Geometric Isomers as Biomarkers in Physicochemical Analysis
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Geometric Isomers as Biomarkers: Background and Objectives
Geometric isomers, a class of structural isomers with identical molecular formulas but different spatial arrangements of atoms, have emerged as promising biomarkers in physicochemical analysis. This field of research has gained significant traction over the past decade, driven by advancements in analytical techniques and a growing understanding of the role these molecules play in biological systems.
The study of geometric isomers as biomarkers is rooted in the fundamental principles of stereochemistry, a branch of chemistry that deals with the three-dimensional arrangement of atoms in molecules. The unique spatial configurations of geometric isomers can lead to distinct biological activities and chemical properties, making them valuable indicators of various physiological and pathological states.
The evolution of this research area has been closely tied to developments in analytical instrumentation, particularly in chromatography and spectroscopy. High-performance liquid chromatography (HPLC) and gas chromatography (GC) coupled with mass spectrometry (MS) have been instrumental in separating and identifying geometric isomers with high precision. Additionally, nuclear magnetic resonance (NMR) spectroscopy has provided crucial insights into the structural characteristics of these molecules.
The primary objective of research in this field is to harness the potential of geometric isomers as sensitive and specific biomarkers for various applications in medicine, environmental science, and food safety. In medical diagnostics, geometric isomers of lipids, proteins, and small molecules are being investigated as potential markers for diseases such as cancer, cardiovascular disorders, and neurodegenerative conditions.
Environmental scientists are exploring the use of geometric isomers to track pollutants and assess ecosystem health. The distinct isomeric ratios of certain compounds can serve as fingerprints for identifying sources of contamination and monitoring environmental changes over time. In the food industry, geometric isomers are being studied as indicators of food quality, authenticity, and processing methods.
A key technological goal in this field is the development of rapid, cost-effective, and highly sensitive analytical methods for detecting and quantifying geometric isomers in complex biological and environmental matrices. This includes the optimization of sample preparation techniques, the enhancement of chromatographic separation methods, and the improvement of mass spectrometric detection limits.
Furthermore, researchers aim to elucidate the mechanisms by which geometric isomers are formed, interconverted, and metabolized in biological systems. Understanding these processes is crucial for interpreting the significance of isomeric ratios in different contexts and for developing targeted interventions based on isomeric biomarkers.
As the field progresses, there is a growing emphasis on integrating geometric isomer analysis with other omics approaches, such as metabolomics and lipidomics. This holistic approach promises to provide a more comprehensive understanding of biological systems and to uncover novel biomarkers with enhanced diagnostic and prognostic value.
The study of geometric isomers as biomarkers is rooted in the fundamental principles of stereochemistry, a branch of chemistry that deals with the three-dimensional arrangement of atoms in molecules. The unique spatial configurations of geometric isomers can lead to distinct biological activities and chemical properties, making them valuable indicators of various physiological and pathological states.
The evolution of this research area has been closely tied to developments in analytical instrumentation, particularly in chromatography and spectroscopy. High-performance liquid chromatography (HPLC) and gas chromatography (GC) coupled with mass spectrometry (MS) have been instrumental in separating and identifying geometric isomers with high precision. Additionally, nuclear magnetic resonance (NMR) spectroscopy has provided crucial insights into the structural characteristics of these molecules.
The primary objective of research in this field is to harness the potential of geometric isomers as sensitive and specific biomarkers for various applications in medicine, environmental science, and food safety. In medical diagnostics, geometric isomers of lipids, proteins, and small molecules are being investigated as potential markers for diseases such as cancer, cardiovascular disorders, and neurodegenerative conditions.
Environmental scientists are exploring the use of geometric isomers to track pollutants and assess ecosystem health. The distinct isomeric ratios of certain compounds can serve as fingerprints for identifying sources of contamination and monitoring environmental changes over time. In the food industry, geometric isomers are being studied as indicators of food quality, authenticity, and processing methods.
A key technological goal in this field is the development of rapid, cost-effective, and highly sensitive analytical methods for detecting and quantifying geometric isomers in complex biological and environmental matrices. This includes the optimization of sample preparation techniques, the enhancement of chromatographic separation methods, and the improvement of mass spectrometric detection limits.
Furthermore, researchers aim to elucidate the mechanisms by which geometric isomers are formed, interconverted, and metabolized in biological systems. Understanding these processes is crucial for interpreting the significance of isomeric ratios in different contexts and for developing targeted interventions based on isomeric biomarkers.
As the field progresses, there is a growing emphasis on integrating geometric isomer analysis with other omics approaches, such as metabolomics and lipidomics. This holistic approach promises to provide a more comprehensive understanding of biological systems and to uncover novel biomarkers with enhanced diagnostic and prognostic value.
Market Demand for Geometric Isomer-Based Biomarkers
The market demand for geometric isomer-based biomarkers has been steadily growing in recent years, driven by advancements in analytical techniques and the increasing need for precise molecular identification in various fields. This demand is particularly pronounced in the pharmaceutical and healthcare sectors, where geometric isomers play a crucial role in drug development and personalized medicine.
In the pharmaceutical industry, geometric isomer-based biomarkers are essential for understanding drug metabolism, efficacy, and potential side effects. The ability to distinguish between different isomeric forms of a compound can significantly impact drug safety and effectiveness. As a result, there is a growing market for analytical tools and methods that can accurately identify and quantify geometric isomers in complex biological samples.
The healthcare sector is another major driver of demand for geometric isomer-based biomarkers. These biomarkers are increasingly used in disease diagnosis, prognosis, and treatment monitoring. For instance, certain geometric isomers of lipids have been identified as potential biomarkers for cardiovascular diseases and cancer. This has led to increased research and development efforts to create diagnostic tests and platforms that can detect and measure these specific isomers.
Environmental monitoring and food safety are emerging areas where geometric isomer-based biomarkers are gaining traction. The ability to detect and differentiate between geometric isomers of pesticides, pollutants, and food additives is becoming increasingly important for regulatory compliance and public health protection. This has created a new market segment for specialized analytical instruments and services.
The biotechnology sector is also contributing to the growing demand for geometric isomer-based biomarkers. As the field of metabolomics expands, researchers are increasingly interested in understanding the role of geometric isomers in cellular processes and metabolic pathways. This has led to a rise in demand for high-resolution analytical techniques capable of distinguishing between closely related isomeric compounds.
Market analysts project that the global market for geometric isomer-based biomarkers and related analytical technologies will continue to grow at a significant rate over the next decade. This growth is expected to be fueled by ongoing research in proteomics, metabolomics, and personalized medicine, as well as the increasing adoption of precision diagnostics in clinical settings.
However, challenges remain in meeting this growing demand. The development of robust, high-throughput methods for geometric isomer analysis is still an active area of research. Additionally, there is a need for standardization and validation of biomarker assays across different platforms and laboratories. These challenges present opportunities for innovation and market growth in the coming years.
In the pharmaceutical industry, geometric isomer-based biomarkers are essential for understanding drug metabolism, efficacy, and potential side effects. The ability to distinguish between different isomeric forms of a compound can significantly impact drug safety and effectiveness. As a result, there is a growing market for analytical tools and methods that can accurately identify and quantify geometric isomers in complex biological samples.
The healthcare sector is another major driver of demand for geometric isomer-based biomarkers. These biomarkers are increasingly used in disease diagnosis, prognosis, and treatment monitoring. For instance, certain geometric isomers of lipids have been identified as potential biomarkers for cardiovascular diseases and cancer. This has led to increased research and development efforts to create diagnostic tests and platforms that can detect and measure these specific isomers.
Environmental monitoring and food safety are emerging areas where geometric isomer-based biomarkers are gaining traction. The ability to detect and differentiate between geometric isomers of pesticides, pollutants, and food additives is becoming increasingly important for regulatory compliance and public health protection. This has created a new market segment for specialized analytical instruments and services.
The biotechnology sector is also contributing to the growing demand for geometric isomer-based biomarkers. As the field of metabolomics expands, researchers are increasingly interested in understanding the role of geometric isomers in cellular processes and metabolic pathways. This has led to a rise in demand for high-resolution analytical techniques capable of distinguishing between closely related isomeric compounds.
Market analysts project that the global market for geometric isomer-based biomarkers and related analytical technologies will continue to grow at a significant rate over the next decade. This growth is expected to be fueled by ongoing research in proteomics, metabolomics, and personalized medicine, as well as the increasing adoption of precision diagnostics in clinical settings.
However, challenges remain in meeting this growing demand. The development of robust, high-throughput methods for geometric isomer analysis is still an active area of research. Additionally, there is a need for standardization and validation of biomarker assays across different platforms and laboratories. These challenges present opportunities for innovation and market growth in the coming years.
Current Challenges in Geometric Isomer Analysis
The analysis of geometric isomers as biomarkers in physicochemical studies faces several significant challenges that hinder progress in this field. One of the primary obstacles is the complexity of isomer separation and identification. Geometric isomers often possess nearly identical physical and chemical properties, making their distinction and quantification exceptionally difficult using conventional analytical techniques.
Chromatographic methods, while widely employed, struggle to achieve complete separation of geometric isomers, especially in complex biological matrices. The similarity in retention times and peak shapes can lead to co-elution and overlapping signals, compromising the accuracy of quantitative analyses. This challenge is particularly pronounced when dealing with trace amounts of isomers in biological samples, where matrix effects can further complicate separation and detection.
Another significant hurdle is the potential for isomerization during sample preparation and analysis. Geometric isomers can interconvert under certain conditions, such as exposure to heat, light, or pH changes. This instability poses a considerable challenge in maintaining the integrity of samples throughout the analytical process, from collection to final measurement. Researchers must develop and validate robust protocols that minimize isomerization risks, which often requires specialized handling and storage conditions.
The lack of standardized methodologies for geometric isomer analysis also presents a challenge. Different laboratories may employ varied approaches, leading to inconsistencies in results and difficulties in comparing data across studies. This absence of harmonized protocols hampers the establishment of reliable reference ranges for isomeric biomarkers and impedes the translation of research findings into clinical applications.
Furthermore, the development of sensitive and specific detection methods for geometric isomers remains an ongoing challenge. While mass spectrometry has emerged as a powerful tool, the differentiation of isomers with identical molecular masses requires advanced techniques such as ion mobility spectrometry or specialized fragmentation methods. These sophisticated approaches often demand significant expertise and resources, limiting their widespread adoption in routine analyses.
The interpretation of geometric isomer data in the context of biomarker research also presents challenges. Establishing clear correlations between isomeric profiles and specific physiological or pathological states requires extensive validation studies and a deep understanding of the biological significance of isomeric variations. This process is further complicated by the potential influence of genetic factors, environmental exposures, and lifestyle variables on isomeric distributions in biological systems.
Chromatographic methods, while widely employed, struggle to achieve complete separation of geometric isomers, especially in complex biological matrices. The similarity in retention times and peak shapes can lead to co-elution and overlapping signals, compromising the accuracy of quantitative analyses. This challenge is particularly pronounced when dealing with trace amounts of isomers in biological samples, where matrix effects can further complicate separation and detection.
Another significant hurdle is the potential for isomerization during sample preparation and analysis. Geometric isomers can interconvert under certain conditions, such as exposure to heat, light, or pH changes. This instability poses a considerable challenge in maintaining the integrity of samples throughout the analytical process, from collection to final measurement. Researchers must develop and validate robust protocols that minimize isomerization risks, which often requires specialized handling and storage conditions.
The lack of standardized methodologies for geometric isomer analysis also presents a challenge. Different laboratories may employ varied approaches, leading to inconsistencies in results and difficulties in comparing data across studies. This absence of harmonized protocols hampers the establishment of reliable reference ranges for isomeric biomarkers and impedes the translation of research findings into clinical applications.
Furthermore, the development of sensitive and specific detection methods for geometric isomers remains an ongoing challenge. While mass spectrometry has emerged as a powerful tool, the differentiation of isomers with identical molecular masses requires advanced techniques such as ion mobility spectrometry or specialized fragmentation methods. These sophisticated approaches often demand significant expertise and resources, limiting their widespread adoption in routine analyses.
The interpretation of geometric isomer data in the context of biomarker research also presents challenges. Establishing clear correlations between isomeric profiles and specific physiological or pathological states requires extensive validation studies and a deep understanding of the biological significance of isomeric variations. This process is further complicated by the potential influence of genetic factors, environmental exposures, and lifestyle variables on isomeric distributions in biological systems.
Existing Methodologies for Geometric Isomer Analysis
01 Identification and analysis of geometric isomers as biomarkers
Geometric isomers can serve as biomarkers for various biological processes and conditions. Advanced analytical techniques are employed to identify and quantify these isomers in biological samples, providing valuable insights into metabolic pathways, disease states, and drug metabolism.- Identification and analysis of geometric isomers as biomarkers: Geometric isomers can serve as biomarkers for various biological processes and conditions. Advanced analytical techniques are employed to identify and quantify these isomers in biological samples, providing valuable information for diagnostic and research purposes.
- Separation and purification of geometric isomers: Methods for separating and purifying geometric isomers are crucial for their use as biomarkers. Techniques such as chromatography, crystallization, and selective adsorption are utilized to isolate specific isomers from complex mixtures, enhancing their effectiveness as biomarkers.
- Synthesis of geometric isomer biomarkers: Novel synthetic routes are developed to produce geometric isomer biomarkers with high purity and yield. These methods often involve stereoselective reactions and catalysts to control the formation of specific isomeric forms, ensuring the reliability of the biomarkers.
- Application of geometric isomers in medical diagnostics: Geometric isomer biomarkers find applications in medical diagnostics, particularly in the detection and monitoring of diseases. These biomarkers can provide early indicators of certain conditions, allowing for timely intervention and treatment.
- Computational modeling and analysis of geometric isomers: Advanced computational techniques are employed to model and analyze geometric isomers as biomarkers. These methods help in predicting isomer behavior, optimizing analytical procedures, and interpreting complex data sets related to geometric isomer biomarkers.
02 Separation and purification of geometric isomers
Methods for separating and purifying geometric isomers are crucial for their use as biomarkers. Techniques such as chromatography, crystallization, and selective complexation are utilized to isolate specific isomers from complex mixtures, enabling their accurate measurement and analysis.Expand Specific Solutions03 Geometric isomers in pharmaceutical applications
Geometric isomers play a significant role in pharmaceutical research and development. They can be used as biomarkers for drug efficacy, metabolism, and toxicity. Understanding the behavior of different geometric isomers helps in optimizing drug design and predicting potential side effects.Expand Specific Solutions04 Computational methods for geometric isomer analysis
Advanced computational techniques are employed to predict, model, and analyze geometric isomers as biomarkers. These methods include molecular dynamics simulations, quantum chemical calculations, and machine learning algorithms, which aid in understanding isomer behavior and interpreting experimental data.Expand Specific Solutions05 Environmental and metabolomic applications of geometric isomer biomarkers
Geometric isomers serve as biomarkers in environmental monitoring and metabolomics studies. They can indicate exposure to specific pollutants, track metabolic changes in organisms, and provide insights into ecosystem health. This application extends to fields such as toxicology, ecology, and personalized medicine.Expand Specific Solutions
Key Players in Geometric Isomer Research
The research on geometric isomers as biomarkers in physicochemical analysis is in an emerging stage, with growing market potential due to its applications in pharmaceutical and biotechnology industries. The global market for biomarkers is expanding rapidly, driven by advancements in precision medicine and personalized healthcare. Companies like AbbVie, Bristol Myers Squibb, and Janssen Pharmaceutica are investing heavily in this field, leveraging their expertise in drug development and molecular analysis. Academic institutions such as Tsinghua University and The Broad Institute are contributing significantly to the fundamental research, while startups like Foghorn Therapeutics are exploring innovative applications. The technology is still evolving, with ongoing efforts to improve sensitivity, specificity, and scalability of isomer-based biomarker detection methods.
The Regents of the University of California
Technical Solution: The University of California has developed advanced techniques for analyzing geometric isomers as biomarkers in physicochemical analysis. Their approach utilizes high-resolution mass spectrometry coupled with liquid chromatography to separate and identify structural isomers[1]. They have implemented novel ion mobility spectrometry methods to enhance isomer differentiation based on shape and size[2]. Additionally, the university has pioneered the use of chiral chromatography columns to separate enantiomers, allowing for precise quantification of specific geometric isomers in complex biological samples[3]. Their research has also focused on developing machine learning algorithms to predict isomer structures from mass spectral data, improving the speed and accuracy of biomarker identification[4].
Strengths: Cutting-edge analytical techniques, access to advanced instrumentation, and interdisciplinary collaboration. Weaknesses: Potential limitations in translating academic research to clinical applications and commercialization.
AbbVie, Inc.
Technical Solution: AbbVie has developed a comprehensive platform for geometric isomer analysis in drug discovery and development. Their approach combines advanced chromatographic techniques with high-resolution mass spectrometry to identify and quantify geometric isomers in pharmaceutical compounds[1]. They have implemented automated workflows for rapid screening of drug candidates, allowing for early detection of potentially problematic isomers[2]. AbbVie's research also focuses on understanding the impact of geometric isomerization on drug efficacy and safety, utilizing in vitro and in vivo models to assess biomarker profiles[3]. Additionally, they have developed novel computational methods to predict isomer formation and stability, aiding in the design of more stable drug formulations[4].
Strengths: Strong integration of analytical techniques with drug development processes, extensive resources for preclinical and clinical studies. Weaknesses: Focus primarily on pharmaceutical applications may limit broader biomarker research.
Innovative Approaches in Geometric Isomer Identification
Erbb/BTK inhibitors
PatentPendingEP4356975A2
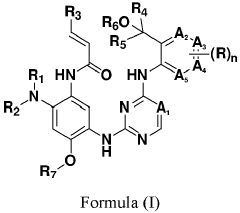
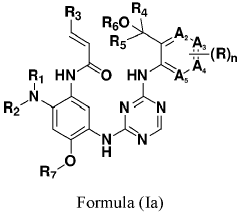
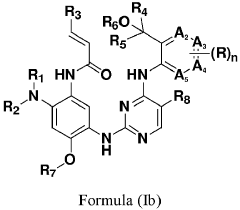
Innovation
- Development of compounds represented by Formula (I) and its pharmaceutically acceptable salts, esters, hydrates, and stereoisomers, which are used in pharmaceutical compositions to inhibit ErbB family kinases and BTK, particularly targeting mutant forms to enhance therapeutic efficacy.
Compounds and uses thereof
PatentPendingUS20230145003A1
Innovation
- Development of specific compounds that modulate the BAF complex by inhibiting BRG1 and/or BRM activity, which can be used alone or in combination with other pharmaceutically active agents to treat disorders like cancer.
Regulatory Framework for Biomarker Development
The regulatory framework for biomarker development in the context of geometric isomers as biomarkers in physicochemical analysis is a complex and evolving landscape. Regulatory agencies worldwide have established guidelines and standards to ensure the validity, reliability, and clinical utility of biomarkers used in various applications, including drug development, diagnostics, and personalized medicine.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing biomarker development and implementation. The FDA has developed specific guidance documents for biomarker qualification, which outline the process for submitting biomarker data for regulatory review and potential use in drug development programs. These guidelines emphasize the importance of analytical validation, clinical validation, and demonstration of clinical utility for biomarkers, including those based on geometric isomers.
The European Medicines Agency (EMA) has also established a framework for biomarker qualification, which shares similarities with the FDA's approach but may have some region-specific requirements. Both agencies emphasize the need for robust scientific evidence to support the use of biomarkers in clinical decision-making and drug development processes.
International organizations, such as the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), have developed guidelines that aim to harmonize regulatory requirements across different regions. These guidelines provide a framework for biomarker development and validation that can be applied globally, facilitating the use of biomarkers in multinational clinical trials and drug development programs.
For geometric isomers as biomarkers in physicochemical analysis, specific regulatory considerations may include the need for standardized analytical methods, quality control procedures, and reference standards. Regulatory agencies may require extensive data on the stability, specificity, and reproducibility of the analytical methods used to detect and quantify geometric isomers as biomarkers.
Additionally, the regulatory framework often addresses the ethical considerations associated with biomarker development and use. This includes guidelines for informed consent, data privacy, and the responsible use of genetic information when applicable to geometric isomer-based biomarkers.
As the field of biomarker research continues to advance, regulatory agencies are adapting their frameworks to keep pace with technological innovations. This includes the development of guidelines for novel biomarker types and analytical platforms, which may be relevant to the use of geometric isomers in physicochemical analysis.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing biomarker development and implementation. The FDA has developed specific guidance documents for biomarker qualification, which outline the process for submitting biomarker data for regulatory review and potential use in drug development programs. These guidelines emphasize the importance of analytical validation, clinical validation, and demonstration of clinical utility for biomarkers, including those based on geometric isomers.
The European Medicines Agency (EMA) has also established a framework for biomarker qualification, which shares similarities with the FDA's approach but may have some region-specific requirements. Both agencies emphasize the need for robust scientific evidence to support the use of biomarkers in clinical decision-making and drug development processes.
International organizations, such as the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), have developed guidelines that aim to harmonize regulatory requirements across different regions. These guidelines provide a framework for biomarker development and validation that can be applied globally, facilitating the use of biomarkers in multinational clinical trials and drug development programs.
For geometric isomers as biomarkers in physicochemical analysis, specific regulatory considerations may include the need for standardized analytical methods, quality control procedures, and reference standards. Regulatory agencies may require extensive data on the stability, specificity, and reproducibility of the analytical methods used to detect and quantify geometric isomers as biomarkers.
Additionally, the regulatory framework often addresses the ethical considerations associated with biomarker development and use. This includes guidelines for informed consent, data privacy, and the responsible use of genetic information when applicable to geometric isomer-based biomarkers.
As the field of biomarker research continues to advance, regulatory agencies are adapting their frameworks to keep pace with technological innovations. This includes the development of guidelines for novel biomarker types and analytical platforms, which may be relevant to the use of geometric isomers in physicochemical analysis.
Ethical Implications of Geometric Isomer Biomarkers
The use of geometric isomers as biomarkers in physicochemical analysis raises several ethical considerations that warrant careful examination. One primary concern is the potential for privacy infringement, as these biomarkers can provide detailed information about an individual's health status, lifestyle, and even genetic predisposition. This level of insight into personal biological data necessitates robust safeguards to protect patient confidentiality and prevent unauthorized access or misuse of sensitive information.
Another ethical implication revolves around the accuracy and reliability of geometric isomer biomarkers. False positives or negatives in diagnostic tests could lead to unnecessary treatments or missed diagnoses, potentially causing physical and emotional harm to patients. Ensuring the precision and reproducibility of these biomarker analyses is crucial to maintain ethical standards in healthcare and research.
The equitable access to geometric isomer biomarker technology is also an important ethical consideration. Advanced analytical techniques may be costly and not readily available in all healthcare settings, potentially exacerbating existing disparities in medical care. This raises questions about fairness and social justice in the distribution of healthcare resources and the potential for widening health inequalities between different socioeconomic groups.
Furthermore, the use of geometric isomer biomarkers in predictive medicine presents ethical challenges related to informed consent and patient autonomy. The ability to forecast future health risks based on current biomarker profiles may lead to difficult decisions regarding preventive treatments or lifestyle changes. Patients must be fully informed about the implications of such predictions and empowered to make autonomous choices about their health management.
The potential for discrimination based on biomarker profiles is another significant ethical concern. Employers, insurers, or other entities might seek access to this information, potentially leading to unfair treatment or denial of opportunities based on predicted health outcomes. Establishing clear regulations and ethical guidelines for the use and disclosure of biomarker data is essential to prevent such discriminatory practices.
Lastly, the development and application of geometric isomer biomarkers raise questions about the responsible use of scientific knowledge. While these biomarkers offer valuable insights for medical diagnosis and treatment, their potential applications in areas such as behavioral prediction or personal identification could infringe on individual rights and societal values. Striking a balance between scientific progress and ethical considerations is crucial to ensure that the benefits of this technology are realized without compromising fundamental human rights and dignity.
Another ethical implication revolves around the accuracy and reliability of geometric isomer biomarkers. False positives or negatives in diagnostic tests could lead to unnecessary treatments or missed diagnoses, potentially causing physical and emotional harm to patients. Ensuring the precision and reproducibility of these biomarker analyses is crucial to maintain ethical standards in healthcare and research.
The equitable access to geometric isomer biomarker technology is also an important ethical consideration. Advanced analytical techniques may be costly and not readily available in all healthcare settings, potentially exacerbating existing disparities in medical care. This raises questions about fairness and social justice in the distribution of healthcare resources and the potential for widening health inequalities between different socioeconomic groups.
Furthermore, the use of geometric isomer biomarkers in predictive medicine presents ethical challenges related to informed consent and patient autonomy. The ability to forecast future health risks based on current biomarker profiles may lead to difficult decisions regarding preventive treatments or lifestyle changes. Patients must be fully informed about the implications of such predictions and empowered to make autonomous choices about their health management.
The potential for discrimination based on biomarker profiles is another significant ethical concern. Employers, insurers, or other entities might seek access to this information, potentially leading to unfair treatment or denial of opportunities based on predicted health outcomes. Establishing clear regulations and ethical guidelines for the use and disclosure of biomarker data is essential to prevent such discriminatory practices.
Lastly, the development and application of geometric isomer biomarkers raise questions about the responsible use of scientific knowledge. While these biomarkers offer valuable insights for medical diagnosis and treatment, their potential applications in areas such as behavioral prediction or personal identification could infringe on individual rights and societal values. Striking a balance between scientific progress and ethical considerations is crucial to ensure that the benefits of this technology are realized without compromising fundamental human rights and dignity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!