How Electrolytic Cells are Reconfiguring Chlorine Production Techniques
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chlorine Production Evolution and Objectives
Chlorine production has undergone significant transformations since its inception in the late 19th century. Initially, the chlor-alkali process relied on mercury cell technology, which dominated the industry for decades. However, environmental concerns and efficiency limitations led to the development of alternative methods. The diaphragm cell technique emerged as a more environmentally friendly option, followed by the membrane cell technology in the 1970s.
The evolution of chlorine production techniques has been driven by several key objectives. Foremost among these is the need for increased energy efficiency. As energy costs represent a substantial portion of production expenses, manufacturers have consistently sought ways to reduce power consumption. This has led to the development of more efficient electrolytic cells and improved membrane technologies.
Environmental sustainability has become another critical goal in chlorine production. The industry has moved away from mercury-based processes due to their potential for environmental contamination. Modern techniques focus on minimizing harmful emissions and waste products, aligning with global environmental regulations and corporate sustainability initiatives.
Product quality and purity have also been central objectives in the evolution of chlorine production. Advancements in electrolytic cell design and membrane technology have enabled the production of higher-grade chlorine with fewer impurities. This has expanded the applications of chlorine in various industries, including water treatment, pharmaceuticals, and electronics manufacturing.
The pursuit of increased production capacity has been a consistent trend throughout the history of chlorine manufacturing. Innovations in cell design, electrode materials, and process control have allowed for larger-scale operations and improved production rates. This has been crucial in meeting the growing global demand for chlorine and its derivatives.
Safety considerations have played a significant role in shaping chlorine production techniques. The industry has continuously worked to develop safer processes and handling methods, reducing the risks associated with chlorine production and transportation. This includes improvements in containment systems, leak detection, and emergency response protocols.
Looking ahead, the chlorine production industry aims to further optimize electrolytic cell technology. Key objectives include developing more durable and efficient electrode materials, enhancing membrane performance, and integrating advanced process control systems. Additionally, there is a growing focus on incorporating renewable energy sources into chlorine production, aligning with global efforts to reduce carbon emissions and promote sustainable industrial practices.
The evolution of chlorine production techniques has been driven by several key objectives. Foremost among these is the need for increased energy efficiency. As energy costs represent a substantial portion of production expenses, manufacturers have consistently sought ways to reduce power consumption. This has led to the development of more efficient electrolytic cells and improved membrane technologies.
Environmental sustainability has become another critical goal in chlorine production. The industry has moved away from mercury-based processes due to their potential for environmental contamination. Modern techniques focus on minimizing harmful emissions and waste products, aligning with global environmental regulations and corporate sustainability initiatives.
Product quality and purity have also been central objectives in the evolution of chlorine production. Advancements in electrolytic cell design and membrane technology have enabled the production of higher-grade chlorine with fewer impurities. This has expanded the applications of chlorine in various industries, including water treatment, pharmaceuticals, and electronics manufacturing.
The pursuit of increased production capacity has been a consistent trend throughout the history of chlorine manufacturing. Innovations in cell design, electrode materials, and process control have allowed for larger-scale operations and improved production rates. This has been crucial in meeting the growing global demand for chlorine and its derivatives.
Safety considerations have played a significant role in shaping chlorine production techniques. The industry has continuously worked to develop safer processes and handling methods, reducing the risks associated with chlorine production and transportation. This includes improvements in containment systems, leak detection, and emergency response protocols.
Looking ahead, the chlorine production industry aims to further optimize electrolytic cell technology. Key objectives include developing more durable and efficient electrode materials, enhancing membrane performance, and integrating advanced process control systems. Additionally, there is a growing focus on incorporating renewable energy sources into chlorine production, aligning with global efforts to reduce carbon emissions and promote sustainable industrial practices.
Market Demand Analysis for Chlorine
The global chlorine market has been experiencing steady growth, driven by increasing demand across various industries. Chlorine, a versatile chemical element, finds applications in water treatment, pharmaceuticals, plastics, and numerous other sectors. The market demand for chlorine is closely tied to economic growth and industrial development.
In recent years, the water treatment sector has emerged as a significant driver of chlorine demand. With growing concerns over water quality and the need for safe drinking water, particularly in developing countries, the use of chlorine in water disinfection processes has increased substantially. This trend is expected to continue as urbanization and population growth put further pressure on water resources.
The plastics industry remains a major consumer of chlorine, particularly in the production of polyvinyl chloride (PVC). The construction sector's recovery in many regions has boosted PVC demand, consequently driving chlorine consumption. Additionally, the automotive and electronics industries contribute to the demand for chlorine-based products, further supporting market growth.
The pharmaceutical sector represents another key area of chlorine demand. Chlorine and its derivatives are essential in the synthesis of various drugs and medical products. As healthcare needs expand globally, particularly in emerging markets, this sector is expected to contribute significantly to chlorine demand growth.
However, the chlorine market faces challenges from environmental concerns and regulatory pressures. There is a growing push for more sustainable and environmentally friendly alternatives in various applications, which could potentially impact chlorine demand in certain sectors. This has led to increased research and development efforts in alternative technologies and production methods.
The geographical distribution of chlorine demand shows variations, with Asia-Pacific leading in consumption due to rapid industrialization and urban development. North America and Europe maintain stable demand, primarily driven by established industrial sectors and water treatment needs.
Market analysts project continued growth in the global chlorine market, albeit at a moderate pace. Factors such as increasing industrial activities in emerging economies, ongoing urbanization, and the critical role of chlorine in water treatment are expected to sustain demand. However, the market must navigate challenges such as environmental regulations and the development of alternative technologies to maintain its growth trajectory.
In recent years, the water treatment sector has emerged as a significant driver of chlorine demand. With growing concerns over water quality and the need for safe drinking water, particularly in developing countries, the use of chlorine in water disinfection processes has increased substantially. This trend is expected to continue as urbanization and population growth put further pressure on water resources.
The plastics industry remains a major consumer of chlorine, particularly in the production of polyvinyl chloride (PVC). The construction sector's recovery in many regions has boosted PVC demand, consequently driving chlorine consumption. Additionally, the automotive and electronics industries contribute to the demand for chlorine-based products, further supporting market growth.
The pharmaceutical sector represents another key area of chlorine demand. Chlorine and its derivatives are essential in the synthesis of various drugs and medical products. As healthcare needs expand globally, particularly in emerging markets, this sector is expected to contribute significantly to chlorine demand growth.
However, the chlorine market faces challenges from environmental concerns and regulatory pressures. There is a growing push for more sustainable and environmentally friendly alternatives in various applications, which could potentially impact chlorine demand in certain sectors. This has led to increased research and development efforts in alternative technologies and production methods.
The geographical distribution of chlorine demand shows variations, with Asia-Pacific leading in consumption due to rapid industrialization and urban development. North America and Europe maintain stable demand, primarily driven by established industrial sectors and water treatment needs.
Market analysts project continued growth in the global chlorine market, albeit at a moderate pace. Factors such as increasing industrial activities in emerging economies, ongoing urbanization, and the critical role of chlorine in water treatment are expected to sustain demand. However, the market must navigate challenges such as environmental regulations and the development of alternative technologies to maintain its growth trajectory.
Electrolytic Cell Technology Status and Challenges
Electrolytic cell technology for chlorine production has made significant strides in recent years, yet it still faces several challenges. The current state of the art primarily revolves around membrane cell technology, which has largely replaced the older mercury and diaphragm cell processes due to its superior energy efficiency and environmental performance.
Membrane cells utilize ion-exchange membranes to separate the anode and cathode compartments, allowing for the production of high-purity chlorine gas and caustic soda solution. This technology has become the industry standard, with continuous improvements in membrane materials and electrode designs leading to increased efficiency and reduced energy consumption.
However, despite these advancements, the chlor-alkali industry still grapples with several key challenges. Energy consumption remains a significant concern, as electrolytic processes are inherently energy-intensive. While membrane cells have improved efficiency, there is still a pressing need for further reductions in energy usage to meet increasingly stringent environmental regulations and economic pressures.
Another major challenge is the durability and lifespan of cell components, particularly the ion-exchange membranes. These membranes are subject to degradation over time, leading to decreased performance and the need for periodic replacement. Improving membrane longevity and resistance to fouling is a key focus area for ongoing research and development efforts.
The industry also faces challenges related to raw material quality and availability. The purity of brine feedstock can significantly impact cell performance and product quality. Ensuring a consistent supply of high-quality brine, especially in regions with limited salt resources, presents logistical and economic challenges for chlorine producers.
From a geographical perspective, the development and adoption of advanced electrolytic cell technologies are not uniform across the globe. While regions like Europe and North America have largely transitioned to membrane cell technology, some developing countries still rely on older, less efficient processes. This disparity in technology adoption creates challenges in terms of global competitiveness and environmental impact.
Looking ahead, the industry is exploring innovative approaches to address these challenges. Research is ongoing into novel electrode materials, such as dimensionally stable anodes with improved catalytic properties, to further enhance energy efficiency. Additionally, there is growing interest in the integration of renewable energy sources to power electrolytic cells, potentially offering a path to more sustainable chlorine production.
In conclusion, while electrolytic cell technology for chlorine production has made significant progress, it continues to face challenges in energy efficiency, component durability, and raw material management. Overcoming these hurdles will be crucial for the industry's future sustainability and competitiveness.
Membrane cells utilize ion-exchange membranes to separate the anode and cathode compartments, allowing for the production of high-purity chlorine gas and caustic soda solution. This technology has become the industry standard, with continuous improvements in membrane materials and electrode designs leading to increased efficiency and reduced energy consumption.
However, despite these advancements, the chlor-alkali industry still grapples with several key challenges. Energy consumption remains a significant concern, as electrolytic processes are inherently energy-intensive. While membrane cells have improved efficiency, there is still a pressing need for further reductions in energy usage to meet increasingly stringent environmental regulations and economic pressures.
Another major challenge is the durability and lifespan of cell components, particularly the ion-exchange membranes. These membranes are subject to degradation over time, leading to decreased performance and the need for periodic replacement. Improving membrane longevity and resistance to fouling is a key focus area for ongoing research and development efforts.
The industry also faces challenges related to raw material quality and availability. The purity of brine feedstock can significantly impact cell performance and product quality. Ensuring a consistent supply of high-quality brine, especially in regions with limited salt resources, presents logistical and economic challenges for chlorine producers.
From a geographical perspective, the development and adoption of advanced electrolytic cell technologies are not uniform across the globe. While regions like Europe and North America have largely transitioned to membrane cell technology, some developing countries still rely on older, less efficient processes. This disparity in technology adoption creates challenges in terms of global competitiveness and environmental impact.
Looking ahead, the industry is exploring innovative approaches to address these challenges. Research is ongoing into novel electrode materials, such as dimensionally stable anodes with improved catalytic properties, to further enhance energy efficiency. Additionally, there is growing interest in the integration of renewable energy sources to power electrolytic cells, potentially offering a path to more sustainable chlorine production.
In conclusion, while electrolytic cell technology for chlorine production has made significant progress, it continues to face challenges in energy efficiency, component durability, and raw material management. Overcoming these hurdles will be crucial for the industry's future sustainability and competitiveness.
Current Electrolytic Cell Solutions
01 Modular design for electrolytic cell reconfiguration
Electrolytic cells can be designed with modular components that allow for easy reconfiguration. This approach enables flexibility in cell arrangement, capacity adjustment, and maintenance. Modular designs can include interchangeable electrodes, separators, and electrolyte chambers, facilitating quick modifications to meet changing production requirements or to optimize performance.- Modular design for electrolytic cell reconfiguration: Electrolytic cells can be designed with modular components that allow for easy reconfiguration. This approach enables flexibility in cell arrangement, capacity adjustment, and maintenance. Modular designs can include interchangeable electrodes, separators, and electrolyte chambers, facilitating quick modifications to meet changing production requirements or to optimize performance.
- Adaptive control systems for electrolytic cells: Implementation of adaptive control systems in electrolytic cells allows for real-time adjustments based on operating conditions. These systems can monitor parameters such as current density, temperature, and electrolyte composition, and automatically reconfigure cell settings to maintain optimal performance. This approach enhances efficiency and product quality while reducing energy consumption.
- Reconfigurable electrode arrangements: Electrolytic cells can be designed with reconfigurable electrode arrangements to optimize current distribution and reaction kinetics. This may involve adjustable electrode spacing, interchangeable electrode materials, or the ability to switch between monopolar and bipolar configurations. Such flexibility allows for process optimization across different electrolytic applications.
- Scalable electrolytic cell systems: Developing scalable electrolytic cell systems allows for easy expansion or reduction of production capacity. These systems can be designed with standardized units that can be added or removed as needed, enabling rapid reconfiguration to meet changing demand. This approach also facilitates maintenance and upgrades without significant downtime.
- Smart electrolyte management systems: Integration of smart electrolyte management systems enables dynamic reconfiguration of electrolyte composition and flow patterns. These systems can adjust electrolyte concentration, pH, and circulation rates in response to changing process conditions or product specifications. This capability enhances process flexibility and product quality control in electrolytic cells.
02 Automated reconfiguration systems
Advanced electrolytic cell systems incorporate automated reconfiguration capabilities. These systems use sensors, actuators, and control algorithms to dynamically adjust cell parameters such as electrode spacing, electrolyte flow, and current distribution. Automated reconfiguration allows for real-time optimization of cell performance, energy efficiency, and product quality without manual intervention.Expand Specific Solutions03 Scalable electrolytic cell designs
Scalable designs for electrolytic cells enable easy expansion or reduction of production capacity. These designs typically feature standardized cell units that can be added or removed from the system. Scalability allows for efficient adaptation to changing market demands or production goals while maintaining optimal performance across different scales of operation.Expand Specific Solutions04 Multi-functional electrolytic cells
Multi-functional electrolytic cells are designed to perform various electrochemical processes within a single unit. These versatile cells can be reconfigured to switch between different electrolytic operations, such as electroplating, electrolysis, and electrorefining. The ability to reconfigure for multiple functions increases the utility and cost-effectiveness of the electrolytic system.Expand Specific Solutions05 Smart electrode systems for reconfiguration
Smart electrode systems incorporate advanced materials and designs that allow for in-situ reconfiguration of electrode properties. These systems may use shape-memory alloys, adjustable surface area electrodes, or electrodes with tunable catalytic properties. Smart electrodes enable optimization of reaction kinetics, selectivity, and energy efficiency without the need for physical cell disassembly.Expand Specific Solutions
Key Players in Electrolytic Cell Industry
The electrolytic cell technology for chlorine production is in a mature stage, with a well-established global market estimated at over $30 billion. Industry leaders like Industrie De Nora, Covestro, and Bayer AG have developed advanced electrolytic cell designs, improving efficiency and reducing environmental impact. The market is characterized by incremental innovations rather than disruptive changes, focusing on optimizing energy consumption and membrane technology. Emerging players such as Moleaer and Dioxide Materials are introducing novel approaches like nanobubble technology and CO2 electrolysis, potentially reshaping future production methods. However, traditional chlor-alkali producers still dominate the market, with ongoing efforts to enhance sustainability and cost-effectiveness in chlorine production techniques.
Industrie De Nora SpA
Technical Solution: Industrie De Nora SpA has developed advanced electrode technologies for chlorine production, focusing on dimensionally stable anodes (DSA) and membrane cell technology. Their NXT electrodes utilize a proprietary coating that enhances chlorine evolution efficiency while reducing power consumption. The company's membrane cell design incorporates zero-gap technology, minimizing the distance between electrodes to improve current efficiency. De Nora has also introduced the NDC electrode series, which offers extended lifetimes and lower operating voltages compared to conventional electrodes [1][3]. Their electrolyzers are designed to operate at high current densities, up to 7 kA/m², allowing for increased production capacity in smaller cell footprints [2].
Strengths: Industry-leading electrode technology, high energy efficiency, and extended electrode lifetimes. Weaknesses: High initial investment costs and potential dependency on specific membrane technologies.
BASF Corp.
Technical Solution: BASF has developed a state-of-the-art membrane cell technology for chlorine production, focusing on energy efficiency and environmental sustainability. Their electrolyzers utilize advanced polymer membranes with optimized ion exchange properties, resulting in high current efficiency and low power consumption. BASF's cell design incorporates a zero-gap configuration and high-performance electrode coatings that minimize voltage drop across the cell. The company has also introduced a novel brine purification system that extends membrane life and improves overall process stability [13][14]. BASF's chlor-alkali technology includes an integrated heat recovery system that captures waste heat from the electrolysis process for use in other plant operations, enhancing overall energy efficiency. Additionally, they have developed a proprietary process control system that uses machine learning algorithms to continuously optimize cell performance based on real-time operating data [15].
Strengths: Integrated process solutions, advanced heat recovery systems, and cutting-edge process control technology. Weaknesses: Potential challenges in adapting to rapidly evolving environmental regulations and competition from specialized electrochemical technology providers.
Core Innovations in Electrolytic Cells
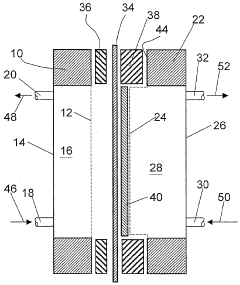
Electrolysis cell, especially for electrochemical production of chlorine
PatentWO2003031690A2
Innovation
- The electrolytic cell design fixes the GDE to a current collector instead of the cation exchange membrane, allowing for easier assembly, reduced risk of damage, and complete surface coverage, with options for gluing, sewing, or using Velcro for attachment, and incorporating sealing elements to prevent slipping and ensure full contact with both the current collector and membrane.
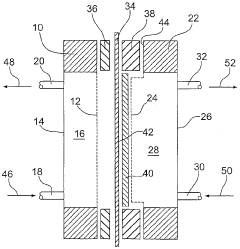
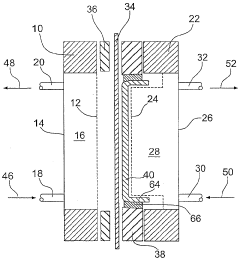
Electrolysis cell, particularly for electrochemically producing chlorine
PatentInactiveEP1417356A2
Innovation
- The electrolytic cell employs an elastic connection between the anode and/or current collector with the frame, using springs or other elastic elements to maintain constant contact between these components, ensuring no gaps form even with pressure differences, thus preventing undesirable voltage increases.
Environmental Impact Assessment
The environmental impact of electrolytic cells in chlorine production is a critical consideration as the industry evolves. These cells have significantly reduced the environmental footprint compared to traditional mercury-based processes. The primary environmental benefits stem from the elimination of mercury emissions and waste, which were major concerns in older production methods. Electrolytic cells operate on a closed-loop system, minimizing the release of harmful substances into the environment.
However, the environmental impact of electrolytic chlorine production is not entirely benign. The process still requires substantial energy input, contributing to indirect carbon emissions if the electricity source is not renewable. The production of chlorine also generates hydrogen as a by-product, which, if not properly utilized or stored, can pose safety and environmental risks.
Water usage and management are crucial aspects of the environmental assessment. While electrolytic cells have improved water efficiency compared to older methods, the process still requires significant amounts of water for brine preparation and cooling. Proper treatment and recycling of process water are essential to minimize environmental impact and conserve resources.
The production of chlorine through electrolysis also involves the use and handling of various chemicals, including sodium chloride and caustic soda. Proper storage, transportation, and disposal of these substances are critical to prevent soil and water contamination. Advanced containment systems and rigorous safety protocols have been developed to mitigate these risks.
One of the most significant environmental considerations is the end-of-life management of electrolytic cells. These cells contain precious metals and other materials that require proper recycling and disposal to prevent environmental contamination and resource waste. The industry has been developing more efficient recycling processes to recover valuable materials and minimize landfill waste.
The shift towards electrolytic cells has also prompted research into more environmentally friendly electrode materials and membrane technologies. These advancements aim to further reduce energy consumption, improve process efficiency, and minimize the use of potentially harmful substances in the production process.
Overall, while electrolytic cells have markedly improved the environmental profile of chlorine production, ongoing efforts are necessary to address remaining challenges and further reduce the industry's ecological footprint. This includes optimizing energy efficiency, enhancing water management practices, and developing more sustainable materials and processes throughout the production lifecycle.
However, the environmental impact of electrolytic chlorine production is not entirely benign. The process still requires substantial energy input, contributing to indirect carbon emissions if the electricity source is not renewable. The production of chlorine also generates hydrogen as a by-product, which, if not properly utilized or stored, can pose safety and environmental risks.
Water usage and management are crucial aspects of the environmental assessment. While electrolytic cells have improved water efficiency compared to older methods, the process still requires significant amounts of water for brine preparation and cooling. Proper treatment and recycling of process water are essential to minimize environmental impact and conserve resources.
The production of chlorine through electrolysis also involves the use and handling of various chemicals, including sodium chloride and caustic soda. Proper storage, transportation, and disposal of these substances are critical to prevent soil and water contamination. Advanced containment systems and rigorous safety protocols have been developed to mitigate these risks.
One of the most significant environmental considerations is the end-of-life management of electrolytic cells. These cells contain precious metals and other materials that require proper recycling and disposal to prevent environmental contamination and resource waste. The industry has been developing more efficient recycling processes to recover valuable materials and minimize landfill waste.
The shift towards electrolytic cells has also prompted research into more environmentally friendly electrode materials and membrane technologies. These advancements aim to further reduce energy consumption, improve process efficiency, and minimize the use of potentially harmful substances in the production process.
Overall, while electrolytic cells have markedly improved the environmental profile of chlorine production, ongoing efforts are necessary to address remaining challenges and further reduce the industry's ecological footprint. This includes optimizing energy efficiency, enhancing water management practices, and developing more sustainable materials and processes throughout the production lifecycle.
Safety Regulations in Chlorine Industry
The chlorine industry operates under stringent safety regulations due to the hazardous nature of chlorine and its production processes. These regulations are designed to protect workers, communities, and the environment from potential risks associated with chlorine manufacturing, storage, and transportation. In the United States, the Occupational Safety and Health Administration (OSHA) sets and enforces standards for workplace safety in chlorine production facilities. These standards include specific requirements for personal protective equipment, emergency response procedures, and exposure limits for chlorine gas.
The Environmental Protection Agency (EPA) also plays a crucial role in regulating the chlorine industry, particularly concerning environmental impacts and risk management. The EPA's Risk Management Program (RMP) requires facilities handling large quantities of chlorine to develop and implement risk management plans to prevent and mitigate potential accidents. These plans must include hazard assessments, prevention programs, and emergency response procedures.
Internationally, the United Nations Environment Programme (UNEP) has established guidelines for the safe production and handling of chlorine. These guidelines emphasize the importance of proper training for workers, regular maintenance of equipment, and the implementation of robust safety management systems. The European Union has implemented REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulations, which apply to chlorine and its derivatives, ensuring their safe use throughout the supply chain.
In recent years, there has been an increased focus on process safety management (PSM) in the chlorine industry. PSM regulations require companies to implement comprehensive safety programs that address all aspects of chlorine production, from design and operation to maintenance and emergency response. This approach aims to prevent major accidents by identifying and controlling potential hazards systematically.
The chlor-alkali industry has also developed its own voluntary initiatives to enhance safety standards. The Chlorine Institute, a global association of chlorine producers, has established recommended practices and guidelines that often exceed regulatory requirements. These include detailed protocols for the safe handling of chlorine containers, emergency preparedness, and risk communication.
As electrolytic cells continue to reconfigure chlorine production techniques, safety regulations are evolving to address new challenges. For instance, the shift towards membrane cell technology has led to updated safety protocols that account for the specific risks associated with this process. Regulatory bodies are also increasingly focusing on cybersecurity measures to protect the digital control systems used in modern chlorine production facilities from potential cyber threats.
The Environmental Protection Agency (EPA) also plays a crucial role in regulating the chlorine industry, particularly concerning environmental impacts and risk management. The EPA's Risk Management Program (RMP) requires facilities handling large quantities of chlorine to develop and implement risk management plans to prevent and mitigate potential accidents. These plans must include hazard assessments, prevention programs, and emergency response procedures.
Internationally, the United Nations Environment Programme (UNEP) has established guidelines for the safe production and handling of chlorine. These guidelines emphasize the importance of proper training for workers, regular maintenance of equipment, and the implementation of robust safety management systems. The European Union has implemented REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulations, which apply to chlorine and its derivatives, ensuring their safe use throughout the supply chain.
In recent years, there has been an increased focus on process safety management (PSM) in the chlorine industry. PSM regulations require companies to implement comprehensive safety programs that address all aspects of chlorine production, from design and operation to maintenance and emergency response. This approach aims to prevent major accidents by identifying and controlling potential hazards systematically.
The chlor-alkali industry has also developed its own voluntary initiatives to enhance safety standards. The Chlorine Institute, a global association of chlorine producers, has established recommended practices and guidelines that often exceed regulatory requirements. These include detailed protocols for the safe handling of chlorine containers, emergency preparedness, and risk communication.
As electrolytic cells continue to reconfigure chlorine production techniques, safety regulations are evolving to address new challenges. For instance, the shift towards membrane cell technology has led to updated safety protocols that account for the specific risks associated with this process. Regulatory bodies are also increasingly focusing on cybersecurity measures to protect the digital control systems used in modern chlorine production facilities from potential cyber threats.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!