Amine-Functionalized Silica Sorbents For Low-Temperature Direct Air Capture
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DAC Technology Background and Objectives
Direct Air Capture (DAC) technology has emerged as a critical approach in carbon dioxide removal strategies, evolving significantly since its conceptual introduction in the late 20th century. Initially considered theoretical, DAC has progressed through laboratory demonstrations to pilot plants and now approaches commercial viability. This evolution reflects growing recognition of the necessity for negative emissions technologies to complement traditional mitigation efforts in addressing climate change.
The development of amine-functionalized silica sorbents represents a pivotal advancement in DAC technology. These materials leverage the chemical affinity between amines and CO2 to enable selective capture from ambient air, where carbon dioxide exists at relatively low concentrations (approximately 420 ppm). Early DAC systems primarily utilized liquid solvents, but solid sorbents have gained prominence due to their potentially lower energy requirements and operational flexibility.
Amine-functionalized silica materials offer particular promise for low-temperature DAC applications, operating effectively at ambient conditions without requiring significant heating for CO2 capture. This characteristic addresses one of the fundamental challenges in DAC technology: minimizing energy consumption while maximizing capture efficiency. The technical objective centers on developing sorbents that demonstrate high CO2 selectivity, substantial capacity, rapid kinetics, and excellent cyclic stability while maintaining cost-effectiveness.
Current research trajectories focus on optimizing several key parameters: amine loading density, pore structure engineering, support material properties, and functionalization methods. Each represents a critical dimension for enhancing overall system performance. The field has progressed from simple impregnation methods to sophisticated molecular grafting techniques that provide greater control over the final material properties.
The technical goals for amine-functionalized silica sorbents include achieving CO2 capture capacities exceeding 2.5 mmol/g under ambient conditions, maintaining performance over thousands of adsorption-desorption cycles, reducing regeneration temperatures below 80°C, and developing scalable synthesis methods compatible with industrial production requirements. These targets align with broader objectives of making DAC economically viable at scale.
Recent technological trends indicate growing interest in hybrid materials that combine multiple capture mechanisms, hierarchical pore structures that optimize mass transfer, and advanced manufacturing techniques that reduce production costs. The convergence of materials science, chemical engineering, and process design continues to drive innovation in this field, with increasing emphasis on system-level optimization rather than isolated material properties.
The development of amine-functionalized silica sorbents represents a pivotal advancement in DAC technology. These materials leverage the chemical affinity between amines and CO2 to enable selective capture from ambient air, where carbon dioxide exists at relatively low concentrations (approximately 420 ppm). Early DAC systems primarily utilized liquid solvents, but solid sorbents have gained prominence due to their potentially lower energy requirements and operational flexibility.
Amine-functionalized silica materials offer particular promise for low-temperature DAC applications, operating effectively at ambient conditions without requiring significant heating for CO2 capture. This characteristic addresses one of the fundamental challenges in DAC technology: minimizing energy consumption while maximizing capture efficiency. The technical objective centers on developing sorbents that demonstrate high CO2 selectivity, substantial capacity, rapid kinetics, and excellent cyclic stability while maintaining cost-effectiveness.
Current research trajectories focus on optimizing several key parameters: amine loading density, pore structure engineering, support material properties, and functionalization methods. Each represents a critical dimension for enhancing overall system performance. The field has progressed from simple impregnation methods to sophisticated molecular grafting techniques that provide greater control over the final material properties.
The technical goals for amine-functionalized silica sorbents include achieving CO2 capture capacities exceeding 2.5 mmol/g under ambient conditions, maintaining performance over thousands of adsorption-desorption cycles, reducing regeneration temperatures below 80°C, and developing scalable synthesis methods compatible with industrial production requirements. These targets align with broader objectives of making DAC economically viable at scale.
Recent technological trends indicate growing interest in hybrid materials that combine multiple capture mechanisms, hierarchical pore structures that optimize mass transfer, and advanced manufacturing techniques that reduce production costs. The convergence of materials science, chemical engineering, and process design continues to drive innovation in this field, with increasing emphasis on system-level optimization rather than isolated material properties.
Market Analysis for Carbon Capture Solutions
The global carbon capture market is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce greenhouse gas emissions. As of 2023, the market size for carbon capture technologies has reached approximately $2.5 billion, with projections indicating a compound annual growth rate of 19.2% through 2030. Direct Air Capture (DAC) technologies, particularly those utilizing amine-functionalized silica sorbents, represent an emerging segment with substantial growth potential.
The demand for low-temperature DAC solutions is particularly strong in regions with ambitious carbon neutrality targets, including the European Union, North America, and increasingly, parts of Asia. Industries with hard-to-abate emissions, such as cement production, steel manufacturing, and power generation, constitute the primary customer base for these technologies. Additionally, the voluntary carbon market, valued at $2 billion in 2022, provides another avenue for commercialization as corporations seek to offset their carbon footprints.
Amine-functionalized silica sorbents for DAC applications address a critical market need for energy-efficient carbon capture solutions that can operate at ambient temperatures. Traditional carbon capture technologies often require significant energy inputs for thermal regeneration, making them economically challenging to implement at scale. The market is increasingly favoring solutions that minimize energy penalties while maximizing capture efficiency.
Government incentives and carbon pricing mechanisms are significantly influencing market dynamics. The U.S. 45Q tax credit, offering up to $180 per ton for DAC with geological storage, has catalyzed investment in this sector. Similarly, the EU Emissions Trading System, with carbon prices exceeding €80 per ton, creates favorable economics for DAC deployment in European markets.
Investor interest in DAC technologies has surged, with venture capital funding in this space reaching $880 million in 2022, a 110% increase from the previous year. Strategic partnerships between technology developers and industrial emitters are becoming increasingly common, facilitating faster commercialization pathways for promising technologies.
Market barriers include high capital costs, with current DAC solutions costing between $250-600 per ton of CO2 captured, and scaling challenges. However, technological improvements in sorbent performance, particularly in the area of amine-functionalized silica materials, are expected to drive costs down to potentially below $100 per ton by 2035, significantly expanding the addressable market.
Customer adoption patterns indicate growing acceptance of DAC technologies, with early adopters primarily being companies with net-zero commitments and operations in jurisdictions with strong carbon pricing signals. The market for amine-based DAC solutions is projected to reach $5.6 billion by 2030, representing a significant opportunity for technology developers in this space.
The demand for low-temperature DAC solutions is particularly strong in regions with ambitious carbon neutrality targets, including the European Union, North America, and increasingly, parts of Asia. Industries with hard-to-abate emissions, such as cement production, steel manufacturing, and power generation, constitute the primary customer base for these technologies. Additionally, the voluntary carbon market, valued at $2 billion in 2022, provides another avenue for commercialization as corporations seek to offset their carbon footprints.
Amine-functionalized silica sorbents for DAC applications address a critical market need for energy-efficient carbon capture solutions that can operate at ambient temperatures. Traditional carbon capture technologies often require significant energy inputs for thermal regeneration, making them economically challenging to implement at scale. The market is increasingly favoring solutions that minimize energy penalties while maximizing capture efficiency.
Government incentives and carbon pricing mechanisms are significantly influencing market dynamics. The U.S. 45Q tax credit, offering up to $180 per ton for DAC with geological storage, has catalyzed investment in this sector. Similarly, the EU Emissions Trading System, with carbon prices exceeding €80 per ton, creates favorable economics for DAC deployment in European markets.
Investor interest in DAC technologies has surged, with venture capital funding in this space reaching $880 million in 2022, a 110% increase from the previous year. Strategic partnerships between technology developers and industrial emitters are becoming increasingly common, facilitating faster commercialization pathways for promising technologies.
Market barriers include high capital costs, with current DAC solutions costing between $250-600 per ton of CO2 captured, and scaling challenges. However, technological improvements in sorbent performance, particularly in the area of amine-functionalized silica materials, are expected to drive costs down to potentially below $100 per ton by 2035, significantly expanding the addressable market.
Customer adoption patterns indicate growing acceptance of DAC technologies, with early adopters primarily being companies with net-zero commitments and operations in jurisdictions with strong carbon pricing signals. The market for amine-based DAC solutions is projected to reach $5.6 billion by 2030, representing a significant opportunity for technology developers in this space.
Current Challenges in Low-Temperature DAC
Despite significant advancements in Direct Air Capture (DAC) technologies, low-temperature DAC systems utilizing amine-functionalized silica sorbents face several critical challenges that impede their widespread commercial deployment. The primary obstacle remains the high energy consumption required for the regeneration process. Even at relatively lower temperatures (80-120°C) compared to high-temperature systems, the energy demands translate to substantial operational costs, making the technology economically unviable for large-scale implementation without significant subsidies or carbon pricing mechanisms.
Material degradation presents another formidable challenge. Amine-functionalized silica sorbents experience performance deterioration over multiple adsorption-desorption cycles due to amine leaching, oxidative degradation, and urea formation. This degradation not only reduces CO₂ capture efficiency but also necessitates frequent sorbent replacement, further increasing operational costs and creating additional waste streams that must be managed.
Moisture management represents a significant technical hurdle. While water can initially enhance CO₂ adsorption through the formation of bicarbonates, excessive humidity can lead to competitive adsorption, reducing overall CO₂ capture capacity. Additionally, water co-adsorption increases the energy required during the regeneration phase, creating a delicate balance that must be optimized for specific environmental conditions.
Scale-up challenges persist in transitioning from laboratory demonstrations to industrial-scale implementations. The design of efficient contactor systems that maximize air-sorbent interaction while minimizing pressure drop remains problematic. Current packed bed configurations suffer from high pressure drops, while fluidized bed systems face issues with particle attrition and increased complexity in sorbent handling.
The economic viability of low-temperature DAC systems is further constrained by high capital expenditures. The specialized equipment required for efficient air handling, precise temperature control during adsorption and desorption cycles, and the substantial quantities of amine-functionalized silica sorbents contribute to prohibitive initial investments, with current cost estimates ranging from $250-600 per ton of CO₂ captured.
Additionally, the environmental footprint of sorbent production presents sustainability concerns. The synthesis of amine-functionalized silica materials involves energy-intensive processes and potentially hazardous chemicals. Life cycle assessments indicate that the environmental benefits of carbon capture may be partially offset by the impacts of sorbent manufacturing, particularly if frequent replacement is necessary due to degradation issues.
Addressing these interconnected challenges requires a multidisciplinary approach combining materials science innovations, process engineering optimizations, and economic incentives to drive the development of next-generation low-temperature DAC technologies with enhanced performance and reduced costs.
Material degradation presents another formidable challenge. Amine-functionalized silica sorbents experience performance deterioration over multiple adsorption-desorption cycles due to amine leaching, oxidative degradation, and urea formation. This degradation not only reduces CO₂ capture efficiency but also necessitates frequent sorbent replacement, further increasing operational costs and creating additional waste streams that must be managed.
Moisture management represents a significant technical hurdle. While water can initially enhance CO₂ adsorption through the formation of bicarbonates, excessive humidity can lead to competitive adsorption, reducing overall CO₂ capture capacity. Additionally, water co-adsorption increases the energy required during the regeneration phase, creating a delicate balance that must be optimized for specific environmental conditions.
Scale-up challenges persist in transitioning from laboratory demonstrations to industrial-scale implementations. The design of efficient contactor systems that maximize air-sorbent interaction while minimizing pressure drop remains problematic. Current packed bed configurations suffer from high pressure drops, while fluidized bed systems face issues with particle attrition and increased complexity in sorbent handling.
The economic viability of low-temperature DAC systems is further constrained by high capital expenditures. The specialized equipment required for efficient air handling, precise temperature control during adsorption and desorption cycles, and the substantial quantities of amine-functionalized silica sorbents contribute to prohibitive initial investments, with current cost estimates ranging from $250-600 per ton of CO₂ captured.
Additionally, the environmental footprint of sorbent production presents sustainability concerns. The synthesis of amine-functionalized silica materials involves energy-intensive processes and potentially hazardous chemicals. Life cycle assessments indicate that the environmental benefits of carbon capture may be partially offset by the impacts of sorbent manufacturing, particularly if frequent replacement is necessary due to degradation issues.
Addressing these interconnected challenges requires a multidisciplinary approach combining materials science innovations, process engineering optimizations, and economic incentives to drive the development of next-generation low-temperature DAC technologies with enhanced performance and reduced costs.
State-of-Art Silica-Amine Sorbent Systems
01 Low-temperature synthesis methods for amine-functionalized silica sorbents
Various methods have been developed for synthesizing amine-functionalized silica sorbents at low temperatures. These methods typically involve the grafting or impregnation of amine compounds onto silica supports under mild temperature conditions. Low-temperature synthesis helps preserve the amine functionality and prevents degradation of the organic components, resulting in higher amine loading and improved CO2 capture performance. These methods often utilize sol-gel processes or post-synthesis modification techniques that can be conducted at ambient or slightly elevated temperatures.- Low-temperature CO2 capture using amine-functionalized silica sorbents: Amine-functionalized silica materials are effective for capturing carbon dioxide at low temperatures. These sorbents demonstrate high CO2 adsorption capacity and selectivity under ambient or sub-ambient temperature conditions. The incorporation of various amine groups onto silica supports creates materials that can efficiently capture CO2 from gas streams without requiring high temperatures, making them energy-efficient for carbon capture applications.
- Synthesis methods for amine-functionalized silica sorbents: Various synthesis approaches are used to create amine-functionalized silica sorbents that operate at low temperatures. These include post-synthesis grafting of amine compounds onto silica surfaces, co-condensation methods during silica formation, and impregnation techniques. The synthesis parameters significantly influence the distribution of amine groups, pore structure, and ultimately the CO2 adsorption performance at low temperatures.
- Structure-property relationships in low-temperature silica sorbents: The relationship between structural properties of amine-functionalized silica materials and their performance as low-temperature sorbents is critical. Factors such as pore size distribution, surface area, amine loading density, and type of amine functionality significantly affect CO2 capture efficiency. Optimizing these parameters enables the development of sorbents with enhanced capacity, selectivity, and stability for low-temperature applications.
- Regeneration of amine-functionalized silica sorbents at low temperatures: Regeneration of amine-functionalized silica sorbents can be achieved at relatively low temperatures compared to traditional sorbents. This characteristic reduces the energy requirements for the overall capture and release cycle. Various methods including temperature swing, pressure swing, and humidity swing approaches are employed to efficiently desorb captured CO2 while maintaining the integrity and performance of the amine functionalities over multiple cycles.
- Applications of low-temperature amine-functionalized silica sorbents: Low-temperature amine-functionalized silica sorbents find applications in various fields including post-combustion carbon capture, direct air capture, natural gas purification, and enclosed space air purification. These materials are particularly valuable in scenarios where thermal energy availability is limited or where temperature-sensitive processes are involved. Their ability to operate efficiently at ambient or sub-ambient conditions makes them suitable for integration with existing industrial processes.
02 CO2 capture applications using amine-functionalized silica at low temperatures
Amine-functionalized silica sorbents are particularly effective for CO2 capture at low temperatures. These materials can adsorb CO2 efficiently from flue gas streams or ambient air at temperatures ranging from ambient to about 100°C. The low-temperature operation provides advantages in terms of energy efficiency and operational costs compared to high-temperature processes. The CO2 capture capacity and selectivity of these sorbents can be tailored by controlling the type and density of amine groups on the silica surface, as well as the pore structure of the support material.Expand Specific Solutions03 Structure-property relationships in low-temperature amine-silica sorbents
The performance of amine-functionalized silica sorbents at low temperatures is strongly influenced by their structural properties. Key factors include the pore size distribution, surface area, and amine density. Mesoporous silica materials with well-defined pore structures provide optimal platforms for amine functionalization, allowing for high amine loading while maintaining accessibility to adsorption sites. The type of amine (primary, secondary, tertiary, or polyamines) and its distribution on the silica surface also significantly affect the adsorption behavior at low temperatures, with primary and secondary amines generally showing better performance for CO2 capture.Expand Specific Solutions04 Regeneration and stability of amine-functionalized silica sorbents
Amine-functionalized silica sorbents can be regenerated at relatively low temperatures compared to traditional sorbents, which contributes to energy efficiency in cyclic adsorption processes. The regeneration typically occurs at temperatures between 70-120°C, which is lower than conventional regeneration temperatures. However, maintaining stability over multiple adsorption-desorption cycles remains a challenge. Strategies to enhance the stability include optimizing the amine loading, using sterically hindered amines, incorporating stabilizing additives, and developing novel silica supports with improved thermal and hydrothermal stability.Expand Specific Solutions05 Hybrid and composite amine-functionalized silica materials
Hybrid and composite materials combining amine-functionalized silica with other components have been developed to enhance performance at low temperatures. These include silica-polymer composites, metal-organic framework (MOF)-silica hybrids, and multi-functional sorbents incorporating both physical and chemical adsorption mechanisms. The addition of metal nanoparticles or metal oxides can introduce catalytic functionality, while polymer incorporation can improve moisture tolerance and mechanical stability. These hybrid materials often demonstrate superior adsorption capacity, selectivity, and stability compared to conventional amine-silica sorbents when operated at low temperatures.Expand Specific Solutions
Leading Organizations in DAC Technology
The direct air capture (DAC) technology market for amine-functionalized silica sorbents is in an early growth phase, characterized by increasing research intensity and commercial deployment. The global DAC market is projected to expand significantly, driven by carbon neutrality goals and climate policies, though currently remains relatively small at under $100 million. From a technological maturity perspective, key players demonstrate varying development stages: Climeworks and Global Thermostat lead with commercial installations, while ExxonMobil and X Development bring substantial R&D capabilities. Academic institutions like Georgia Tech, University of California, and Shanghai Jiao Tong University contribute fundamental research advancements. The competitive landscape features both specialized DAC companies and diversified energy corporations investing in this technology, indicating growing recognition of its strategic importance in carbon management solutions.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil has developed a comprehensive approach to amine-functionalized silica sorbents for direct air capture focusing on scalability and integration with existing industrial infrastructure. Their technology employs specially engineered silica supports with controlled porosity and surface chemistry, functionalized with proprietary amine formulations that balance CO2 selectivity, capacity, and regeneration energy requirements. The company's approach includes a dual-function sorbent system where primary amines provide high capture rates at low CO2 concentrations while secondary and tertiary amines contribute to overall capacity and regeneration efficiency. ExxonMobil's process operates in a temperature swing adsorption cycle with adsorption at 25-40°C and regeneration at 90-110°C, enabling integration with industrial waste heat streams. A distinguishing feature is their fluidized bed contactor design that maximizes air-sorbent contact while minimizing pressure drop and energy consumption. The system incorporates moisture management technology to handle water co-adsorption effects, maintaining performance across varying humidity conditions. ExxonMobil has demonstrated their technology at pilot scale, capturing several tons of CO2 per day with projected costs of $100-150 per ton at commercial scale when integrated with existing industrial facilities[7][8].
Strengths: Extensive resources and engineering expertise for rapid scaling; integration capability with existing industrial infrastructure; sophisticated moisture management systems; advanced process optimization reducing parasitic energy loads. Weaknesses: Higher regeneration temperatures (90-110°C) than some competitors; technology remains primarily at pilot scale rather than commercial deployment; potential sorbent degradation from contaminants in industrial environments.
Climeworks AG
Technical Solution: Climeworks has developed a proprietary direct air capture (DAC) technology using amine-functionalized solid sorbents that operate at relatively low temperatures (80-100°C). Their approach employs a modular design with specialized adsorption filters containing porous granulates modified with amines that selectively capture CO2 from ambient air. The process works through temperature-vacuum swing adsorption (TVSA), where fans draw air through collectors containing the sorbent. Once saturated, the collectors are heated to 80-100°C while under vacuum to release concentrated CO2. Climeworks' technology is commercially deployed in multiple facilities, including their Orca plant in Iceland which captures 4,000 tons of CO2 annually, and their larger Mammoth plant under development with a planned capacity of 36,000 tons per year. Their sorbent materials are specifically engineered for durability, allowing thousands of adsorption-desorption cycles without significant degradation of capture capacity[1][2].
Strengths: Commercially proven technology with operational plants; modular design allows for scalability; integration with geothermal energy in Iceland reduces energy costs; sorbent durability enables long operational lifetime. Weaknesses: Relatively high energy requirements for regeneration compared to theoretical minimums; current costs remain high at approximately $600-800 per ton of CO2 captured; limited by geographical constraints for optimal renewable energy access.
Key Innovations in Amine-Silica Chemistry
Method of treatment ammonia contaminated solid sorbent
PatentWO2025136089A1
Innovation
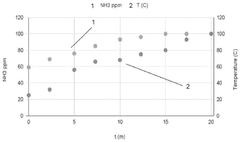
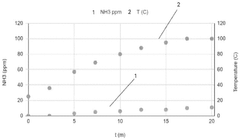
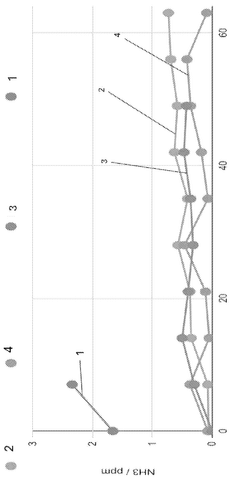
- A method involving contacting the ammonia-contaminated solid amine-functionalized sorbent with an aqueous medium while stirring, followed by drying, to reduce ammonia concentrations to less than 5 ppm, thereby enhancing CO2 adsorption capacity and minimizing ammonia emissions.
Energy-efficient and stable modified amines and methods for co 2 separation
PatentWO2024216296A1
Innovation
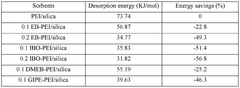
- A sorbent chemical comprising an amine functionalized with an epoxide chemical, such as polyethylenimine or tetraethylenepentamine, impregnated onto a mesoporous silica support, combined with microwave heating to enhance desorption efficiency and reduce energy requirements.
Environmental Impact Assessment
The environmental implications of amine-functionalized silica sorbents for direct air capture (DAC) technology must be comprehensively evaluated to ensure sustainable implementation. These sorbents, while promising for carbon dioxide removal, require careful assessment across their entire lifecycle.
Primary environmental benefits stem from the technology's carbon negative potential. Each ton of CO2 captured and permanently sequestered represents a net reduction in atmospheric greenhouse gases. Low-temperature operation (below 100°C) significantly reduces energy requirements compared to high-temperature DAC systems, potentially lowering the overall carbon footprint of the capture process when powered by renewable energy sources.
However, several environmental concerns warrant attention. The production of functionalized silica materials involves chemical synthesis processes that may generate hazardous waste streams. Silica mining and processing create land disturbances and habitat disruption, while amine production typically relies on petrochemical feedstocks with associated extraction impacts. Quantitative life cycle assessment studies indicate that manufacturing impacts must be amortized over multiple adsorption-desorption cycles to achieve net environmental benefits.
Water usage presents another critical consideration. While amine-functionalized silica sorbents demonstrate improved performance in humid conditions compared to some alternatives, the regeneration process may require significant water inputs depending on system design. In water-stressed regions, this could exacerbate existing resource pressures, necessitating careful site selection and water management strategies.
Long-term environmental risks include potential amine degradation and leaching. Under operational conditions, amines may slowly degrade, potentially releasing volatile organic compounds or ammonia. Proper containment systems and monitoring protocols are essential to prevent these emissions. Additionally, disposal or recycling pathways for spent sorbents must be established to prevent environmental contamination at end-of-life.
The spatial footprint of DAC facilities utilizing these sorbents must also be considered. Large-scale implementation would require substantial land area, potentially competing with other land uses. However, the modular nature of adsorption-based systems allows for flexible deployment in various settings, including integration with existing industrial facilities to utilize waste heat.
Regulatory frameworks for environmental monitoring and compliance are still evolving for DAC technologies. Establishing robust environmental management systems, including regular monitoring of air and water quality surrounding DAC facilities, will be essential for responsible deployment and public acceptance of this emerging carbon removal approach.
Primary environmental benefits stem from the technology's carbon negative potential. Each ton of CO2 captured and permanently sequestered represents a net reduction in atmospheric greenhouse gases. Low-temperature operation (below 100°C) significantly reduces energy requirements compared to high-temperature DAC systems, potentially lowering the overall carbon footprint of the capture process when powered by renewable energy sources.
However, several environmental concerns warrant attention. The production of functionalized silica materials involves chemical synthesis processes that may generate hazardous waste streams. Silica mining and processing create land disturbances and habitat disruption, while amine production typically relies on petrochemical feedstocks with associated extraction impacts. Quantitative life cycle assessment studies indicate that manufacturing impacts must be amortized over multiple adsorption-desorption cycles to achieve net environmental benefits.
Water usage presents another critical consideration. While amine-functionalized silica sorbents demonstrate improved performance in humid conditions compared to some alternatives, the regeneration process may require significant water inputs depending on system design. In water-stressed regions, this could exacerbate existing resource pressures, necessitating careful site selection and water management strategies.
Long-term environmental risks include potential amine degradation and leaching. Under operational conditions, amines may slowly degrade, potentially releasing volatile organic compounds or ammonia. Proper containment systems and monitoring protocols are essential to prevent these emissions. Additionally, disposal or recycling pathways for spent sorbents must be established to prevent environmental contamination at end-of-life.
The spatial footprint of DAC facilities utilizing these sorbents must also be considered. Large-scale implementation would require substantial land area, potentially competing with other land uses. However, the modular nature of adsorption-based systems allows for flexible deployment in various settings, including integration with existing industrial facilities to utilize waste heat.
Regulatory frameworks for environmental monitoring and compliance are still evolving for DAC technologies. Establishing robust environmental management systems, including regular monitoring of air and water quality surrounding DAC facilities, will be essential for responsible deployment and public acceptance of this emerging carbon removal approach.
Techno-Economic Analysis
The techno-economic analysis of amine-functionalized silica sorbents for low-temperature direct air capture (DAC) reveals significant economic considerations that influence commercial viability. Current cost estimates for DAC using these materials range from $250-600 per ton of CO2 captured, substantially higher than point-source carbon capture technologies which typically cost $50-100 per ton.
Capital expenditure represents approximately 60-70% of total costs, primarily attributed to the infrastructure required for air handling and contactor systems. The sorbent material itself constitutes only 10-15% of capital costs, though its performance characteristics dramatically impact operational expenses through energy requirements and replacement frequency.
Energy consumption remains a critical economic factor, with thermal energy for sorbent regeneration accounting for 40-60% of operational costs. Amine-functionalized silica sorbents typically require 2.5-4.0 GJ of thermal energy per ton of CO2 captured, though recent innovations have demonstrated potential reductions to 2.0-2.5 GJ/ton through improved material design and process optimization.
Sorbent lifetime significantly impacts economic feasibility. Current amine-silica materials maintain performance for 1,000-3,000 cycles before requiring replacement, translating to 1-3 years of operational life. Extending this to 5,000+ cycles could reduce overall capture costs by 15-25%, highlighting the importance of developing degradation-resistant formulations.
Scale economies present substantial opportunities for cost reduction. Modeling suggests that scaling from pilot (1,000 tons CO2/year) to commercial scale (1 million tons CO2/year) could reduce costs by 30-50% through improved process integration, heat recovery systems, and manufacturing efficiencies for sorbent materials.
Policy incentives significantly influence economic viability. Carbon pricing mechanisms, tax credits (such as the 45Q tax credit in the US offering $85/ton for DAC with sequestration), and regulatory frameworks create essential revenue streams that bridge the gap between current costs and market competitiveness.
Sensitivity analysis indicates that improvements in three key parameters could reduce costs below $200/ton: increasing CO2 working capacity from current 1.5-2.5 mmol/g to >3.5 mmol/g; extending sorbent lifetime beyond 5,000 cycles; and reducing regeneration energy requirements below 2.0 GJ/ton. These targets represent the primary focus areas for ongoing research and development efforts.
Capital expenditure represents approximately 60-70% of total costs, primarily attributed to the infrastructure required for air handling and contactor systems. The sorbent material itself constitutes only 10-15% of capital costs, though its performance characteristics dramatically impact operational expenses through energy requirements and replacement frequency.
Energy consumption remains a critical economic factor, with thermal energy for sorbent regeneration accounting for 40-60% of operational costs. Amine-functionalized silica sorbents typically require 2.5-4.0 GJ of thermal energy per ton of CO2 captured, though recent innovations have demonstrated potential reductions to 2.0-2.5 GJ/ton through improved material design and process optimization.
Sorbent lifetime significantly impacts economic feasibility. Current amine-silica materials maintain performance for 1,000-3,000 cycles before requiring replacement, translating to 1-3 years of operational life. Extending this to 5,000+ cycles could reduce overall capture costs by 15-25%, highlighting the importance of developing degradation-resistant formulations.
Scale economies present substantial opportunities for cost reduction. Modeling suggests that scaling from pilot (1,000 tons CO2/year) to commercial scale (1 million tons CO2/year) could reduce costs by 30-50% through improved process integration, heat recovery systems, and manufacturing efficiencies for sorbent materials.
Policy incentives significantly influence economic viability. Carbon pricing mechanisms, tax credits (such as the 45Q tax credit in the US offering $85/ton for DAC with sequestration), and regulatory frameworks create essential revenue streams that bridge the gap between current costs and market competitiveness.
Sensitivity analysis indicates that improvements in three key parameters could reduce costs below $200/ton: increasing CO2 working capacity from current 1.5-2.5 mmol/g to >3.5 mmol/g; extending sorbent lifetime beyond 5,000 cycles; and reducing regeneration energy requirements below 2.0 GJ/ton. These targets represent the primary focus areas for ongoing research and development efforts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!