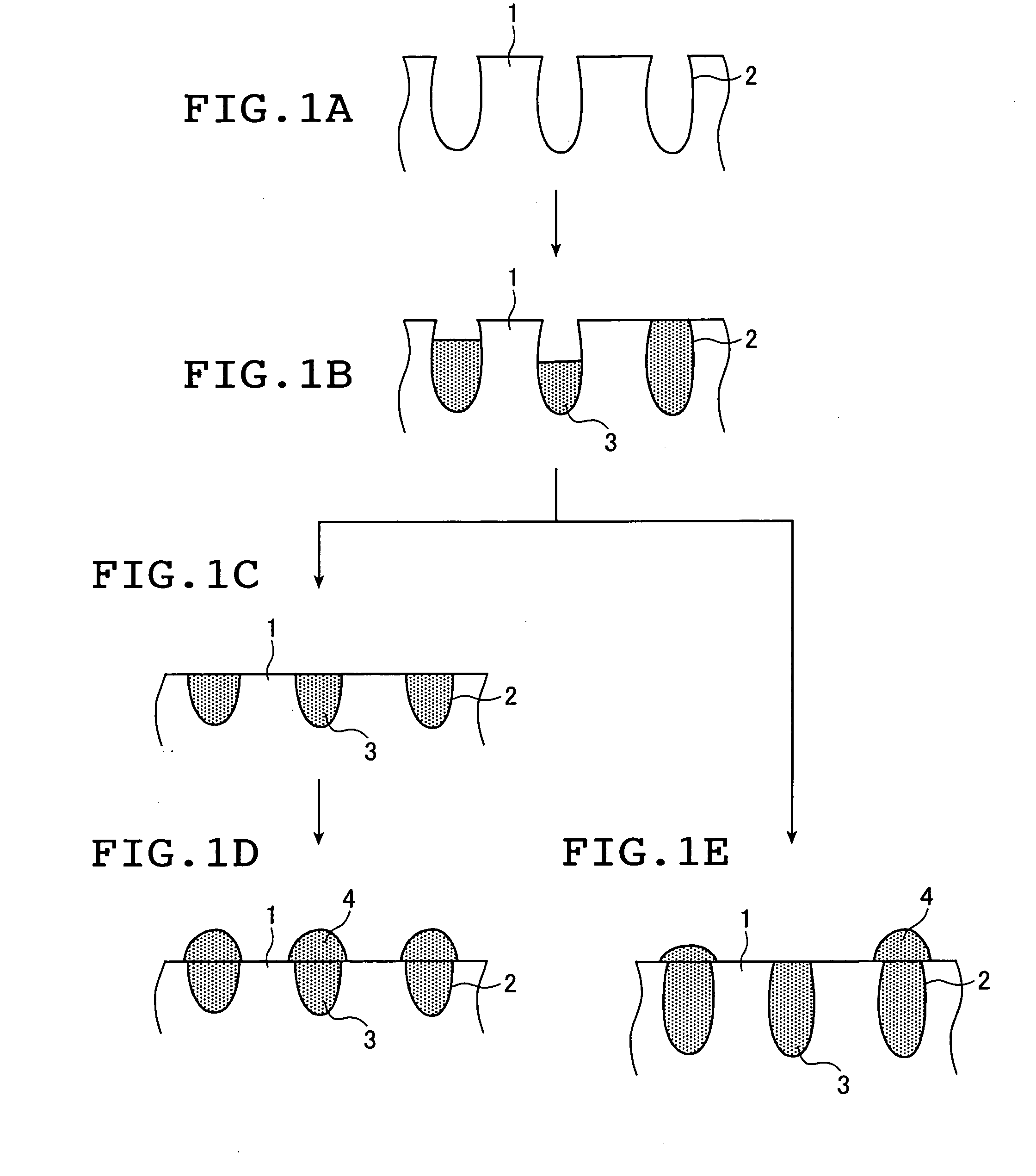
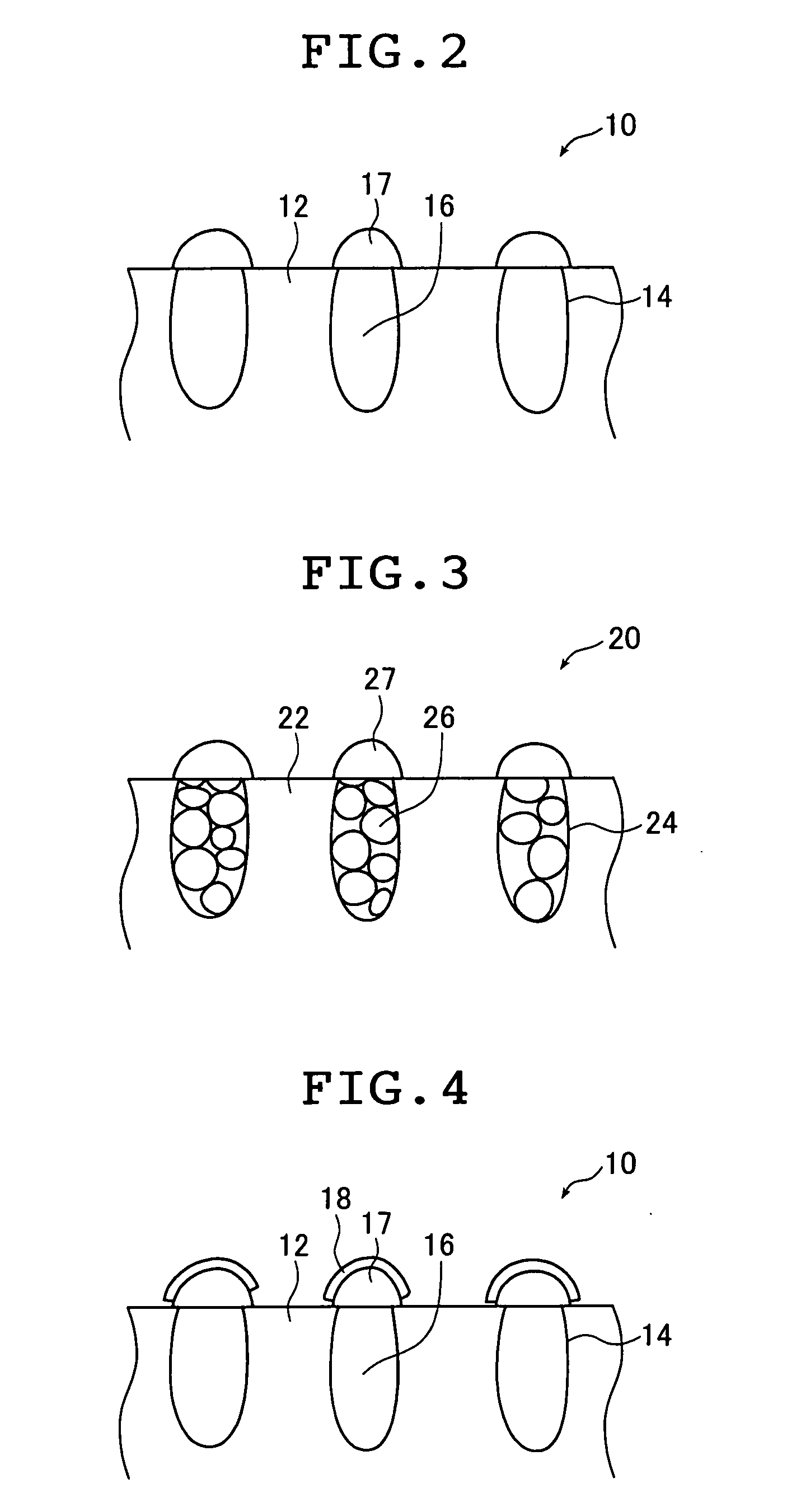
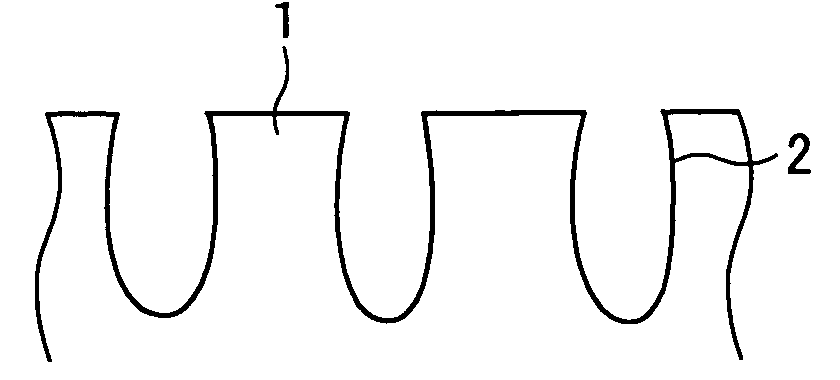Impact of Surface Plasmon Resonance in Electrolytic Cell Applications
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SPR in Electrolytic Cells: Background and Objectives
Surface Plasmon Resonance (SPR) has emerged as a powerful analytical technique with significant potential in electrolytic cell applications. This technology, which exploits the oscillation of electrons at the interface between a metal and a dielectric, has evolved from its initial discovery in the early 20th century to become a versatile tool in various scientific and industrial fields.
The development of SPR technology can be traced back to the observations of Wood's anomalies in 1902, followed by the theoretical explanations provided by Fano in 1941. However, it was not until the 1960s that the phenomenon was fully understood and its potential applications began to be explored. The seminal work of Otto, Kretschmann, and Raether in the late 1960s laid the foundation for modern SPR techniques.
In the context of electrolytic cells, SPR offers unique capabilities for real-time, label-free monitoring of electrochemical processes at the electrode-electrolyte interface. This application represents a convergence of two important fields: surface plasmonics and electrochemistry. The integration of SPR with electrolytic cells has opened up new avenues for studying electron transfer reactions, adsorption phenomena, and interfacial dynamics with unprecedented sensitivity and temporal resolution.
The primary objective of incorporating SPR into electrolytic cell applications is to enhance our understanding of electrochemical processes at the molecular level. This includes the ability to detect and quantify minute changes in the refractive index near the electrode surface, which can be correlated with electrochemical events such as redox reactions, ion adsorption, and the formation of electrical double layers.
Furthermore, the application of SPR in electrolytic cells aims to address several key challenges in electrochemistry. These include the need for in situ characterization of electrode surfaces, real-time monitoring of reaction kinetics, and the development of more efficient and selective electrochemical sensors. The non-invasive nature of SPR measurements makes it particularly attractive for studying delicate electrochemical systems without perturbing the equilibrium.
As research in this field progresses, the goal is to develop more sophisticated SPR-based electrolytic cell systems that can provide multidimensional information about electrochemical processes. This includes combining SPR with other spectroscopic techniques to obtain complementary data, as well as developing novel plasmonic materials and nanostructures to enhance sensitivity and selectivity.
The integration of SPR with electrolytic cells also aligns with broader technological trends, such as the miniaturization of analytical devices and the push towards green chemistry. By enabling more efficient and precise electrochemical analyses, SPR has the potential to contribute to the development of more sustainable industrial processes and advanced energy storage systems.
The development of SPR technology can be traced back to the observations of Wood's anomalies in 1902, followed by the theoretical explanations provided by Fano in 1941. However, it was not until the 1960s that the phenomenon was fully understood and its potential applications began to be explored. The seminal work of Otto, Kretschmann, and Raether in the late 1960s laid the foundation for modern SPR techniques.
In the context of electrolytic cells, SPR offers unique capabilities for real-time, label-free monitoring of electrochemical processes at the electrode-electrolyte interface. This application represents a convergence of two important fields: surface plasmonics and electrochemistry. The integration of SPR with electrolytic cells has opened up new avenues for studying electron transfer reactions, adsorption phenomena, and interfacial dynamics with unprecedented sensitivity and temporal resolution.
The primary objective of incorporating SPR into electrolytic cell applications is to enhance our understanding of electrochemical processes at the molecular level. This includes the ability to detect and quantify minute changes in the refractive index near the electrode surface, which can be correlated with electrochemical events such as redox reactions, ion adsorption, and the formation of electrical double layers.
Furthermore, the application of SPR in electrolytic cells aims to address several key challenges in electrochemistry. These include the need for in situ characterization of electrode surfaces, real-time monitoring of reaction kinetics, and the development of more efficient and selective electrochemical sensors. The non-invasive nature of SPR measurements makes it particularly attractive for studying delicate electrochemical systems without perturbing the equilibrium.
As research in this field progresses, the goal is to develop more sophisticated SPR-based electrolytic cell systems that can provide multidimensional information about electrochemical processes. This includes combining SPR with other spectroscopic techniques to obtain complementary data, as well as developing novel plasmonic materials and nanostructures to enhance sensitivity and selectivity.
The integration of SPR with electrolytic cells also aligns with broader technological trends, such as the miniaturization of analytical devices and the push towards green chemistry. By enabling more efficient and precise electrochemical analyses, SPR has the potential to contribute to the development of more sustainable industrial processes and advanced energy storage systems.
Market Analysis for SPR-Enhanced Electrolytic Applications
The market for Surface Plasmon Resonance (SPR) enhanced electrolytic applications is experiencing significant growth, driven by the increasing demand for more efficient and sustainable electrochemical processes across various industries. The integration of SPR technology into electrolytic cells has opened up new possibilities for enhancing reaction rates, reducing energy consumption, and improving overall process efficiency.
In the energy sector, SPR-enhanced electrolytic applications are gaining traction in hydrogen production through water splitting. The ability of SPR to concentrate electromagnetic fields at metal-dielectric interfaces has shown promising results in boosting the efficiency of electrocatalysts, potentially reducing the cost of green hydrogen production. This aligns with the global push towards a hydrogen-based economy, creating a substantial market opportunity.
The environmental remediation industry is another key market for SPR-enhanced electrolytic applications. Water treatment processes, particularly those involving the degradation of persistent organic pollutants, benefit from the enhanced oxidation capabilities provided by SPR. Municipal water treatment plants and industrial wastewater facilities are increasingly exploring these technologies to meet stricter environmental regulations and improve treatment efficacy.
In the chemical manufacturing sector, SPR-enhanced electrolytic processes are being investigated for their potential to improve the selectivity and yield of electrochemical synthesis reactions. This could lead to more efficient production of fine chemicals, pharmaceuticals, and specialty materials, driving adoption in the chemical industry.
The electronics industry is also showing interest in SPR-enhanced electrolytic applications, particularly in the field of electroplating and surface finishing. The ability to control and enhance metal deposition at the nanoscale offers potential improvements in the manufacturing of advanced electronic components and printed circuit boards.
Market analysts predict a compound annual growth rate (CAGR) for SPR-enhanced electrolytic applications to be in the double digits over the next five years. This growth is supported by increasing research and development investments from both academic institutions and industrial players, as well as government initiatives promoting clean energy technologies and environmental protection.
However, the market faces challenges in terms of scalability and cost-effectiveness. While laboratory-scale demonstrations have shown promising results, translating these into large-scale industrial applications remains a significant hurdle. Additionally, the high initial investment required for implementing SPR-enhanced electrolytic systems may slow adoption rates, particularly among smaller enterprises.
Despite these challenges, the long-term market outlook for SPR-enhanced electrolytic applications remains positive. As the technology matures and economies of scale are achieved, it is expected to play a crucial role in advancing sustainable industrial processes and clean energy technologies, positioning itself as a key enabler in the transition towards a more environmentally friendly and efficient industrial landscape.
In the energy sector, SPR-enhanced electrolytic applications are gaining traction in hydrogen production through water splitting. The ability of SPR to concentrate electromagnetic fields at metal-dielectric interfaces has shown promising results in boosting the efficiency of electrocatalysts, potentially reducing the cost of green hydrogen production. This aligns with the global push towards a hydrogen-based economy, creating a substantial market opportunity.
The environmental remediation industry is another key market for SPR-enhanced electrolytic applications. Water treatment processes, particularly those involving the degradation of persistent organic pollutants, benefit from the enhanced oxidation capabilities provided by SPR. Municipal water treatment plants and industrial wastewater facilities are increasingly exploring these technologies to meet stricter environmental regulations and improve treatment efficacy.
In the chemical manufacturing sector, SPR-enhanced electrolytic processes are being investigated for their potential to improve the selectivity and yield of electrochemical synthesis reactions. This could lead to more efficient production of fine chemicals, pharmaceuticals, and specialty materials, driving adoption in the chemical industry.
The electronics industry is also showing interest in SPR-enhanced electrolytic applications, particularly in the field of electroplating and surface finishing. The ability to control and enhance metal deposition at the nanoscale offers potential improvements in the manufacturing of advanced electronic components and printed circuit boards.
Market analysts predict a compound annual growth rate (CAGR) for SPR-enhanced electrolytic applications to be in the double digits over the next five years. This growth is supported by increasing research and development investments from both academic institutions and industrial players, as well as government initiatives promoting clean energy technologies and environmental protection.
However, the market faces challenges in terms of scalability and cost-effectiveness. While laboratory-scale demonstrations have shown promising results, translating these into large-scale industrial applications remains a significant hurdle. Additionally, the high initial investment required for implementing SPR-enhanced electrolytic systems may slow adoption rates, particularly among smaller enterprises.
Despite these challenges, the long-term market outlook for SPR-enhanced electrolytic applications remains positive. As the technology matures and economies of scale are achieved, it is expected to play a crucial role in advancing sustainable industrial processes and clean energy technologies, positioning itself as a key enabler in the transition towards a more environmentally friendly and efficient industrial landscape.
Current Challenges in SPR Integration with Electrolytic Cells
The integration of Surface Plasmon Resonance (SPR) technology with electrolytic cells presents several significant challenges that researchers and engineers are currently grappling with. One of the primary obstacles is the inherent incompatibility between the sensitive optical components required for SPR and the harsh electrochemical environment of electrolytic cells.
The corrosive nature of electrolytes and the presence of evolving gases can rapidly degrade the delicate metallic films used in SPR sensing. This degradation not only affects the longevity of the SPR sensors but also compromises the accuracy and reliability of measurements over time. Researchers are exploring various protective coatings and materials to enhance the durability of SPR components in electrolytic environments, but finding a solution that doesn't interfere with SPR sensitivity remains a significant challenge.
Another major hurdle is the integration of SPR optics with the electrodes and other components of electrolytic cells. The physical constraints of combining these two systems often lead to compromises in either the electrochemical performance or the SPR sensing capabilities. Miniaturization efforts are underway to create more compact and integrated designs, but these often come at the cost of reduced sensitivity or limited electrochemical control.
The dynamic nature of electrolytic processes also poses challenges for SPR measurements. Rapid changes in local refractive index due to evolving gases, concentration gradients, and temperature fluctuations can introduce noise and artifacts in SPR signals. Developing robust algorithms and data processing techniques to distinguish between these effects and the actual analyte-induced responses is an ongoing area of research.
Furthermore, the limited penetration depth of the evanescent field in SPR (typically around 100-200 nm) restricts its applicability to processes occurring very close to the electrode surface. This limitation makes it challenging to study bulk electrolyte behavior or reactions occurring further from the electrode interface. Researchers are exploring various modifications to extend the sensing range, such as long-range SPR or coupled plasmon-waveguide resonance, but each approach comes with its own set of integration challenges.
Lastly, the cost and complexity of integrating SPR systems with electrolytic cells remain significant barriers to widespread adoption. High-precision optical components and specialized surface preparations increase the overall system cost, while the expertise required to operate and interpret SPR data in electrochemical contexts limits its accessibility to non-specialists. Efforts to develop more user-friendly and cost-effective integrated systems are ongoing, but bridging the gap between SPR's potential and practical implementation in electrolytic cell applications continues to be a formidable challenge.
The corrosive nature of electrolytes and the presence of evolving gases can rapidly degrade the delicate metallic films used in SPR sensing. This degradation not only affects the longevity of the SPR sensors but also compromises the accuracy and reliability of measurements over time. Researchers are exploring various protective coatings and materials to enhance the durability of SPR components in electrolytic environments, but finding a solution that doesn't interfere with SPR sensitivity remains a significant challenge.
Another major hurdle is the integration of SPR optics with the electrodes and other components of electrolytic cells. The physical constraints of combining these two systems often lead to compromises in either the electrochemical performance or the SPR sensing capabilities. Miniaturization efforts are underway to create more compact and integrated designs, but these often come at the cost of reduced sensitivity or limited electrochemical control.
The dynamic nature of electrolytic processes also poses challenges for SPR measurements. Rapid changes in local refractive index due to evolving gases, concentration gradients, and temperature fluctuations can introduce noise and artifacts in SPR signals. Developing robust algorithms and data processing techniques to distinguish between these effects and the actual analyte-induced responses is an ongoing area of research.
Furthermore, the limited penetration depth of the evanescent field in SPR (typically around 100-200 nm) restricts its applicability to processes occurring very close to the electrode surface. This limitation makes it challenging to study bulk electrolyte behavior or reactions occurring further from the electrode interface. Researchers are exploring various modifications to extend the sensing range, such as long-range SPR or coupled plasmon-waveguide resonance, but each approach comes with its own set of integration challenges.
Lastly, the cost and complexity of integrating SPR systems with electrolytic cells remain significant barriers to widespread adoption. High-precision optical components and specialized surface preparations increase the overall system cost, while the expertise required to operate and interpret SPR data in electrochemical contexts limits its accessibility to non-specialists. Efforts to develop more user-friendly and cost-effective integrated systems are ongoing, but bridging the gap between SPR's potential and practical implementation in electrolytic cell applications continues to be a formidable challenge.
Existing SPR-Electrolytic Cell Integration Approaches
01 Surface Plasmon Resonance (SPR) sensing techniques
SPR is a powerful optical sensing technique used for detecting and analyzing biomolecular interactions. It relies on the excitation of surface plasmons at the interface between a metal film and a dielectric medium. This technique allows for label-free, real-time monitoring of molecular binding events with high sensitivity.- Surface Plasmon Resonance (SPR) sensing techniques: SPR is a widely used optical sensing technique for detecting and analyzing biomolecular interactions. It utilizes the phenomenon of surface plasmon resonance to measure changes in refractive index near a metal surface, typically gold or silver. This technique allows for label-free, real-time monitoring of molecular binding events and is extensively used in various fields such as biochemistry, pharmaceutical research, and environmental monitoring.
- SPR imaging and microscopy: SPR imaging and microscopy techniques combine the principles of SPR with imaging capabilities to provide spatial information about molecular interactions. These methods allow for the visualization and mapping of binding events across a surface, enabling high-throughput screening and multiplexed analysis. Advanced SPR imaging systems can achieve high spatial resolution and sensitivity, making them valuable tools for studying complex biological systems and developing biosensors.
- SPR-based biosensors and immunoassays: SPR technology is extensively used in the development of biosensors and immunoassays for detecting and quantifying various analytes, including proteins, nucleic acids, and small molecules. These sensors offer high sensitivity, specificity, and rapid detection capabilities, making them valuable tools in medical diagnostics, food safety testing, and environmental monitoring. SPR-based immunoassays can provide quantitative measurements of analyte concentrations without the need for labeling.
- Nanostructured SPR sensors: Nanostructured materials and surfaces are being explored to enhance the sensitivity and performance of SPR sensors. These include nanoparticles, nanoholes, and nanostructured metal films that can support localized surface plasmon resonance (LSPR) or exhibit unique optical properties. Nanostructured SPR sensors can achieve higher sensitivity, lower detection limits, and improved spatial resolution compared to conventional SPR systems, opening up new possibilities for ultra-sensitive detection and miniaturized sensing devices.
- Data analysis and signal processing in SPR: Advanced data analysis and signal processing techniques are crucial for extracting meaningful information from SPR measurements. This includes methods for noise reduction, baseline correction, and kinetic analysis of binding events. Machine learning and artificial intelligence approaches are being applied to SPR data analysis to improve the accuracy and efficiency of data interpretation, enabling more robust and automated analysis of complex molecular interactions and large-scale screening experiments.
02 SPR biosensor design and fabrication
The development of SPR biosensors involves careful design and fabrication of the sensing platform. This includes the selection of appropriate materials for the metal film (typically gold), optimization of the film thickness, and integration with microfluidic systems. Advanced fabrication techniques are employed to create nanostructured surfaces that enhance sensitivity and specificity.Expand Specific Solutions03 Signal processing and data analysis in SPR
Effective signal processing and data analysis are crucial for extracting meaningful information from SPR measurements. This involves developing algorithms for noise reduction, baseline correction, and kinetic analysis. Advanced data analysis techniques, such as machine learning and artificial intelligence, are being applied to improve the interpretation of SPR data.Expand Specific Solutions04 Applications of SPR in biomedical research and diagnostics
SPR technology has found widespread applications in biomedical research and diagnostics. It is used for studying protein-protein interactions, drug discovery, antibody characterization, and the development of point-of-care diagnostic devices. The technique's ability to provide real-time, label-free detection makes it valuable for various biomedical applications.Expand Specific Solutions05 Advancements in SPR instrumentation
Ongoing advancements in SPR instrumentation focus on improving sensitivity, throughput, and miniaturization. This includes the development of portable SPR devices, integration with other analytical techniques, and the use of novel light sources and detectors. These improvements aim to expand the applicability of SPR technology in various fields.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The surface plasmon resonance (SPR) technology in electrolytic cell applications is in a growth phase, with increasing market size and evolving technological maturity. The global market for SPR-based systems is expanding, driven by applications in biosensing, material science, and electrochemistry. Key players like Applied Materials and CSEM are advancing the technology, while academic institutions such as Tsinghua University and University of Manitoba are contributing to fundamental research. The technology's maturity is progressing, with companies like Beihang University and Sun Yat-Sen University developing novel SPR-based electrolytic cell designs. However, challenges in scalability and integration with existing systems remain, indicating room for further innovation and market development.
Applied Materials, Inc.
Technical Solution: Applied Materials has developed advanced surface plasmon resonance (SPR) sensors for electrolytic cell applications. Their technology utilizes nanoscale metal structures to enhance the sensitivity and selectivity of electrochemical measurements. The company's SPR-based sensors employ a thin gold film on a glass substrate, with precisely engineered nanostructures to optimize plasmon excitation[1]. This design allows for real-time monitoring of electrochemical reactions with unprecedented accuracy. The sensors can detect minute changes in the refractive index near the electrode surface, enabling the study of interfacial processes in electrolytic cells. Applied Materials has also integrated their SPR technology with microfluidic systems, allowing for high-throughput analysis of electrolyte compositions and reaction kinetics[3].
Strengths: High sensitivity and selectivity, real-time monitoring capabilities, and integration with microfluidic systems. Weaknesses: Relatively high cost of implementation and complexity in data interpretation for some applications.
Tsinghua University
Technical Solution: Tsinghua University has made significant advancements in applying surface plasmon resonance (SPR) to electrolytic cell applications. Their research team has developed a novel SPR-based sensing platform that combines plasmonic nanostructures with electrochemical techniques. This platform utilizes gold nanoparticle arrays on a transparent conductive substrate, allowing for simultaneous optical and electrochemical measurements[2]. The university's approach enables the study of electron transfer processes at the electrode-electrolyte interface with high spatial and temporal resolution. They have demonstrated the ability to monitor redox reactions, ion adsorption, and surface catalysis in real-time. Tsinghua's technology has shown particular promise in the field of energy storage, where it has been used to optimize electrolyte compositions for lithium-ion batteries[4].
Strengths: High-resolution measurements, simultaneous optical and electrochemical analysis, and applications in energy storage. Weaknesses: Limited scalability for large-scale industrial processes and potential interference from complex electrolyte compositions.
Breakthrough SPR Technologies for Electrolytic Processes
Microstructures and method of manufacture
PatentInactiveUS20060060472A1
Innovation
- A process involving anodizing an aluminum member to form a layer with micropores, sealing with metal, surface treatment to reduce surface roughness, and electrodeposition to form metal particles, ensuring they are close and uniformly distributed.
Use of electromagnetic excitation or light-matter interactions to generate or exchange thermal, kinetic, electronic or photonic energy
PatentInactiveUS20080271778A1
Innovation
- The use of electromagnetic excitation or light-matter interactions to generate localized thermal conditions and control energy states in metallic nanostructures, enabling the development of photovoltaic and thermophotovoltaic cells with improved power conversion efficiency, lower fabrication costs, and reduced environmental impact by employing non-toxic, organic, and ecologically stable elements, and using plasmon resonant frequencies to enhance light absorption and energy conversion.
Environmental Impact of SPR in Electrolytic Applications
Surface Plasmon Resonance (SPR) in electrolytic cell applications has significant environmental implications that warrant careful consideration. The integration of SPR technology in electrolytic processes offers potential benefits in terms of enhanced efficiency and sensitivity, but it also raises concerns about its ecological footprint.
One of the primary environmental advantages of SPR in electrolytic applications is the potential reduction in energy consumption. By improving the sensitivity and specificity of electrochemical reactions, SPR can lead to more efficient processes that require less electrical input. This reduction in energy demand can translate to lower greenhouse gas emissions associated with power generation, particularly in regions heavily reliant on fossil fuels.
However, the manufacturing of SPR-enabled electrolytic cells may involve the use of rare earth metals and other scarce resources. The extraction and processing of these materials can have substantial environmental impacts, including habitat destruction, water pollution, and increased carbon emissions. It is crucial to consider the entire life cycle of SPR-enhanced electrolytic systems when assessing their overall environmental impact.
The improved selectivity offered by SPR in electrolytic applications can lead to reduced waste generation. By enabling more precise control over electrochemical reactions, unwanted by-products can be minimized, resulting in cleaner effluents and less environmental contamination. This aspect is particularly relevant in industries such as wastewater treatment and chemical manufacturing, where the reduction of harmful by-products is a key environmental concern.
The durability and longevity of SPR-enhanced electrolytic cells also play a role in their environmental impact. If these systems prove to be more robust and longer-lasting than conventional electrolytic cells, it could lead to a reduction in the frequency of replacements and associated waste. However, the complex nature of SPR components may present challenges in terms of recyclability and end-of-life management.
In the context of environmental monitoring and remediation, SPR in electrolytic applications shows promise for detecting and removing pollutants from water and soil. The enhanced sensitivity of SPR-based sensors could enable the detection of contaminants at lower concentrations, potentially leading to more effective and timely environmental protection measures.
The scalability of SPR technology in electrolytic applications is another factor to consider when assessing its environmental impact. As the technology matures and becomes more widely adopted, economies of scale may lead to more efficient production processes and reduced environmental costs per unit. However, the widespread deployment of this technology could also lead to increased demand for the materials used in SPR components, potentially exacerbating resource extraction issues.
One of the primary environmental advantages of SPR in electrolytic applications is the potential reduction in energy consumption. By improving the sensitivity and specificity of electrochemical reactions, SPR can lead to more efficient processes that require less electrical input. This reduction in energy demand can translate to lower greenhouse gas emissions associated with power generation, particularly in regions heavily reliant on fossil fuels.
However, the manufacturing of SPR-enabled electrolytic cells may involve the use of rare earth metals and other scarce resources. The extraction and processing of these materials can have substantial environmental impacts, including habitat destruction, water pollution, and increased carbon emissions. It is crucial to consider the entire life cycle of SPR-enhanced electrolytic systems when assessing their overall environmental impact.
The improved selectivity offered by SPR in electrolytic applications can lead to reduced waste generation. By enabling more precise control over electrochemical reactions, unwanted by-products can be minimized, resulting in cleaner effluents and less environmental contamination. This aspect is particularly relevant in industries such as wastewater treatment and chemical manufacturing, where the reduction of harmful by-products is a key environmental concern.
The durability and longevity of SPR-enhanced electrolytic cells also play a role in their environmental impact. If these systems prove to be more robust and longer-lasting than conventional electrolytic cells, it could lead to a reduction in the frequency of replacements and associated waste. However, the complex nature of SPR components may present challenges in terms of recyclability and end-of-life management.
In the context of environmental monitoring and remediation, SPR in electrolytic applications shows promise for detecting and removing pollutants from water and soil. The enhanced sensitivity of SPR-based sensors could enable the detection of contaminants at lower concentrations, potentially leading to more effective and timely environmental protection measures.
The scalability of SPR technology in electrolytic applications is another factor to consider when assessing its environmental impact. As the technology matures and becomes more widely adopted, economies of scale may lead to more efficient production processes and reduced environmental costs per unit. However, the widespread deployment of this technology could also lead to increased demand for the materials used in SPR components, potentially exacerbating resource extraction issues.
Scalability and Industrial Implementation Considerations
The scalability and industrial implementation of Surface Plasmon Resonance (SPR) in electrolytic cell applications present both opportunities and challenges. As the technology transitions from laboratory-scale experiments to large-scale industrial processes, several key considerations must be addressed.
Firstly, the scaling up of SPR-enhanced electrolytic cells requires careful engineering of the plasmonic structures. While nanoscale plasmonic features can be readily fabricated for small-scale devices, producing large-area plasmonic surfaces with consistent properties poses significant manufacturing challenges. Advanced fabrication techniques such as roll-to-roll nanoimprinting or large-area lithography may need to be developed or adapted to meet industrial production demands.
The integration of SPR components into existing electrolytic cell designs is another crucial aspect. Industrial-scale cells often operate under harsh conditions, including high temperatures, corrosive environments, and intense electric fields. Ensuring the long-term stability and performance of plasmonic structures under these conditions is essential for practical implementation. This may necessitate the development of protective coatings or novel material combinations that can withstand industrial operating conditions while maintaining SPR functionality.
Cost considerations play a vital role in the industrial adoption of SPR-enhanced electrolytic cells. While the technology promises improved efficiency and selectivity, the initial investment in plasmonic materials and specialized fabrication equipment must be balanced against the expected performance gains. A thorough cost-benefit analysis, considering factors such as increased product yield, reduced energy consumption, and potential savings in downstream processing, is crucial for justifying the implementation of SPR technology in industrial settings.
The scalability of SPR effects themselves must also be carefully evaluated. Laboratory-scale demonstrations often involve ideal conditions and optimized geometries. Translating these results to large-scale systems may reveal unexpected challenges or diminishing returns. Comprehensive modeling and pilot-scale testing are essential to predict and optimize the performance of SPR-enhanced electrolytic cells at industrial scales.
Lastly, the integration of SPR technology into existing industrial processes requires consideration of the entire production chain. This includes adapting upstream and downstream processes to accommodate the unique characteristics of SPR-enhanced electrolysis, as well as developing new quality control and monitoring systems specific to plasmonic-enhanced reactions. Training of personnel and development of new operational protocols will also be necessary to ensure the effective implementation and maintenance of this advanced technology in industrial settings.
Firstly, the scaling up of SPR-enhanced electrolytic cells requires careful engineering of the plasmonic structures. While nanoscale plasmonic features can be readily fabricated for small-scale devices, producing large-area plasmonic surfaces with consistent properties poses significant manufacturing challenges. Advanced fabrication techniques such as roll-to-roll nanoimprinting or large-area lithography may need to be developed or adapted to meet industrial production demands.
The integration of SPR components into existing electrolytic cell designs is another crucial aspect. Industrial-scale cells often operate under harsh conditions, including high temperatures, corrosive environments, and intense electric fields. Ensuring the long-term stability and performance of plasmonic structures under these conditions is essential for practical implementation. This may necessitate the development of protective coatings or novel material combinations that can withstand industrial operating conditions while maintaining SPR functionality.
Cost considerations play a vital role in the industrial adoption of SPR-enhanced electrolytic cells. While the technology promises improved efficiency and selectivity, the initial investment in plasmonic materials and specialized fabrication equipment must be balanced against the expected performance gains. A thorough cost-benefit analysis, considering factors such as increased product yield, reduced energy consumption, and potential savings in downstream processing, is crucial for justifying the implementation of SPR technology in industrial settings.
The scalability of SPR effects themselves must also be carefully evaluated. Laboratory-scale demonstrations often involve ideal conditions and optimized geometries. Translating these results to large-scale systems may reveal unexpected challenges or diminishing returns. Comprehensive modeling and pilot-scale testing are essential to predict and optimize the performance of SPR-enhanced electrolytic cells at industrial scales.
Lastly, the integration of SPR technology into existing industrial processes requires consideration of the entire production chain. This includes adapting upstream and downstream processes to accommodate the unique characteristics of SPR-enhanced electrolysis, as well as developing new quality control and monitoring systems specific to plasmonic-enhanced reactions. Training of personnel and development of new operational protocols will also be necessary to ensure the effective implementation and maintenance of this advanced technology in industrial settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!