing market potential as industries seek to optimize energy efficiency and process control. The market size is expanding, driven by applications in various sectors including energy storage, electrochemistry, and industrial manufacturing. Technologically, the field is advancing rapidly, with companies like Siemens Energy Global GmbH & Co. KG and Evonik Litarion GmbH leading in innovation. Academic institutions such as Tianjin University and The Hong Kong Polytechnic University are contributing significant research. While not yet fully mature, the technology is progressing towards practical applications, with companies like MaxCyte, Inc. and Nanovis LLC exploring novel approaches in related fields.
Siemens Energy Global GmbH & Co. KG
Technical Solution: Siemens Energy has developed a comprehensive approach to managing electric field fluctuations in large-scale industrial electrolytic cells. Their solution incorporates advanced electromagnetic modeling to predict and mitigate the impact of field variations on cell performance. Siemens has implemented adaptive power supply systems that can rapidly adjust to changing field conditions, maintaining optimal electrolyte behavior[7]. The company has also pioneered the use of smart electrode designs that incorporate field-sensing elements, allowing for real-time monitoring and control of local electric field distributions within the cell. Furthermore, Siemens has developed sophisticated software algorithms that can optimize cell operation parameters in response to both internal and external field fluctuations, maximizing efficiency and product quality[8].
Strengths: Expertise in large-scale industrial applications. Integrated approach combining hardware, software, and process optimization. Weaknesses: Solutions may be complex and costly to implement in smaller-scale operations.
Tianjin University
Technical Solution: Researchers at Tianjin University have made significant advancements in understanding and controlling the influence of electric field fluctuations on electrolytic cell performance. Their approach focuses on fundamental studies of ion transport mechanisms under varying field conditions. The university has developed novel in-situ characterization techniques that allow for real-time observation of electrolyte behavior during field fluctuations[9]. This has led to the creation of predictive models that can accurately simulate cell performance under a wide range of field conditions. Additionally, Tianjin University has explored the use of nanostructured electrode materials that exhibit enhanced stability in fluctuating electric fields, potentially leading to more robust electrolytic cell designs[10].
Strengths: Strong focus on fundamental research and theoretical modeling. Development of advanced characterization techniques. Weaknesses: May face challenges in translating academic research into practical industrial solutions.
Innovations in Electric Field Stability
Application of electric and electromagnetic fields in solid state fermentation to manufacture edible mycelium-based products
PatentPendingEP4588363A1
Innovation
- Applying electrical and/or static magnetic fields, and/or extremely low electromagnetic fields during solid-state fermentation to influence enzyme activity and fungal growth by altering DNA structure and calcium ion distribution, using equipment like Helmholtz or Merritt coils to generate controlled fields.
Method and apparatus for treating tumors using low strength electric fields
PatentInactiveUS20080132963A1
Innovation
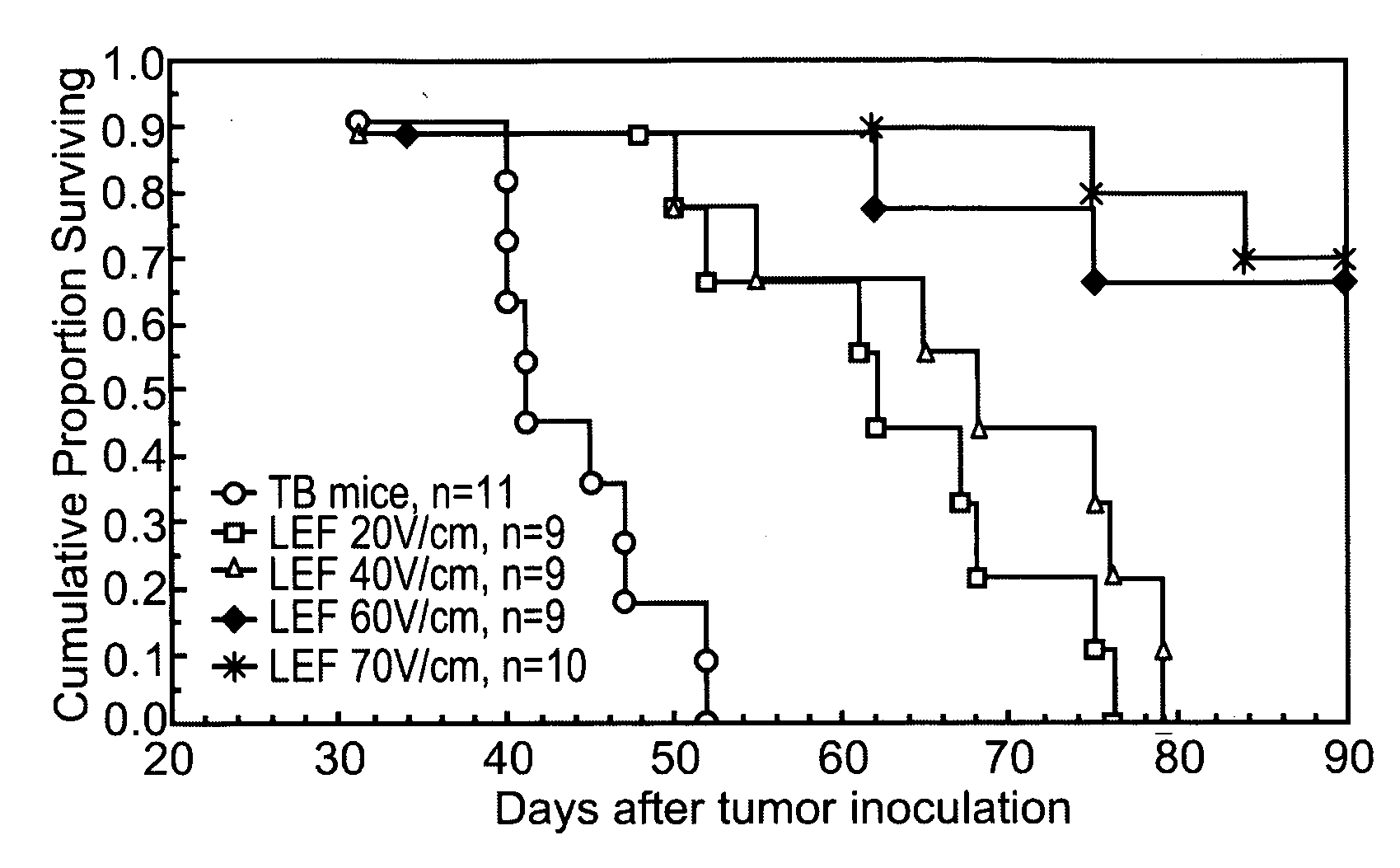
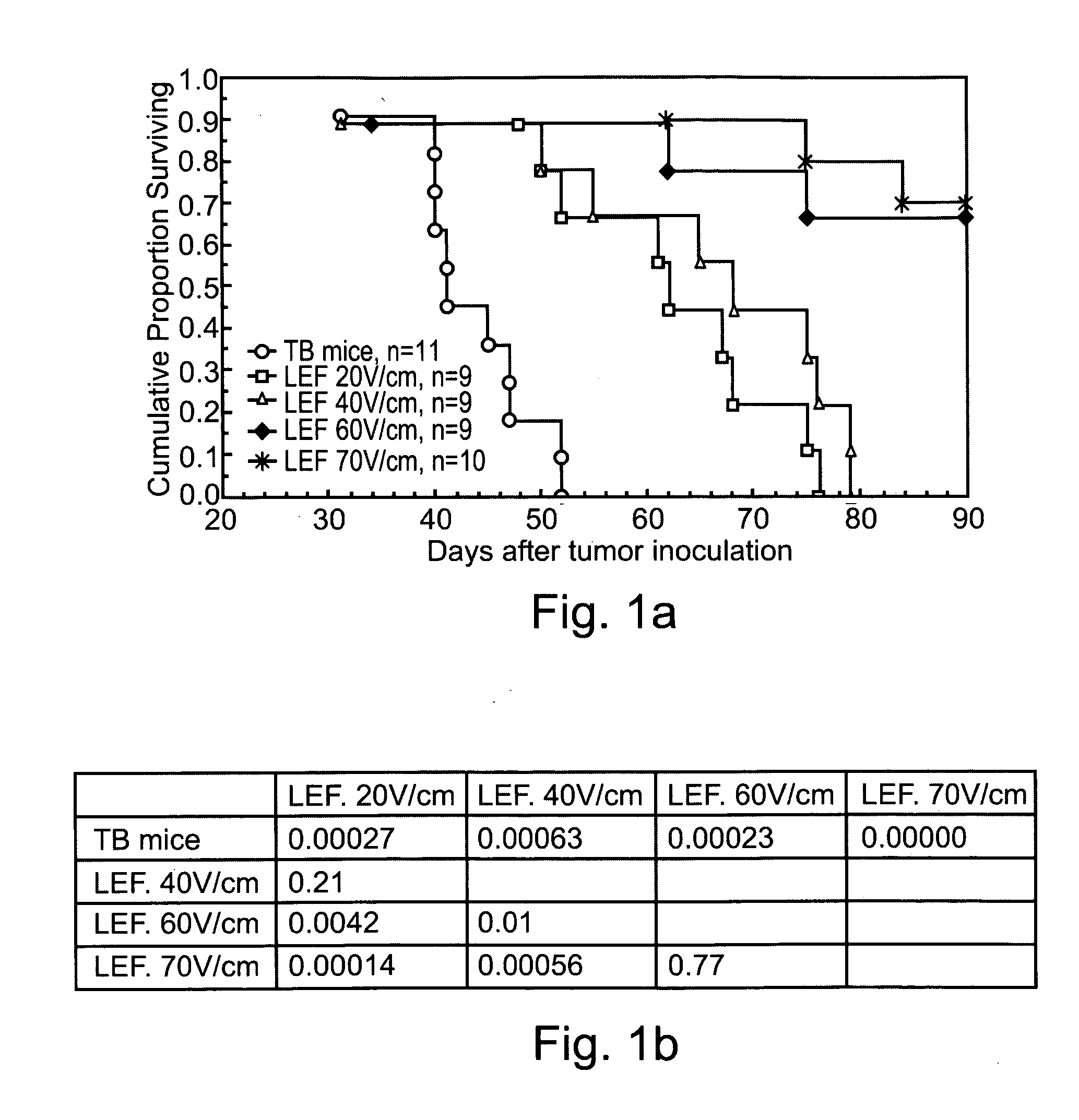
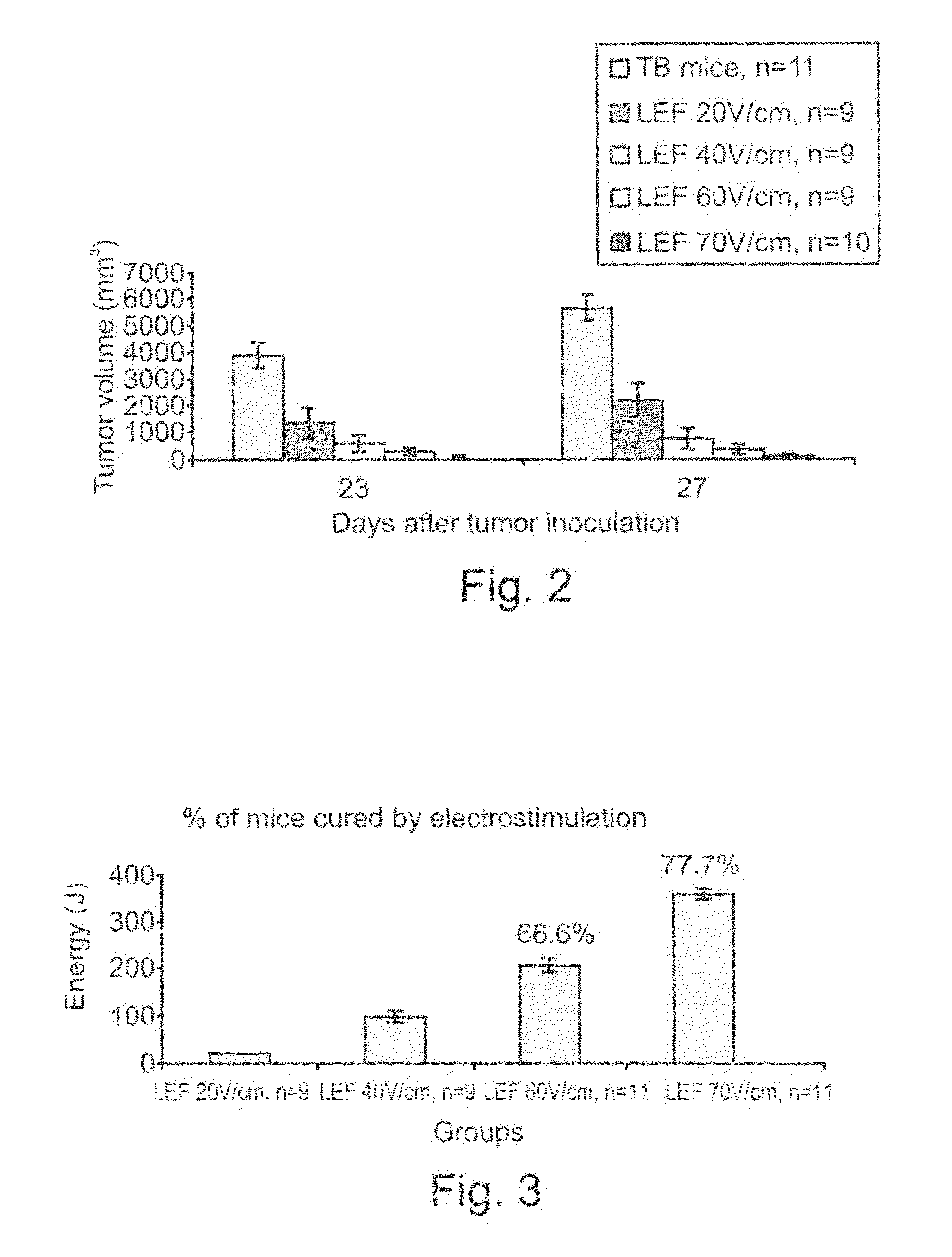
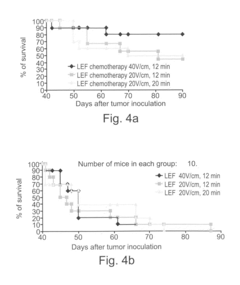
- Application of low-strength pulsed electric fields (20-70 V/cm) with specific repetition frequencies and pulse widths to induce endocytosis-mediated cell death and enhance immune responses in tumors, potentially with adjunct cytotoxic agents like bleomycin, 5-fluorouracil, or taxol, using an apparatus with controlled electrode placement and power delivery.
Environmental Impact of Electrolytic Processes
Electrolytic processes, while essential in various industrial applications, have significant environmental implications that warrant careful consideration. The environmental impact of these processes primarily stems from energy consumption, resource utilization, and potential pollutant emissions. One of the most pressing concerns is the high energy demand of electrolytic cells, which often relies on fossil fuel-based electricity generation, contributing to greenhouse gas emissions and climate change.
The production of chlorine and sodium hydroxide through the chlor-alkali process, a common electrolytic application, exemplifies these environmental challenges. This process consumes substantial amounts of electricity and may result in the release of chlorine gas, a potent environmental hazard, if not properly managed. Additionally, the use of mercury cells in some older chlor-alkali plants has led to mercury contamination in surrounding ecosystems, affecting both aquatic and terrestrial life.
Water usage and wastewater discharge present another significant environmental concern. Electrolytic processes often require large volumes of water for cooling and as a medium for electrolysis. The resulting wastewater may contain dissolved metals, salts, and other contaminants that can harm aquatic ecosystems if not adequately treated before release. Moreover, the extraction and processing of raw materials used in electrolytic processes, such as bauxite for aluminum production, can lead to habitat destruction, soil erosion, and biodiversity loss in mining areas.
The disposal of spent electrolytes and electrode materials also poses environmental risks. These materials may contain heavy metals and other toxic substances that can leach into soil and groundwater if not properly handled. In the case of lithium-ion battery production, the extraction of lithium and cobalt has raised concerns about water depletion and pollution in mining regions, particularly in water-stressed areas.
However, it is important to note that electrolytic processes also play a crucial role in environmental protection and sustainable technologies. For instance, electrolysis is fundamental in the production of hydrogen for fuel cells, which offer a clean alternative to fossil fuels in transportation and energy storage. Similarly, electrochemical water treatment technologies are increasingly used for the removal of contaminants from wastewater, contributing to water conservation efforts.
To mitigate the environmental impact of electrolytic processes, industries are adopting cleaner technologies and more efficient practices. These include the development of membrane cell technology to replace mercury cells in chlor-alkali production, implementation of closed-loop systems to reduce water consumption and wastewater discharge, and the use of renewable energy sources to power electrolytic cells. Additionally, advancements in electrode materials and cell designs are improving energy efficiency and reducing the overall environmental footprint of these processes.
The production of chlorine and sodium hydroxide through the chlor-alkali process, a common electrolytic application, exemplifies these environmental challenges. This process consumes substantial amounts of electricity and may result in the release of chlorine gas, a potent environmental hazard, if not properly managed. Additionally, the use of mercury cells in some older chlor-alkali plants has led to mercury contamination in surrounding ecosystems, affecting both aquatic and terrestrial life.
Water usage and wastewater discharge present another significant environmental concern. Electrolytic processes often require large volumes of water for cooling and as a medium for electrolysis. The resulting wastewater may contain dissolved metals, salts, and other contaminants that can harm aquatic ecosystems if not adequately treated before release. Moreover, the extraction and processing of raw materials used in electrolytic processes, such as bauxite for aluminum production, can lead to habitat destruction, soil erosion, and biodiversity loss in mining areas.
The disposal of spent electrolytes and electrode materials also poses environmental risks. These materials may contain heavy metals and other toxic substances that can leach into soil and groundwater if not properly handled. In the case of lithium-ion battery production, the extraction of lithium and cobalt has raised concerns about water depletion and pollution in mining regions, particularly in water-stressed areas.
However, it is important to note that electrolytic processes also play a crucial role in environmental protection and sustainable technologies. For instance, electrolysis is fundamental in the production of hydrogen for fuel cells, which offer a clean alternative to fossil fuels in transportation and energy storage. Similarly, electrochemical water treatment technologies are increasingly used for the removal of contaminants from wastewater, contributing to water conservation efforts.
To mitigate the environmental impact of electrolytic processes, industries are adopting cleaner technologies and more efficient practices. These include the development of membrane cell technology to replace mercury cells in chlor-alkali production, implementation of closed-loop systems to reduce water consumption and wastewater discharge, and the use of renewable energy sources to power electrolytic cells. Additionally, advancements in electrode materials and cell designs are improving energy efficiency and reducing the overall environmental footprint of these processes.
Energy Efficiency in Electrolytic Systems
Energy efficiency in electrolytic systems has become a critical focus in the pursuit of sustainable industrial processes. The performance of electrolytic cells is significantly influenced by electric field fluctuations, which can impact both energy consumption and product yield. These fluctuations arise from various sources, including power supply instabilities, electrode degradation, and changes in electrolyte composition.
Recent advancements in power electronics have led to the development of more stable and precise power supplies, which can mitigate some of the electric field fluctuations. However, challenges remain in maintaining consistent field strength across large-scale industrial electrolytic cells. Researchers have identified that even minor fluctuations can lead to substantial energy losses and reduced product quality over time.
One promising approach to improving energy efficiency involves the implementation of real-time monitoring and control systems. These systems utilize advanced sensors to detect minute changes in electric field strength and distribution within the cell. By continuously adjusting power input and electrolyte parameters, these systems can maintain optimal operating conditions, thereby minimizing energy waste and maximizing product yield.
Another area of focus is the development of novel electrode materials and designs that are more resistant to degradation and can maintain consistent performance under fluctuating conditions. Nanostructured electrodes, for instance, have shown potential in providing more stable electric fields and improved energy efficiency.
The integration of artificial intelligence and machine learning algorithms into electrolytic cell management systems is also gaining traction. These technologies can predict and preemptively adjust for potential fluctuations based on historical data and real-time inputs, further enhancing energy efficiency and process stability.
As industries strive to meet increasingly stringent environmental regulations and reduce operational costs, the optimization of energy efficiency in electrolytic systems remains a key priority. The ongoing research and development in this field are not only improving the performance of existing electrolytic processes but also opening up new possibilities for more sustainable industrial applications.
Recent advancements in power electronics have led to the development of more stable and precise power supplies, which can mitigate some of the electric field fluctuations. However, challenges remain in maintaining consistent field strength across large-scale industrial electrolytic cells. Researchers have identified that even minor fluctuations can lead to substantial energy losses and reduced product quality over time.
One promising approach to improving energy efficiency involves the implementation of real-time monitoring and control systems. These systems utilize advanced sensors to detect minute changes in electric field strength and distribution within the cell. By continuously adjusting power input and electrolyte parameters, these systems can maintain optimal operating conditions, thereby minimizing energy waste and maximizing product yield.
Another area of focus is the development of novel electrode materials and designs that are more resistant to degradation and can maintain consistent performance under fluctuating conditions. Nanostructured electrodes, for instance, have shown potential in providing more stable electric fields and improved energy efficiency.
The integration of artificial intelligence and machine learning algorithms into electrolytic cell management systems is also gaining traction. These technologies can predict and preemptively adjust for potential fluctuations based on historical data and real-time inputs, further enhancing energy efficiency and process stability.
As industries strive to meet increasingly stringent environmental regulations and reduce operational costs, the optimization of energy efficiency in electrolytic systems remains a key priority. The ongoing research and development in this field are not only improving the performance of existing electrolytic processes but also opening up new possibilities for more sustainable industrial applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!