The Role of Electrolytic Cells in Clean Hydrogen Fuel Systems
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolytic Cells in H2 Fuel: Background and Objectives
Electrolytic cells have emerged as a pivotal technology in the pursuit of clean hydrogen fuel systems, marking a significant milestone in the global transition towards sustainable energy solutions. The development of these cells can be traced back to the early 19th century, with Sir William Nicholson and Anthony Carlisle's groundbreaking discovery of water electrolysis in 1800. This fundamental process has since evolved into a cornerstone of modern hydrogen production techniques.
The trajectory of electrolytic cell technology has been characterized by continuous refinement and innovation, driven by the increasing demand for efficient and environmentally friendly energy sources. In recent decades, the urgency of addressing climate change and reducing dependence on fossil fuels has accelerated research and development in this field. The primary objective of current electrolytic cell technology is to achieve high-efficiency hydrogen production while minimizing energy input and operational costs.
As we delve into the present landscape, electrolytic cells are poised to play a crucial role in realizing the vision of a hydrogen-based economy. The technology aims to overcome several key challenges, including improving energy efficiency, reducing production costs, and scaling up for industrial applications. These objectives align with broader global initiatives to decarbonize energy systems and mitigate the environmental impact of traditional fuel sources.
The evolution of electrolytic cell technology is closely intertwined with advancements in materials science, electrochemistry, and renewable energy integration. Current research focuses on developing novel electrode materials, optimizing cell designs, and enhancing the overall system efficiency. The ultimate goal is to establish electrolytic hydrogen production as a viable and competitive alternative to fossil fuel-based hydrogen production methods.
In the context of clean hydrogen fuel systems, electrolytic cells offer several distinct advantages. They enable the production of high-purity hydrogen without direct carbon emissions, making them an attractive option for environmentally conscious industries and governments. Furthermore, their ability to operate using renewable electricity sources, such as solar and wind power, positions them as a key enabler of the green hydrogen revolution.
As we look towards the future, the role of electrolytic cells in clean hydrogen fuel systems is expected to expand significantly. Technological breakthroughs in this field have the potential to reshape global energy landscapes, offering a pathway to sustainable transportation, industrial processes, and energy storage solutions. The ongoing research and development efforts aim to unlock the full potential of electrolytic cells, paving the way for a cleaner, more sustainable energy future.
The trajectory of electrolytic cell technology has been characterized by continuous refinement and innovation, driven by the increasing demand for efficient and environmentally friendly energy sources. In recent decades, the urgency of addressing climate change and reducing dependence on fossil fuels has accelerated research and development in this field. The primary objective of current electrolytic cell technology is to achieve high-efficiency hydrogen production while minimizing energy input and operational costs.
As we delve into the present landscape, electrolytic cells are poised to play a crucial role in realizing the vision of a hydrogen-based economy. The technology aims to overcome several key challenges, including improving energy efficiency, reducing production costs, and scaling up for industrial applications. These objectives align with broader global initiatives to decarbonize energy systems and mitigate the environmental impact of traditional fuel sources.
The evolution of electrolytic cell technology is closely intertwined with advancements in materials science, electrochemistry, and renewable energy integration. Current research focuses on developing novel electrode materials, optimizing cell designs, and enhancing the overall system efficiency. The ultimate goal is to establish electrolytic hydrogen production as a viable and competitive alternative to fossil fuel-based hydrogen production methods.
In the context of clean hydrogen fuel systems, electrolytic cells offer several distinct advantages. They enable the production of high-purity hydrogen without direct carbon emissions, making them an attractive option for environmentally conscious industries and governments. Furthermore, their ability to operate using renewable electricity sources, such as solar and wind power, positions them as a key enabler of the green hydrogen revolution.
As we look towards the future, the role of electrolytic cells in clean hydrogen fuel systems is expected to expand significantly. Technological breakthroughs in this field have the potential to reshape global energy landscapes, offering a pathway to sustainable transportation, industrial processes, and energy storage solutions. The ongoing research and development efforts aim to unlock the full potential of electrolytic cells, paving the way for a cleaner, more sustainable energy future.
Market Analysis for Clean Hydrogen Fuel Systems
The clean hydrogen fuel systems market is experiencing significant growth, driven by the increasing global focus on decarbonization and sustainable energy solutions. As governments and industries worldwide seek to reduce carbon emissions, hydrogen fuel cells have emerged as a promising alternative to traditional fossil fuels, particularly in transportation and industrial applications.
The market for clean hydrogen fuel systems is projected to expand rapidly in the coming years, with a compound annual growth rate (CAGR) expected to exceed 20% through 2030. This growth is primarily attributed to the rising demand for zero-emission vehicles, stringent environmental regulations, and substantial investments in hydrogen infrastructure development.
The transportation sector represents a key market segment for clean hydrogen fuel systems, with fuel cell electric vehicles (FCEVs) gaining traction in both passenger and commercial applications. Major automotive manufacturers are investing heavily in FCEV technology, recognizing its potential to complement battery electric vehicles in the transition to sustainable mobility.
Industrial applications, including power generation, chemical production, and steel manufacturing, are also driving market growth. These sectors are exploring hydrogen as a means to reduce their carbon footprint and meet increasingly stringent emissions targets. The potential for green hydrogen, produced through electrolysis powered by renewable energy sources, is particularly attractive for industries seeking to achieve carbon neutrality.
Geographically, Asia-Pacific is expected to dominate the clean hydrogen fuel systems market, led by countries such as Japan, South Korea, and China. These nations have implemented supportive policies and substantial investments in hydrogen infrastructure. Europe is also emerging as a significant market, with countries like Germany, France, and the United Kingdom setting ambitious targets for hydrogen adoption across various sectors.
Despite the promising outlook, several challenges remain for the widespread adoption of clean hydrogen fuel systems. These include the high cost of hydrogen production, limited infrastructure for distribution and storage, and the need for technological advancements to improve efficiency and reduce costs. Addressing these challenges will be crucial for realizing the full market potential of clean hydrogen fuel systems.
In conclusion, the market analysis for clean hydrogen fuel systems reveals a rapidly growing sector with significant opportunities across multiple industries. As technology advances and costs decrease, the market is poised for substantial expansion, playing a crucial role in the global transition to a low-carbon economy.
The market for clean hydrogen fuel systems is projected to expand rapidly in the coming years, with a compound annual growth rate (CAGR) expected to exceed 20% through 2030. This growth is primarily attributed to the rising demand for zero-emission vehicles, stringent environmental regulations, and substantial investments in hydrogen infrastructure development.
The transportation sector represents a key market segment for clean hydrogen fuel systems, with fuel cell electric vehicles (FCEVs) gaining traction in both passenger and commercial applications. Major automotive manufacturers are investing heavily in FCEV technology, recognizing its potential to complement battery electric vehicles in the transition to sustainable mobility.
Industrial applications, including power generation, chemical production, and steel manufacturing, are also driving market growth. These sectors are exploring hydrogen as a means to reduce their carbon footprint and meet increasingly stringent emissions targets. The potential for green hydrogen, produced through electrolysis powered by renewable energy sources, is particularly attractive for industries seeking to achieve carbon neutrality.
Geographically, Asia-Pacific is expected to dominate the clean hydrogen fuel systems market, led by countries such as Japan, South Korea, and China. These nations have implemented supportive policies and substantial investments in hydrogen infrastructure. Europe is also emerging as a significant market, with countries like Germany, France, and the United Kingdom setting ambitious targets for hydrogen adoption across various sectors.
Despite the promising outlook, several challenges remain for the widespread adoption of clean hydrogen fuel systems. These include the high cost of hydrogen production, limited infrastructure for distribution and storage, and the need for technological advancements to improve efficiency and reduce costs. Addressing these challenges will be crucial for realizing the full market potential of clean hydrogen fuel systems.
In conclusion, the market analysis for clean hydrogen fuel systems reveals a rapidly growing sector with significant opportunities across multiple industries. As technology advances and costs decrease, the market is poised for substantial expansion, playing a crucial role in the global transition to a low-carbon economy.
Current Challenges in Electrolytic Cell Technology
Electrolytic cell technology plays a crucial role in clean hydrogen fuel systems, yet it faces several significant challenges that hinder its widespread adoption and efficiency. One of the primary issues is the high cost of materials used in electrolyzers, particularly the catalysts. Noble metals like platinum and iridium are commonly used due to their excellent catalytic properties, but their scarcity and high price make large-scale implementation economically challenging.
Energy efficiency remains a major concern in electrolytic cell technology. Current systems typically operate at 60-80% efficiency, with substantial energy losses occurring during the electrolysis process. This inefficiency not only increases operational costs but also reduces the overall environmental benefits of hydrogen as a clean fuel source. Improving energy efficiency is critical for making hydrogen production more competitive with traditional fossil fuel-based methods.
Durability and longevity of electrolytic cells present another significant challenge. The harsh operating conditions, including high temperatures and corrosive environments, can lead to degradation of cell components over time. This degradation results in decreased performance and increased maintenance costs, affecting the long-term viability of hydrogen production systems.
Scaling up electrolytic cell technology for industrial-scale hydrogen production is a complex challenge. Current systems are often limited in size and output, making it difficult to meet the growing demand for hydrogen fuel. Developing larger, more efficient electrolyzers while maintaining performance and reliability is a key focus area for researchers and engineers in the field.
Water purity requirements pose an additional challenge, particularly in regions where clean water resources are scarce. Electrolytic cells typically require high-purity water to prevent contamination and ensure optimal performance. Developing systems that can operate effectively with lower-quality water inputs could significantly expand the potential applications of this technology.
Integration with renewable energy sources is another critical challenge. To maximize the environmental benefits of hydrogen fuel, electrolytic cells should ideally be powered by renewable electricity. However, the intermittent nature of renewable sources like solar and wind creates challenges in maintaining consistent hydrogen production. Developing advanced control systems and energy storage solutions to balance these fluctuations is essential for creating truly sustainable hydrogen fuel systems.
Lastly, safety concerns surrounding hydrogen production and storage continue to be a significant challenge. The highly flammable nature of hydrogen requires robust safety measures and specialized infrastructure, which can increase the complexity and cost of implementing electrolytic cell technology on a large scale.
Energy efficiency remains a major concern in electrolytic cell technology. Current systems typically operate at 60-80% efficiency, with substantial energy losses occurring during the electrolysis process. This inefficiency not only increases operational costs but also reduces the overall environmental benefits of hydrogen as a clean fuel source. Improving energy efficiency is critical for making hydrogen production more competitive with traditional fossil fuel-based methods.
Durability and longevity of electrolytic cells present another significant challenge. The harsh operating conditions, including high temperatures and corrosive environments, can lead to degradation of cell components over time. This degradation results in decreased performance and increased maintenance costs, affecting the long-term viability of hydrogen production systems.
Scaling up electrolytic cell technology for industrial-scale hydrogen production is a complex challenge. Current systems are often limited in size and output, making it difficult to meet the growing demand for hydrogen fuel. Developing larger, more efficient electrolyzers while maintaining performance and reliability is a key focus area for researchers and engineers in the field.
Water purity requirements pose an additional challenge, particularly in regions where clean water resources are scarce. Electrolytic cells typically require high-purity water to prevent contamination and ensure optimal performance. Developing systems that can operate effectively with lower-quality water inputs could significantly expand the potential applications of this technology.
Integration with renewable energy sources is another critical challenge. To maximize the environmental benefits of hydrogen fuel, electrolytic cells should ideally be powered by renewable electricity. However, the intermittent nature of renewable sources like solar and wind creates challenges in maintaining consistent hydrogen production. Developing advanced control systems and energy storage solutions to balance these fluctuations is essential for creating truly sustainable hydrogen fuel systems.
Lastly, safety concerns surrounding hydrogen production and storage continue to be a significant challenge. The highly flammable nature of hydrogen requires robust safety measures and specialized infrastructure, which can increase the complexity and cost of implementing electrolytic cell technology on a large scale.
Existing Electrolytic Cell Solutions for H2 Production
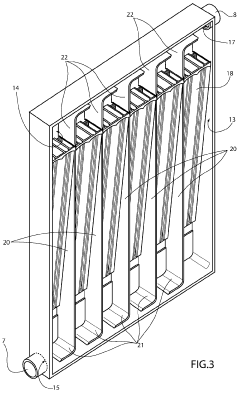
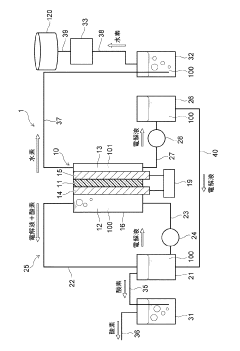
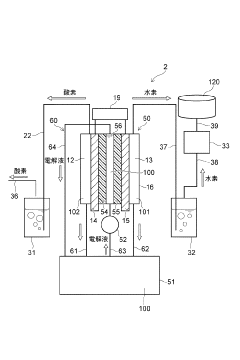
01 Electrolytic cell design and components
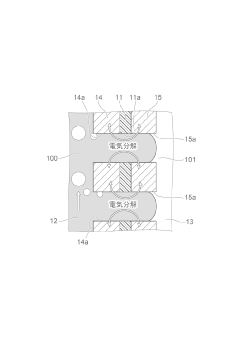
Electrolytic cells are designed with specific components to facilitate electrochemical reactions. These components typically include electrodes, electrolyte solutions, and separators. The design of these cells can vary based on the intended application, such as fuel cells, batteries, or industrial electrolysis processes. Innovations in cell design focus on improving efficiency, durability, and performance.- Electrolytic cell design and structure: Electrolytic cells are designed with specific structures to facilitate efficient electrolysis. These designs may include specialized electrodes, membranes, and compartments to optimize the electrochemical reactions. The structure of the cell can be tailored to suit different applications, such as water electrolysis, metal production, or chemical synthesis.
- Electrode materials and configurations: The choice of electrode materials and their configurations play a crucial role in the performance of electrolytic cells. Various materials, such as metals, alloys, and coated substrates, are used to enhance conductivity, catalytic activity, and durability. Electrode configurations may include planar, mesh, or three-dimensional structures to maximize surface area and reaction efficiency.
- Electrolyte composition and management: The composition and management of electrolytes are essential for optimal cell performance. This includes selecting appropriate electrolyte materials, maintaining proper concentration, and managing temperature and pH. Electrolyte circulation and replenishment systems may be incorporated to ensure consistent performance and longevity of the electrolytic cell.
- Control and monitoring systems: Advanced control and monitoring systems are implemented in electrolytic cells to optimize performance and ensure safety. These systems may include sensors for measuring voltage, current, temperature, and electrolyte properties. Automated control mechanisms can adjust operating parameters in real-time to maintain optimal conditions and prevent issues such as overheating or electrode degradation.
- Applications and specialized cell designs: Electrolytic cells are used in various applications, each requiring specialized designs. These may include cells for hydrogen production, chlor-alkali processes, metal refining, or wastewater treatment. Specialized designs can incorporate features such as gas separation membranes, bipolar electrodes, or continuous flow systems to meet specific application requirements and improve overall efficiency.
02 Electrode materials and configurations
The choice of electrode materials and their configurations play a crucial role in the performance of electrolytic cells. Research focuses on developing novel electrode materials with enhanced conductivity, stability, and catalytic properties. Electrode configurations may include planar, cylindrical, or more complex geometries to optimize surface area and reaction kinetics.Expand Specific Solutions03 Electrolyte composition and management
The electrolyte composition is critical for ion transport and overall cell performance. Innovations in this area include developing new electrolyte formulations, improving ionic conductivity, and managing electrolyte degradation. Electrolyte management systems may also be incorporated to maintain optimal concentration and prevent unwanted side reactions.Expand Specific Solutions04 Control and monitoring systems
Advanced control and monitoring systems are essential for optimizing electrolytic cell performance and safety. These systems may include sensors for temperature, pressure, and concentration monitoring, as well as automated control mechanisms for adjusting operating parameters. Innovations in this area focus on improving real-time data analysis and predictive maintenance capabilities.Expand Specific Solutions05 Applications and specialized electrolytic cells
Electrolytic cells find applications in various industries, including energy storage, chemical production, and water treatment. Specialized cells are developed for specific applications, such as chlor-alkali production, metal refining, or hydrogen generation. Research in this area focuses on tailoring cell designs and materials to meet the unique requirements of each application, improving efficiency and reducing environmental impact.Expand Specific Solutions
Key Players in Electrolytic Cell Manufacturing
The electrolytic cell market for clean hydrogen fuel systems is in a growth phase, driven by increasing demand for sustainable energy solutions. The market size is expanding rapidly, with projections indicating significant growth in the coming years. Technologically, the field is advancing quickly, with companies like Toyota Motor Corp., FuelCell Energy, and Nuvera Fuel Cells leading innovation. These firms are developing more efficient and cost-effective electrolytic cell technologies, focusing on improving durability, performance, and scalability. While established players dominate, emerging companies like QuantumSphere are introducing novel catalyst materials, potentially disrupting the market. The competitive landscape is characterized by a mix of automotive giants, specialized fuel cell manufacturers, and research-driven startups, all vying for market share in this promising clean energy sector.
Toyota Motor Corp.
Technical Solution: Toyota has been at the forefront of hydrogen fuel cell technology for vehicles. Their approach to clean hydrogen fuel systems involves both on-board hydrogen production and the use of pre-produced hydrogen. For on-board production, Toyota has developed a compact water electrolysis system that can generate hydrogen directly in the vehicle. This system uses a solid polymer electrolyte membrane and operates at lower temperatures compared to high-temperature electrolysis[4]. Toyota's latest fuel cell stack, used in the Mirai model, achieves a power output density of 3.1 kW/L, a 50% increase over the previous generation[5]. The company has also invested in large-scale hydrogen production facilities, partnering with various energy companies to establish hydrogen supply chains[6].
Strengths: Advanced on-board hydrogen production technology, established fuel cell vehicle production, and strong hydrogen infrastructure partnerships. Weaknesses: Reliance on platinum catalysts in fuel cells, which increases costs.
FuelCell Energy, Inc.
Technical Solution: FuelCell Energy has developed a proprietary Solid Oxide Electrolysis Cell (SOEC) technology for clean hydrogen production. Their SOEC system operates at high temperatures (700-800°C) to split water molecules into hydrogen and oxygen using electricity. The company's electrolysis stacks can produce hydrogen at a rate of up to 1,200 kg per day[1]. FuelCell Energy's SOEC technology integrates seamlessly with renewable energy sources, allowing for the production of green hydrogen. They have also implemented a reversible solid oxide fuel cell (RSOFC) system that can switch between electrolysis and fuel cell modes, providing flexibility in energy storage and generation[2][3].
Strengths: High efficiency due to high-temperature operation, scalable production capacity, and integration with renewable energy. Weaknesses: High initial capital costs and the need for high-temperature resistant materials.
Innovative Electrolyte Materials and Catalysts
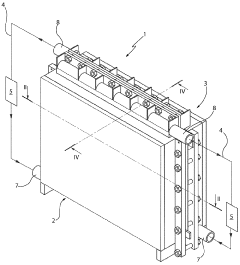
Electrolytic cell for the production of h2
PatentWO2023144802A1
Innovation
- The electrolytic cell design eliminates the need for a salt bridge or semi-permeable membrane by using a metal sheet with holes and slits, where water solution recirculation and degassers ensure automatic gas separation, with the electrolysis occurring during the second passage through the metal sheet, and hydraulic resistance layers ensure uniform fluid distribution for efficient gas generation.
Electrolytic cell and hydrogen production device
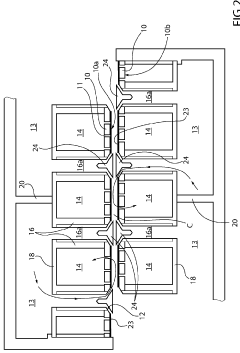
PatentInactiveJP2019090087A
Innovation
- The electrolytic cell is designed with a housing divided into an anode-side and cathode-side cells by a diaphragm, where the cathode electrode is predominantly in contact with the gas phase and the anode electrode with the electrolytic solution, minimizing the mixing of oxygen into the hydrogen gas.
Environmental Impact of Electrolytic H2 Production
The environmental impact of electrolytic hydrogen production is a critical consideration in the development of clean hydrogen fuel systems. While hydrogen itself is a clean-burning fuel, the process of producing it through electrolysis can have significant environmental implications, both positive and negative.
One of the primary environmental benefits of electrolytic hydrogen production is its potential to reduce greenhouse gas emissions. When powered by renewable energy sources such as solar or wind, electrolysis can produce hydrogen with minimal carbon footprint. This clean hydrogen can then be used in fuel cells to generate electricity or power vehicles, effectively displacing fossil fuels and reducing overall carbon emissions.
However, the environmental impact of electrolytic hydrogen production is heavily dependent on the source of electricity used to power the process. If the electricity is derived from fossil fuels, the carbon footprint of hydrogen production can be substantial, potentially negating its benefits as a clean fuel. Therefore, the integration of renewable energy sources is crucial for maximizing the environmental benefits of electrolytic hydrogen production.
Water consumption is another important environmental consideration. Electrolysis requires significant amounts of water as a feedstock, which could strain local water resources, particularly in water-scarce regions. However, advancements in water recycling and the use of seawater for electrolysis are being developed to mitigate this impact.
The production and disposal of electrolytic cell components also contribute to the environmental footprint. The manufacturing of electrodes, membranes, and other cell materials involves resource extraction and energy-intensive processes. Additionally, the eventual disposal or recycling of these components must be carefully managed to minimize environmental impact.
Land use is another factor to consider, especially when scaling up hydrogen production. Large-scale electrolysis facilities and associated renewable energy infrastructure can require significant land area, potentially competing with other land uses or impacting local ecosystems.
On the positive side, electrolytic hydrogen production can contribute to grid stability and energy storage solutions. By utilizing excess renewable energy during peak production periods, electrolysis can help balance the grid and store energy in the form of hydrogen for later use, reducing the need for fossil fuel-based peaker plants.
In conclusion, while electrolytic hydrogen production offers significant potential for clean energy systems, its environmental impact is complex and multifaceted. Maximizing its benefits while minimizing negative impacts requires careful consideration of energy sources, water management, material lifecycle, and land use. As technology advances and production scales up, ongoing assessment and optimization of these environmental factors will be crucial for ensuring the sustainability of hydrogen fuel systems.
One of the primary environmental benefits of electrolytic hydrogen production is its potential to reduce greenhouse gas emissions. When powered by renewable energy sources such as solar or wind, electrolysis can produce hydrogen with minimal carbon footprint. This clean hydrogen can then be used in fuel cells to generate electricity or power vehicles, effectively displacing fossil fuels and reducing overall carbon emissions.
However, the environmental impact of electrolytic hydrogen production is heavily dependent on the source of electricity used to power the process. If the electricity is derived from fossil fuels, the carbon footprint of hydrogen production can be substantial, potentially negating its benefits as a clean fuel. Therefore, the integration of renewable energy sources is crucial for maximizing the environmental benefits of electrolytic hydrogen production.
Water consumption is another important environmental consideration. Electrolysis requires significant amounts of water as a feedstock, which could strain local water resources, particularly in water-scarce regions. However, advancements in water recycling and the use of seawater for electrolysis are being developed to mitigate this impact.
The production and disposal of electrolytic cell components also contribute to the environmental footprint. The manufacturing of electrodes, membranes, and other cell materials involves resource extraction and energy-intensive processes. Additionally, the eventual disposal or recycling of these components must be carefully managed to minimize environmental impact.
Land use is another factor to consider, especially when scaling up hydrogen production. Large-scale electrolysis facilities and associated renewable energy infrastructure can require significant land area, potentially competing with other land uses or impacting local ecosystems.
On the positive side, electrolytic hydrogen production can contribute to grid stability and energy storage solutions. By utilizing excess renewable energy during peak production periods, electrolysis can help balance the grid and store energy in the form of hydrogen for later use, reducing the need for fossil fuel-based peaker plants.
In conclusion, while electrolytic hydrogen production offers significant potential for clean energy systems, its environmental impact is complex and multifaceted. Maximizing its benefits while minimizing negative impacts requires careful consideration of energy sources, water management, material lifecycle, and land use. As technology advances and production scales up, ongoing assessment and optimization of these environmental factors will be crucial for ensuring the sustainability of hydrogen fuel systems.
Regulatory Framework for Clean Hydrogen Technologies
The regulatory framework for clean hydrogen technologies plays a crucial role in shaping the development and implementation of electrolytic cells in clean hydrogen fuel systems. As governments worldwide recognize the potential of hydrogen as a clean energy carrier, they are establishing comprehensive regulations to ensure safety, environmental protection, and market competitiveness.
At the international level, organizations such as the International Organization for Standardization (ISO) and the International Electrotechnical Commission (IEC) are developing standards for hydrogen production, storage, and utilization. These standards provide a foundation for national and regional regulatory bodies to build upon, ensuring consistency and interoperability across borders.
In the United States, the Department of Energy (DOE) has taken a leading role in developing regulations for clean hydrogen technologies. The DOE's Hydrogen and Fuel Cell Technologies Office has established guidelines for hydrogen production, including specific requirements for electrolytic cells. These regulations cover aspects such as efficiency standards, safety protocols, and environmental impact assessments.
The European Union has also made significant strides in creating a regulatory framework for clean hydrogen. The European Commission's Hydrogen Strategy outlines a comprehensive approach to hydrogen development, including regulations for electrolytic cell technologies. The EU's Renewable Energy Directive (RED II) sets targets for renewable hydrogen production, incentivizing the use of electrolytic cells powered by renewable energy sources.
Safety regulations are a critical component of the regulatory framework for clean hydrogen technologies. Agencies such as the Occupational Safety and Health Administration (OSHA) in the US and the European Agency for Safety and Health at Work (EU-OSHA) have established guidelines for the safe operation of electrolytic cells and hydrogen handling systems.
Environmental regulations also play a significant role in shaping the development of clean hydrogen technologies. Many jurisdictions require life cycle assessments and environmental impact studies for hydrogen production facilities, including those utilizing electrolytic cells. These regulations aim to ensure that the entire hydrogen production process, from raw material extraction to end-use, meets stringent environmental standards.
As the clean hydrogen industry continues to evolve, regulatory frameworks are expected to adapt and expand. Future regulations may focus on areas such as grid integration of electrolytic cells, carbon intensity standards for hydrogen production, and incentives for green hydrogen production using renewable energy sources. The ongoing development of these regulatory frameworks will be crucial in supporting the growth and widespread adoption of clean hydrogen technologies, including electrolytic cells in fuel systems.
At the international level, organizations such as the International Organization for Standardization (ISO) and the International Electrotechnical Commission (IEC) are developing standards for hydrogen production, storage, and utilization. These standards provide a foundation for national and regional regulatory bodies to build upon, ensuring consistency and interoperability across borders.
In the United States, the Department of Energy (DOE) has taken a leading role in developing regulations for clean hydrogen technologies. The DOE's Hydrogen and Fuel Cell Technologies Office has established guidelines for hydrogen production, including specific requirements for electrolytic cells. These regulations cover aspects such as efficiency standards, safety protocols, and environmental impact assessments.
The European Union has also made significant strides in creating a regulatory framework for clean hydrogen. The European Commission's Hydrogen Strategy outlines a comprehensive approach to hydrogen development, including regulations for electrolytic cell technologies. The EU's Renewable Energy Directive (RED II) sets targets for renewable hydrogen production, incentivizing the use of electrolytic cells powered by renewable energy sources.
Safety regulations are a critical component of the regulatory framework for clean hydrogen technologies. Agencies such as the Occupational Safety and Health Administration (OSHA) in the US and the European Agency for Safety and Health at Work (EU-OSHA) have established guidelines for the safe operation of electrolytic cells and hydrogen handling systems.
Environmental regulations also play a significant role in shaping the development of clean hydrogen technologies. Many jurisdictions require life cycle assessments and environmental impact studies for hydrogen production facilities, including those utilizing electrolytic cells. These regulations aim to ensure that the entire hydrogen production process, from raw material extraction to end-use, meets stringent environmental standards.
As the clean hydrogen industry continues to evolve, regulatory frameworks are expected to adapt and expand. Future regulations may focus on areas such as grid integration of electrolytic cells, carbon intensity standards for hydrogen production, and incentives for green hydrogen production using renewable energy sources. The ongoing development of these regulatory frameworks will be crucial in supporting the growth and widespread adoption of clean hydrogen technologies, including electrolytic cells in fuel systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!