Electrolytic Cells and Their Role in the Hydrogen Economy
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolytic Cell Tech Evolution and Objectives
Electrolytic cells have played a pivotal role in the evolution of hydrogen production technologies, marking significant milestones in the journey towards a sustainable hydrogen economy. The development of these cells can be traced back to the early 19th century, with Sir William Nicholson and Anthony Carlisle's groundbreaking discovery of water electrolysis in 1800. This discovery laid the foundation for future advancements in electrolytic hydrogen production.
Throughout the 20th century, electrolytic cell technology underwent substantial improvements, driven by the increasing demand for industrial hydrogen production. The introduction of alkaline electrolyzers in the 1920s represented a major leap forward, offering higher efficiency and scalability compared to earlier designs. These systems became the backbone of large-scale hydrogen production for various industrial applications, including ammonia synthesis and petroleum refining.
The 1960s and 1970s saw the emergence of proton exchange membrane (PEM) electrolyzers, which promised higher current densities and improved efficiency. This technology, initially developed for space applications, gradually found its way into terrestrial use, offering advantages such as compact design and rapid response times. The continuous refinement of PEM technology has been crucial in addressing the intermittent nature of renewable energy sources, making it increasingly relevant in the context of green hydrogen production.
In recent years, the focus has shifted towards developing more efficient and cost-effective electrolytic cells to support the growing hydrogen economy. High-temperature electrolysis, utilizing solid oxide electrolyte cells, has emerged as a promising technology for achieving higher overall system efficiency. This approach leverages waste heat from industrial processes or high-temperature nuclear reactors to reduce the electrical energy required for hydrogen production.
The primary objective of current electrolytic cell research is to overcome the key challenges hindering widespread adoption. These include reducing capital costs, improving durability and efficiency, and scaling up production capacity. Researchers are exploring advanced materials, such as novel catalysts and membranes, to enhance performance and longevity while reducing reliance on precious metals.
Another critical goal is the integration of electrolytic cells with renewable energy sources to produce green hydrogen at scale. This involves developing flexible and responsive electrolysis systems capable of operating efficiently under variable power inputs from wind and solar sources. The ultimate aim is to establish a sustainable and economically viable pathway for large-scale hydrogen production, supporting the transition to a low-carbon energy system.
As the hydrogen economy continues to evolve, electrolytic cells are expected to play an increasingly central role. The technology roadmap focuses on achieving cost parity with fossil fuel-based hydrogen production methods, targeting a significant reduction in the levelized cost of hydrogen by 2030. This ambitious objective necessitates continued innovation in cell design, materials science, and system integration to position electrolytic hydrogen as a cornerstone of future energy systems.
Throughout the 20th century, electrolytic cell technology underwent substantial improvements, driven by the increasing demand for industrial hydrogen production. The introduction of alkaline electrolyzers in the 1920s represented a major leap forward, offering higher efficiency and scalability compared to earlier designs. These systems became the backbone of large-scale hydrogen production for various industrial applications, including ammonia synthesis and petroleum refining.
The 1960s and 1970s saw the emergence of proton exchange membrane (PEM) electrolyzers, which promised higher current densities and improved efficiency. This technology, initially developed for space applications, gradually found its way into terrestrial use, offering advantages such as compact design and rapid response times. The continuous refinement of PEM technology has been crucial in addressing the intermittent nature of renewable energy sources, making it increasingly relevant in the context of green hydrogen production.
In recent years, the focus has shifted towards developing more efficient and cost-effective electrolytic cells to support the growing hydrogen economy. High-temperature electrolysis, utilizing solid oxide electrolyte cells, has emerged as a promising technology for achieving higher overall system efficiency. This approach leverages waste heat from industrial processes or high-temperature nuclear reactors to reduce the electrical energy required for hydrogen production.
The primary objective of current electrolytic cell research is to overcome the key challenges hindering widespread adoption. These include reducing capital costs, improving durability and efficiency, and scaling up production capacity. Researchers are exploring advanced materials, such as novel catalysts and membranes, to enhance performance and longevity while reducing reliance on precious metals.
Another critical goal is the integration of electrolytic cells with renewable energy sources to produce green hydrogen at scale. This involves developing flexible and responsive electrolysis systems capable of operating efficiently under variable power inputs from wind and solar sources. The ultimate aim is to establish a sustainable and economically viable pathway for large-scale hydrogen production, supporting the transition to a low-carbon energy system.
As the hydrogen economy continues to evolve, electrolytic cells are expected to play an increasingly central role. The technology roadmap focuses on achieving cost parity with fossil fuel-based hydrogen production methods, targeting a significant reduction in the levelized cost of hydrogen by 2030. This ambitious objective necessitates continued innovation in cell design, materials science, and system integration to position electrolytic hydrogen as a cornerstone of future energy systems.
Hydrogen Economy Market Analysis
The hydrogen economy market is experiencing significant growth and transformation, driven by the increasing global focus on decarbonization and sustainable energy solutions. As countries and industries strive to reduce their carbon footprint, hydrogen has emerged as a versatile and clean energy carrier with the potential to revolutionize various sectors, including transportation, industry, and power generation.
The market for hydrogen-related technologies and applications is projected to expand rapidly in the coming years. This growth is fueled by several factors, including government policies and incentives promoting clean energy adoption, advancements in hydrogen production and storage technologies, and the increasing cost-competitiveness of hydrogen solutions compared to traditional fossil fuel-based alternatives.
One of the key drivers of the hydrogen economy is the transportation sector, particularly in the heavy-duty vehicle segment. Fuel cell electric vehicles (FCEVs) are gaining traction as a viable alternative to battery electric vehicles for long-range and high-payload applications. Major automotive manufacturers are investing heavily in hydrogen fuel cell technology, with several commercial FCEV models already available in the market.
The industrial sector represents another significant market opportunity for hydrogen. Industries such as steel production, chemical manufacturing, and refining are exploring hydrogen as a means to reduce their carbon emissions and meet increasingly stringent environmental regulations. Green hydrogen, produced through electrolysis using renewable energy sources, is particularly attractive for these applications.
In the power generation sector, hydrogen is being considered as a potential solution for long-term energy storage and grid balancing. As the share of intermittent renewable energy sources in the electricity mix increases, hydrogen can play a crucial role in storing excess energy during periods of high production and providing power during low production periods.
The market for electrolytic cells, a key component in hydrogen production through water electrolysis, is expected to grow substantially as the demand for green hydrogen increases. Technological advancements in electrolysis, such as improved efficiency and reduced costs, are making green hydrogen production more economically viable and competitive with traditional hydrogen production methods.
Geographically, several regions are emerging as leaders in the hydrogen economy. Europe, particularly countries like Germany and the Netherlands, has set ambitious targets for hydrogen adoption and is investing heavily in infrastructure development. Asia-Pacific, led by Japan and South Korea, is also at the forefront of hydrogen technology development and deployment, especially in the transportation sector.
The market for hydrogen-related technologies and applications is projected to expand rapidly in the coming years. This growth is fueled by several factors, including government policies and incentives promoting clean energy adoption, advancements in hydrogen production and storage technologies, and the increasing cost-competitiveness of hydrogen solutions compared to traditional fossil fuel-based alternatives.
One of the key drivers of the hydrogen economy is the transportation sector, particularly in the heavy-duty vehicle segment. Fuel cell electric vehicles (FCEVs) are gaining traction as a viable alternative to battery electric vehicles for long-range and high-payload applications. Major automotive manufacturers are investing heavily in hydrogen fuel cell technology, with several commercial FCEV models already available in the market.
The industrial sector represents another significant market opportunity for hydrogen. Industries such as steel production, chemical manufacturing, and refining are exploring hydrogen as a means to reduce their carbon emissions and meet increasingly stringent environmental regulations. Green hydrogen, produced through electrolysis using renewable energy sources, is particularly attractive for these applications.
In the power generation sector, hydrogen is being considered as a potential solution for long-term energy storage and grid balancing. As the share of intermittent renewable energy sources in the electricity mix increases, hydrogen can play a crucial role in storing excess energy during periods of high production and providing power during low production periods.
The market for electrolytic cells, a key component in hydrogen production through water electrolysis, is expected to grow substantially as the demand for green hydrogen increases. Technological advancements in electrolysis, such as improved efficiency and reduced costs, are making green hydrogen production more economically viable and competitive with traditional hydrogen production methods.
Geographically, several regions are emerging as leaders in the hydrogen economy. Europe, particularly countries like Germany and the Netherlands, has set ambitious targets for hydrogen adoption and is investing heavily in infrastructure development. Asia-Pacific, led by Japan and South Korea, is also at the forefront of hydrogen technology development and deployment, especially in the transportation sector.
Electrolytic Cell Challenges and Limitations
Electrolytic cells, while promising for hydrogen production in the green energy transition, face several significant challenges and limitations. The primary obstacle is the high energy consumption required for the electrolysis process, which impacts overall efficiency and economic viability. Current electrolytic systems typically operate at 70-80% efficiency, with substantial energy losses occurring as heat. This inefficiency translates to higher operational costs and reduced competitiveness compared to traditional hydrogen production methods.
Material degradation presents another critical challenge. The harsh electrochemical environment within electrolytic cells leads to corrosion and degradation of electrodes and other components. This not only reduces cell performance over time but also necessitates frequent maintenance and replacement, increasing long-term operational costs. The development of more durable materials that can withstand these conditions without compromising performance is an ongoing area of research.
Scalability remains a significant hurdle for widespread adoption of electrolytic hydrogen production. While small-scale electrolyzers have demonstrated effectiveness, scaling up to industrial levels presents engineering and economic challenges. Large-scale systems must maintain efficiency and reliability while managing increased complexity and potential safety concerns associated with hydrogen production and storage at scale.
The intermittent nature of renewable energy sources, such as wind and solar, poses challenges for electrolytic hydrogen production. Electrolyzers perform optimally under steady-state conditions, but fluctuating power inputs can lead to reduced efficiency and increased wear on components. Developing electrolytic systems that can operate efficiently under variable power conditions is crucial for integrating hydrogen production with renewable energy sources.
Water purity requirements present another limitation. Electrolyzers typically require high-purity water to prevent contamination and degradation of cell components. In regions where water scarcity is an issue, the need for water purification adds to the overall cost and complexity of hydrogen production systems. Developing electrolytic technologies that can operate with lower-quality water inputs could significantly expand their applicability.
Lastly, the current high cost of electrolytic hydrogen production compared to conventional methods remains a significant barrier to widespread adoption. While costs have decreased in recent years, further reductions are necessary to make green hydrogen economically competitive with fossil fuel-derived hydrogen. This challenge is compounded by the need for substantial infrastructure investments to support large-scale hydrogen production, distribution, and utilization.
Material degradation presents another critical challenge. The harsh electrochemical environment within electrolytic cells leads to corrosion and degradation of electrodes and other components. This not only reduces cell performance over time but also necessitates frequent maintenance and replacement, increasing long-term operational costs. The development of more durable materials that can withstand these conditions without compromising performance is an ongoing area of research.
Scalability remains a significant hurdle for widespread adoption of electrolytic hydrogen production. While small-scale electrolyzers have demonstrated effectiveness, scaling up to industrial levels presents engineering and economic challenges. Large-scale systems must maintain efficiency and reliability while managing increased complexity and potential safety concerns associated with hydrogen production and storage at scale.
The intermittent nature of renewable energy sources, such as wind and solar, poses challenges for electrolytic hydrogen production. Electrolyzers perform optimally under steady-state conditions, but fluctuating power inputs can lead to reduced efficiency and increased wear on components. Developing electrolytic systems that can operate efficiently under variable power conditions is crucial for integrating hydrogen production with renewable energy sources.
Water purity requirements present another limitation. Electrolyzers typically require high-purity water to prevent contamination and degradation of cell components. In regions where water scarcity is an issue, the need for water purification adds to the overall cost and complexity of hydrogen production systems. Developing electrolytic technologies that can operate with lower-quality water inputs could significantly expand their applicability.
Lastly, the current high cost of electrolytic hydrogen production compared to conventional methods remains a significant barrier to widespread adoption. While costs have decreased in recent years, further reductions are necessary to make green hydrogen economically competitive with fossil fuel-derived hydrogen. This challenge is compounded by the need for substantial infrastructure investments to support large-scale hydrogen production, distribution, and utilization.
Current Electrolytic Cell Solutions
01 Electrolyte composition and membrane technology
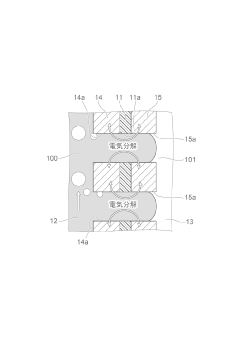
Advancements in electrolyte compositions and membrane technologies for electrolytic cells. This includes the development of novel electrolyte formulations and improved membrane materials to enhance cell efficiency, durability, and performance in various applications such as fuel cells and electrolyzers.- Electrolytic cell design and components: Electrolytic cells are designed with specific components to facilitate electrochemical reactions. These components typically include electrodes (anode and cathode), electrolyte, and a separator. The design of these cells can vary based on the intended application, such as fuel cells, batteries, or industrial electrolysis processes. Innovations in cell design focus on improving efficiency, durability, and performance.
- Electrode materials and configurations: The choice of electrode materials and their configurations play a crucial role in the performance of electrolytic cells. Research focuses on developing novel electrode materials with enhanced conductivity, stability, and catalytic activity. Electrode configurations may include planar, cylindrical, or more complex geometries to optimize surface area and reaction kinetics.
- Electrolyte composition and management: The electrolyte in an electrolytic cell serves as the medium for ion transport between electrodes. Innovations in this area include developing new electrolyte compositions, improving ion conductivity, and managing electrolyte levels and distribution within the cell. Proper electrolyte management is essential for maintaining cell performance and longevity.
- Control systems and monitoring: Advanced control systems and monitoring techniques are employed to optimize the operation of electrolytic cells. These systems may include sensors for measuring voltage, current, temperature, and other parameters. Real-time monitoring and control help maintain optimal operating conditions, prevent degradation, and enhance overall efficiency of the electrolytic process.
- Applications and specialized cell types: Electrolytic cells find applications in various fields, including energy storage, chemical production, and water treatment. Specialized cell types are developed for specific applications, such as chlor-alkali cells for chlorine production, fuel cells for energy conversion, and electrolyzers for hydrogen production. These specialized cells often incorporate unique design features and materials tailored to their specific requirements.
02 Electrode design and materials
Innovations in electrode design and materials for electrolytic cells. This encompasses the development of new electrode structures, coatings, and catalysts to improve reaction kinetics, reduce overpotentials, and increase the overall efficiency of electrochemical processes.Expand Specific Solutions03 Cell configuration and stack design
Advancements in the configuration and design of electrolytic cells and cell stacks. This includes optimizing cell geometry, flow patterns, and stack arrangements to improve mass transport, reduce internal resistance, and enhance overall system performance for various electrochemical applications.Expand Specific Solutions04 Control systems and monitoring
Development of advanced control systems and monitoring techniques for electrolytic cells. This involves the integration of sensors, data analytics, and intelligent control algorithms to optimize cell operation, detect faults, and improve overall system efficiency and reliability.Expand Specific Solutions05 Specialized applications and processes
Innovations in electrolytic cells for specialized applications and processes. This includes the development of cells for specific electrochemical reactions, such as water electrolysis, chlor-alkali production, metal recovery, and advanced energy storage systems, tailored to meet unique industry requirements.Expand Specific Solutions
Key Players in Electrolytic Cell Industry
The electrolytic cell market for hydrogen production is in a growth phase, driven by increasing focus on the hydrogen economy. The global market size is projected to expand significantly in the coming years, with estimates ranging from $0.5 to $2 billion by 2026. Technologically, the field is advancing rapidly, with companies like Umicore, Robert Bosch, and ThyssenKrupp Uhde Chlorine Engineers leading innovation in electrolysis efficiency and scalability. Emerging players such as EvolOH and Verdagy are also making strides in novel electrolysis technologies. While PEM and alkaline electrolysis are mature, newer technologies like solid oxide electrolysis are still evolving, indicating a dynamic competitive landscape with potential for further technological breakthroughs.
ThyssenKrupp Uhde Chlorine Engineers GmbH
Technical Solution: ThyssenKrupp Uhde Chlorine Engineers GmbH has developed advanced electrolysis technologies for hydrogen production. Their water electrolysis systems use both alkaline and PEM (Proton Exchange Membrane) technologies. The company's 20MW alkaline water electrolysis plant can produce up to 3,000 Nm³/h of hydrogen[1]. Their electrolyzers are designed for large-scale industrial applications, with a focus on green hydrogen production using renewable energy sources. The company has also invested in developing more efficient electrodes and membranes to improve the overall efficiency of the electrolysis process[2].
Strengths: Extensive experience in large-scale industrial electrolysis, proven technology for green hydrogen production. Weaknesses: High capital costs for large-scale plants, dependence on renewable energy availability for truly green hydrogen production.
Fortescue Future Industries Pty Ltd.
Technical Solution: Fortescue Future Industries (FFI) is actively developing electrolysis technology for large-scale green hydrogen production. They are working on advanced electrolyzer designs that aim to reduce costs and increase efficiency. FFI has announced plans to build one of the world's largest electrolyzer manufacturing facilities in Queensland, Australia, with a capacity to produce up to 2 GW of electrolyzers annually[3]. The company is also investing in integrating renewable energy sources directly with their electrolysis systems to ensure a consistent supply of green hydrogen. FFI's approach includes developing modular and scalable electrolyzer units that can be deployed in various industrial settings[4].
Strengths: Strong financial backing, ambitious plans for large-scale production, focus on cost reduction and efficiency improvements. Weaknesses: Relatively new entrant in the field, technology still in development stages.
Innovative Electrolysis Techniques
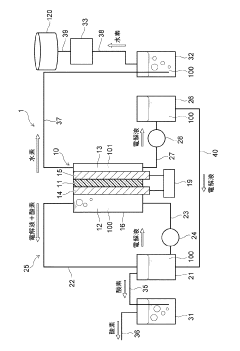
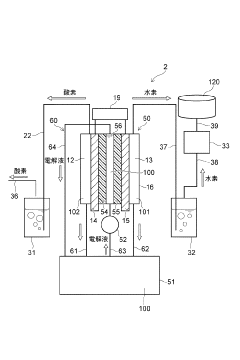
Electrolytic cell and hydrogen production device
PatentInactiveJP2019090087A
Innovation
- The electrolytic cell is designed with a housing divided into an anode-side and cathode-side cells by a diaphragm, where the cathode electrode is predominantly in contact with the gas phase and the anode electrode with the electrolytic solution, minimizing the mixing of oxygen into the hydrogen gas.
Electrolysis Cell for the Production of Hydrogen
PatentInactiveUS20090223814A1
Innovation
- Utilization of A/C current for water electrolysis, which is unconventional compared to typical DC electrolysis systems.
- Incorporation of hydrogen-rich compounds (e.g., cellulose, sucrose, chlorophyll) in the electrolyte to potentially enhance hydrogen yield.
- Flexibility in electrode materials and configurations to adapt to various cell output requirements.
Environmental Impact of Electrolysis
The environmental impact of electrolysis in the context of the hydrogen economy is a critical consideration for the sustainable development of this technology. While hydrogen production through electrolysis offers a promising pathway to clean energy, its environmental footprint must be carefully assessed and managed.
One of the primary environmental concerns associated with electrolysis is the source of electricity used to power the process. If the electricity is derived from fossil fuels, the overall carbon footprint of hydrogen production remains significant. However, when coupled with renewable energy sources such as solar or wind power, electrolysis can become a truly low-carbon solution for hydrogen generation.
Water consumption is another important environmental factor to consider. Electrolysis requires substantial amounts of water, which could potentially strain local water resources in areas where water scarcity is an issue. Advanced water management techniques and the use of seawater or wastewater as feedstock are being explored to mitigate this impact.
The production and disposal of electrolytic cell components also contribute to the environmental impact. Many electrolyzers use rare earth elements and precious metals as catalysts, which have their own environmental and social implications in terms of mining and processing. Developing more sustainable materials and improving recycling processes for these components are crucial areas of ongoing research.
Oxygen, a byproduct of water electrolysis, is typically released into the atmosphere. While oxygen itself is not a pollutant, large-scale hydrogen production could potentially alter local atmospheric compositions. This aspect requires further study to understand any long-term ecological effects.
Land use is another consideration, particularly for large-scale electrolysis facilities. The integration of hydrogen production with existing renewable energy infrastructure could help minimize additional land requirements and associated ecosystem disruptions.
The environmental benefits of electrolysis in the hydrogen economy are substantial when considering the potential reduction in greenhouse gas emissions from various sectors. Hydrogen produced through clean electrolysis can replace fossil fuels in transportation, industry, and heating, leading to significant reductions in carbon dioxide and other pollutants.
As the technology advances, life cycle assessments (LCAs) are becoming increasingly important to quantify the full environmental impact of electrolytic hydrogen production. These assessments consider all stages from raw material extraction to end-of-life disposal, providing a comprehensive view of the technology's sustainability.
In conclusion, while electrolysis presents challenges in terms of resource use and potential local environmental impacts, its role in enabling a clean hydrogen economy offers substantial net environmental benefits. Ongoing research and development efforts are focused on optimizing the process to minimize negative impacts while maximizing its potential as a key enabler of a sustainable energy future.
One of the primary environmental concerns associated with electrolysis is the source of electricity used to power the process. If the electricity is derived from fossil fuels, the overall carbon footprint of hydrogen production remains significant. However, when coupled with renewable energy sources such as solar or wind power, electrolysis can become a truly low-carbon solution for hydrogen generation.
Water consumption is another important environmental factor to consider. Electrolysis requires substantial amounts of water, which could potentially strain local water resources in areas where water scarcity is an issue. Advanced water management techniques and the use of seawater or wastewater as feedstock are being explored to mitigate this impact.
The production and disposal of electrolytic cell components also contribute to the environmental impact. Many electrolyzers use rare earth elements and precious metals as catalysts, which have their own environmental and social implications in terms of mining and processing. Developing more sustainable materials and improving recycling processes for these components are crucial areas of ongoing research.
Oxygen, a byproduct of water electrolysis, is typically released into the atmosphere. While oxygen itself is not a pollutant, large-scale hydrogen production could potentially alter local atmospheric compositions. This aspect requires further study to understand any long-term ecological effects.
Land use is another consideration, particularly for large-scale electrolysis facilities. The integration of hydrogen production with existing renewable energy infrastructure could help minimize additional land requirements and associated ecosystem disruptions.
The environmental benefits of electrolysis in the hydrogen economy are substantial when considering the potential reduction in greenhouse gas emissions from various sectors. Hydrogen produced through clean electrolysis can replace fossil fuels in transportation, industry, and heating, leading to significant reductions in carbon dioxide and other pollutants.
As the technology advances, life cycle assessments (LCAs) are becoming increasingly important to quantify the full environmental impact of electrolytic hydrogen production. These assessments consider all stages from raw material extraction to end-of-life disposal, providing a comprehensive view of the technology's sustainability.
In conclusion, while electrolysis presents challenges in terms of resource use and potential local environmental impacts, its role in enabling a clean hydrogen economy offers substantial net environmental benefits. Ongoing research and development efforts are focused on optimizing the process to minimize negative impacts while maximizing its potential as a key enabler of a sustainable energy future.
Economic Viability of Hydrogen Production
The economic viability of hydrogen production through electrolysis is a critical factor in determining the role of electrolytic cells in the hydrogen economy. Current cost estimates for green hydrogen production range from $2.50 to $6.80 per kilogram, with the lower end representing the most optimized production scenarios. These costs are still significantly higher than the target price of $1 to $2 per kilogram, which is considered necessary for widespread adoption and competitiveness with fossil fuels.
The primary cost drivers in electrolytic hydrogen production are electricity, capital expenditure for electrolyzers, and operational expenses. Electricity costs typically account for 50-80% of the total production cost, making access to cheap, renewable energy sources crucial for economic viability. Regions with abundant solar or wind resources have a distinct advantage in this regard.
Capital costs for electrolyzers have been decreasing steadily, with prices falling by around 60% since 2010. Current prices range from $500 to $1,000 per kilowatt of capacity, depending on the technology and scale. Further reductions are expected as manufacturing scales up and technologies mature, with projections suggesting costs could fall below $200 per kilowatt by 2030.
Operational costs, including maintenance and replacement of components, also play a significant role in the overall economics. Improving the durability and efficiency of electrolyzers can substantially reduce these costs over time. Current electrolyzers have lifespans of 40,000 to 80,000 hours, but increasing this to 100,000 hours or more would significantly improve the economic outlook.
The economic viability of hydrogen production is also influenced by policy support and market demand. Government incentives, carbon pricing mechanisms, and mandates for clean energy can significantly improve the competitiveness of green hydrogen. As demand grows in sectors such as heavy industry, transportation, and energy storage, economies of scale are expected to further drive down costs.
Projections suggest that green hydrogen could become cost-competitive with fossil fuel-derived hydrogen in many regions by 2030, assuming continued technological improvements and supportive policies. However, achieving true economic viability will require a holistic approach, including optimizing the entire value chain from production to end-use applications.
The primary cost drivers in electrolytic hydrogen production are electricity, capital expenditure for electrolyzers, and operational expenses. Electricity costs typically account for 50-80% of the total production cost, making access to cheap, renewable energy sources crucial for economic viability. Regions with abundant solar or wind resources have a distinct advantage in this regard.
Capital costs for electrolyzers have been decreasing steadily, with prices falling by around 60% since 2010. Current prices range from $500 to $1,000 per kilowatt of capacity, depending on the technology and scale. Further reductions are expected as manufacturing scales up and technologies mature, with projections suggesting costs could fall below $200 per kilowatt by 2030.
Operational costs, including maintenance and replacement of components, also play a significant role in the overall economics. Improving the durability and efficiency of electrolyzers can substantially reduce these costs over time. Current electrolyzers have lifespans of 40,000 to 80,000 hours, but increasing this to 100,000 hours or more would significantly improve the economic outlook.
The economic viability of hydrogen production is also influenced by policy support and market demand. Government incentives, carbon pricing mechanisms, and mandates for clean energy can significantly improve the competitiveness of green hydrogen. As demand grows in sectors such as heavy industry, transportation, and energy storage, economies of scale are expected to further drive down costs.
Projections suggest that green hydrogen could become cost-competitive with fossil fuel-derived hydrogen in many regions by 2030, assuming continued technological improvements and supportive policies. However, achieving true economic viability will require a holistic approach, including optimizing the entire value chain from production to end-use applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!