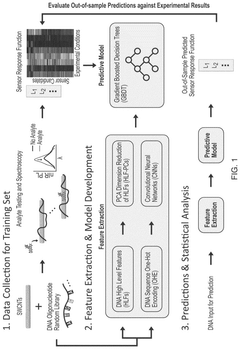
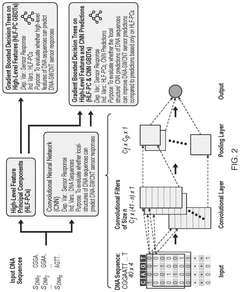
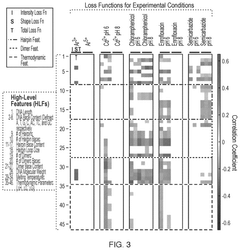
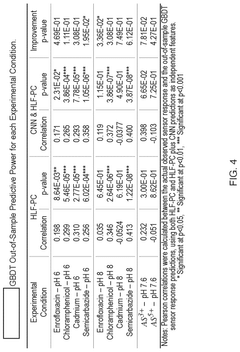
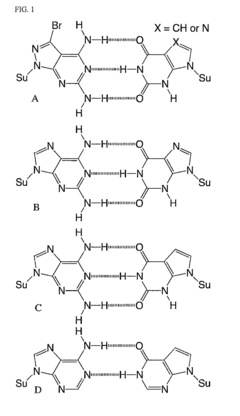
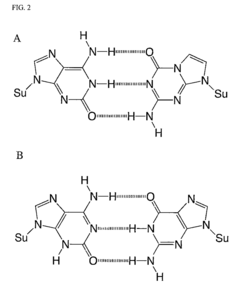
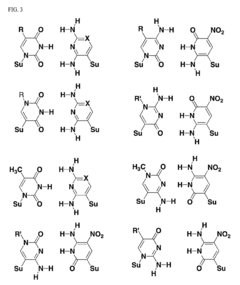
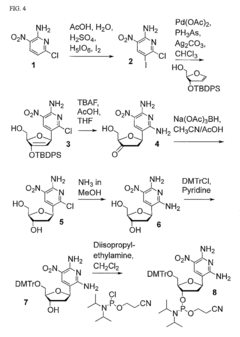
Molecular Recognition of Geometric Isomers in Enzyme Engineering
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Enzyme Engineering Background and Objectives
Enzyme engineering has emerged as a powerful tool in biotechnology, enabling the modification and optimization of enzymes for various industrial and medical applications. The field has evolved significantly over the past few decades, driven by advances in molecular biology, protein engineering, and computational methods. The primary focus of enzyme engineering is to enhance the catalytic properties, stability, and specificity of enzymes, making them more suitable for specific applications.
The study of molecular recognition of geometric isomers in enzyme engineering represents a crucial area of research, as it addresses the fundamental challenge of enzyme selectivity. Geometric isomers, also known as stereoisomers, are molecules with the same molecular formula but different spatial arrangements of atoms. The ability of enzymes to distinguish between these isomers is critical for many biological processes and industrial applications.
The evolution of enzyme engineering has been marked by several key milestones. Initially, random mutagenesis and directed evolution techniques were employed to generate enzyme variants with improved properties. As our understanding of protein structure and function deepened, rational design approaches gained prominence, allowing for more targeted modifications. Recent years have seen the integration of computational methods, machine learning, and high-throughput screening techniques, significantly accelerating the enzyme engineering process.
The primary objectives of research on molecular recognition of geometric isomers in enzyme engineering are multifaceted. Firstly, there is a need to elucidate the underlying mechanisms by which enzymes recognize and differentiate between geometric isomers. This involves investigating the structural and chemical factors that contribute to enzyme-substrate interactions and selectivity.
Secondly, researchers aim to develop strategies for engineering enzymes with enhanced selectivity towards specific geometric isomers. This is particularly important in industries such as pharmaceuticals, where the production of enantiopure compounds is crucial. By improving enzyme selectivity, it becomes possible to increase the efficiency and yield of desired isomers while minimizing the formation of unwanted byproducts.
Another key objective is to expand the repertoire of enzymes capable of recognizing and acting upon geometric isomers. This involves exploring novel enzyme scaffolds and engineering existing enzymes to accept a broader range of substrates. Such advancements could open up new possibilities for biocatalysis in various industrial processes.
Furthermore, there is a growing emphasis on developing computational tools and predictive models to guide enzyme engineering efforts. These tools aim to streamline the design process, reduce experimental iterations, and accelerate the development of enzymes with desired properties. The integration of machine learning algorithms and molecular dynamics simulations is expected to play a crucial role in achieving this objective.
The study of molecular recognition of geometric isomers in enzyme engineering represents a crucial area of research, as it addresses the fundamental challenge of enzyme selectivity. Geometric isomers, also known as stereoisomers, are molecules with the same molecular formula but different spatial arrangements of atoms. The ability of enzymes to distinguish between these isomers is critical for many biological processes and industrial applications.
The evolution of enzyme engineering has been marked by several key milestones. Initially, random mutagenesis and directed evolution techniques were employed to generate enzyme variants with improved properties. As our understanding of protein structure and function deepened, rational design approaches gained prominence, allowing for more targeted modifications. Recent years have seen the integration of computational methods, machine learning, and high-throughput screening techniques, significantly accelerating the enzyme engineering process.
The primary objectives of research on molecular recognition of geometric isomers in enzyme engineering are multifaceted. Firstly, there is a need to elucidate the underlying mechanisms by which enzymes recognize and differentiate between geometric isomers. This involves investigating the structural and chemical factors that contribute to enzyme-substrate interactions and selectivity.
Secondly, researchers aim to develop strategies for engineering enzymes with enhanced selectivity towards specific geometric isomers. This is particularly important in industries such as pharmaceuticals, where the production of enantiopure compounds is crucial. By improving enzyme selectivity, it becomes possible to increase the efficiency and yield of desired isomers while minimizing the formation of unwanted byproducts.
Another key objective is to expand the repertoire of enzymes capable of recognizing and acting upon geometric isomers. This involves exploring novel enzyme scaffolds and engineering existing enzymes to accept a broader range of substrates. Such advancements could open up new possibilities for biocatalysis in various industrial processes.
Furthermore, there is a growing emphasis on developing computational tools and predictive models to guide enzyme engineering efforts. These tools aim to streamline the design process, reduce experimental iterations, and accelerate the development of enzymes with desired properties. The integration of machine learning algorithms and molecular dynamics simulations is expected to play a crucial role in achieving this objective.
Market Analysis for Isomer-Specific Enzymes
The market for isomer-specific enzymes has been experiencing significant growth in recent years, driven by increasing demand across various industries, particularly in pharmaceuticals, fine chemicals, and biotechnology. This specialized enzyme market is projected to continue its upward trajectory due to the rising need for precise and efficient catalysts in asymmetric synthesis and chiral resolution processes.
In the pharmaceutical sector, isomer-specific enzymes play a crucial role in the production of enantiopure drugs, which often exhibit superior therapeutic efficacy and reduced side effects compared to their racemic counterparts. The growing emphasis on developing single-enantiomer drugs has created a substantial market opportunity for enzymes capable of recognizing and selectively processing geometric isomers.
The fine chemicals industry has also emerged as a key consumer of isomer-specific enzymes, utilizing them in the synthesis of high-value intermediates and specialty chemicals. These enzymes offer advantages over traditional chemical catalysts, including higher selectivity, milder reaction conditions, and reduced environmental impact, aligning with the industry's shift towards greener and more sustainable production methods.
Biotechnology applications, such as biocatalysis and industrial bioprocessing, represent another significant market segment for isomer-specific enzymes. The ability of these enzymes to perform highly selective transformations under mild conditions makes them attractive for various biotech processes, including the production of biofuels, bioplastics, and other bio-based materials.
Geographically, North America and Europe currently dominate the market for isomer-specific enzymes, owing to their well-established pharmaceutical and biotechnology industries. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing investments in biotechnology and the rapid expansion of the pharmaceutical sector in countries like China and India.
The market is characterized by a mix of large multinational enzyme producers and specialized biotech companies focusing on enzyme engineering and optimization. Key players are investing heavily in research and development to expand their product portfolios and improve enzyme performance, particularly in terms of selectivity, stability, and efficiency under industrial conditions.
Despite the positive outlook, challenges remain in the widespread adoption of isomer-specific enzymes. These include the high costs associated with enzyme development and production, as well as the need for specialized expertise in enzyme engineering and application. However, ongoing advancements in protein engineering, directed evolution, and computational design are expected to address these challenges, potentially leading to more cost-effective and versatile isomer-specific enzymes in the future.
In the pharmaceutical sector, isomer-specific enzymes play a crucial role in the production of enantiopure drugs, which often exhibit superior therapeutic efficacy and reduced side effects compared to their racemic counterparts. The growing emphasis on developing single-enantiomer drugs has created a substantial market opportunity for enzymes capable of recognizing and selectively processing geometric isomers.
The fine chemicals industry has also emerged as a key consumer of isomer-specific enzymes, utilizing them in the synthesis of high-value intermediates and specialty chemicals. These enzymes offer advantages over traditional chemical catalysts, including higher selectivity, milder reaction conditions, and reduced environmental impact, aligning with the industry's shift towards greener and more sustainable production methods.
Biotechnology applications, such as biocatalysis and industrial bioprocessing, represent another significant market segment for isomer-specific enzymes. The ability of these enzymes to perform highly selective transformations under mild conditions makes them attractive for various biotech processes, including the production of biofuels, bioplastics, and other bio-based materials.
Geographically, North America and Europe currently dominate the market for isomer-specific enzymes, owing to their well-established pharmaceutical and biotechnology industries. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing investments in biotechnology and the rapid expansion of the pharmaceutical sector in countries like China and India.
The market is characterized by a mix of large multinational enzyme producers and specialized biotech companies focusing on enzyme engineering and optimization. Key players are investing heavily in research and development to expand their product portfolios and improve enzyme performance, particularly in terms of selectivity, stability, and efficiency under industrial conditions.
Despite the positive outlook, challenges remain in the widespread adoption of isomer-specific enzymes. These include the high costs associated with enzyme development and production, as well as the need for specialized expertise in enzyme engineering and application. However, ongoing advancements in protein engineering, directed evolution, and computational design are expected to address these challenges, potentially leading to more cost-effective and versatile isomer-specific enzymes in the future.
Current Challenges in Geometric Isomer Recognition
The recognition of geometric isomers in enzyme engineering presents several significant challenges that researchers are currently grappling with. One of the primary difficulties lies in the subtle structural differences between geometric isomers, which often have identical molecular formulas and similar physical properties. This similarity makes it challenging for enzymes to distinguish between these isomers effectively.
A major hurdle in this field is the development of highly specific and selective enzymes capable of recognizing and binding to only one geometric isomer while excluding its counterpart. The design of such enzymes requires a deep understanding of the molecular interactions at play and the ability to engineer precise binding pockets that can discriminate based on spatial arrangement rather than chemical composition.
Another challenge is the dynamic nature of enzyme-substrate interactions. Geometric isomers can sometimes interconvert under certain conditions, making it difficult to maintain selectivity throughout the enzymatic process. Researchers must consider how to stabilize the desired isomer and prevent unwanted isomerization during recognition and subsequent catalysis.
The complexity of the cellular environment adds another layer of difficulty to geometric isomer recognition. Enzymes must be able to function effectively in the presence of numerous other molecules, some of which may have similar structures or properties to the target isomers. This requires the development of recognition mechanisms that are not only specific but also robust enough to operate in crowded and diverse molecular landscapes.
Furthermore, the scalability of enzyme-based recognition systems for geometric isomers poses a significant challenge. While laboratory-scale experiments may demonstrate successful discrimination, translating these results to industrial-scale applications often encounters obstacles related to enzyme stability, efficiency, and cost-effectiveness.
Computational modeling and prediction of enzyme-isomer interactions remain challenging due to the need for high-resolution structural data and accurate simulation of complex molecular dynamics. Improving these computational tools is crucial for accelerating the design and optimization of enzymes capable of geometric isomer recognition.
Lastly, the integration of geometric isomer recognition into broader enzymatic pathways and cascades presents its own set of challenges. Researchers must consider how the recognition step affects subsequent reactions and how to maintain selectivity throughout multi-step processes. This requires a systems-level approach to enzyme engineering that considers not just individual recognition events but the entire biochemical context in which they occur.
A major hurdle in this field is the development of highly specific and selective enzymes capable of recognizing and binding to only one geometric isomer while excluding its counterpart. The design of such enzymes requires a deep understanding of the molecular interactions at play and the ability to engineer precise binding pockets that can discriminate based on spatial arrangement rather than chemical composition.
Another challenge is the dynamic nature of enzyme-substrate interactions. Geometric isomers can sometimes interconvert under certain conditions, making it difficult to maintain selectivity throughout the enzymatic process. Researchers must consider how to stabilize the desired isomer and prevent unwanted isomerization during recognition and subsequent catalysis.
The complexity of the cellular environment adds another layer of difficulty to geometric isomer recognition. Enzymes must be able to function effectively in the presence of numerous other molecules, some of which may have similar structures or properties to the target isomers. This requires the development of recognition mechanisms that are not only specific but also robust enough to operate in crowded and diverse molecular landscapes.
Furthermore, the scalability of enzyme-based recognition systems for geometric isomers poses a significant challenge. While laboratory-scale experiments may demonstrate successful discrimination, translating these results to industrial-scale applications often encounters obstacles related to enzyme stability, efficiency, and cost-effectiveness.
Computational modeling and prediction of enzyme-isomer interactions remain challenging due to the need for high-resolution structural data and accurate simulation of complex molecular dynamics. Improving these computational tools is crucial for accelerating the design and optimization of enzymes capable of geometric isomer recognition.
Lastly, the integration of geometric isomer recognition into broader enzymatic pathways and cascades presents its own set of challenges. Researchers must consider how the recognition step affects subsequent reactions and how to maintain selectivity throughout multi-step processes. This requires a systems-level approach to enzyme engineering that considers not just individual recognition events but the entire biochemical context in which they occur.
Existing Approaches for Isomer Discrimination
01 Molecular recognition in biosensors and diagnostic devices
Molecular recognition techniques are utilized in the development of biosensors and diagnostic devices. These technologies employ specific molecular interactions to detect and identify target molecules, enabling rapid and accurate analysis in various fields such as healthcare, environmental monitoring, and food safety.- Biosensors for molecular recognition: Biosensors utilize molecular recognition principles for detecting specific molecules. These devices often incorporate biological components such as enzymes, antibodies, or nucleic acids to selectively bind target molecules. The recognition event is then transduced into a measurable signal, enabling detection and quantification of analytes in various fields including healthcare, environmental monitoring, and food safety.
- Aptamer-based molecular recognition: Aptamers are synthetic oligonucleotides that can bind specifically to target molecules. They are used in molecular recognition applications due to their high affinity and selectivity. Aptamer-based systems are employed in various fields, including diagnostics, therapeutics, and analytical chemistry, offering advantages such as stability, ease of synthesis, and the ability to be modified for improved functionality.
- Molecular imprinting for recognition: Molecular imprinting technology creates synthetic materials with recognition sites complementary to target molecules in shape, size, and functional groups. These molecularly imprinted polymers (MIPs) can selectively bind to specific analytes, mimicking natural recognition elements. MIPs are used in various applications, including separation processes, sensors, and drug delivery systems.
- Mass spectrometry for molecular recognition: Mass spectrometry techniques are employed for molecular recognition and characterization. These methods can identify and quantify molecules based on their mass-to-charge ratios, providing high sensitivity and specificity. Advanced mass spectrometry approaches, such as tandem MS and ion mobility MS, enhance the ability to recognize and differentiate between similar molecules in complex mixtures.
- Nanomaterial-based molecular recognition: Nanomaterials, such as nanoparticles, quantum dots, and carbon nanotubes, are utilized in molecular recognition applications. These materials offer unique properties like high surface area, tunable surface chemistry, and optical or electrical characteristics that can enhance recognition sensitivity and selectivity. Nanomaterial-based recognition systems are applied in areas including biosensing, environmental monitoring, and drug delivery.
02 Aptamer-based molecular recognition systems
Aptamers are synthetic oligonucleotides that can bind specifically to target molecules. They are used in molecular recognition applications due to their high affinity and selectivity. Aptamer-based systems are employed in various fields, including drug delivery, biosensing, and environmental monitoring.Expand Specific Solutions03 Molecular imprinting for selective recognition
Molecular imprinting technology involves creating synthetic materials with specific recognition sites for target molecules. This approach is used to develop selective adsorbents, sensors, and separation materials for various applications in analytical chemistry, environmental science, and biotechnology.Expand Specific Solutions04 Nanomaterial-based molecular recognition systems
Nanomaterials, such as nanoparticles, nanotubes, and nanowires, are utilized in molecular recognition applications. These materials offer unique properties that enhance sensitivity and selectivity in detecting and binding target molecules, leading to improved performance in sensors and analytical devices.Expand Specific Solutions05 Molecular recognition in protein-ligand interactions
Understanding and manipulating protein-ligand interactions are crucial in drug discovery and development. Molecular recognition techniques are employed to study these interactions, design new drugs, and optimize existing ones for improved efficacy and reduced side effects.Expand Specific Solutions
Key Players in Enzyme Engineering Industry
The research on molecular recognition of geometric isomers in enzyme engineering is in a developing stage, with significant potential for growth. The market size is expanding as industries recognize the importance of precise molecular recognition in various applications. Technologically, the field is advancing rapidly, with companies like Agilent Technologies and Pacific Biosciences leading in analytical instrumentation. Universities such as South China University of Technology and Zhejiang University are contributing valuable research. Smaller specialized firms like Ningbo Meisai Biological Engineering and X-Body are focusing on biocatalysis and antibody discovery, respectively. The involvement of diverse players indicates a maturing field with room for innovation and commercial development.
The Regents of the University of California
Technical Solution: The University of California has developed advanced computational methods for predicting enzyme-substrate interactions in geometric isomers. Their approach combines molecular dynamics simulations with machine learning algorithms to model the binding pocket geometry and electrostatic interactions[1]. This allows for accurate prediction of enzyme selectivity for specific isomers. They have also engineered novel enzymes with enhanced recognition of target geometric isomers by introducing mutations that alter the binding pocket shape[2]. Additionally, they utilize directed evolution techniques to further optimize enzyme specificity for desired isomeric forms[3].
Strengths: Cutting-edge computational modeling and protein engineering capabilities. Access to extensive research facilities and interdisciplinary expertise. Weaknesses: Potential challenges in scaling up to industrial applications. Academic focus may limit commercial development.
Pacific Biosciences of California, Inc.
Technical Solution: Pacific Biosciences has developed a novel single-molecule real-time (SMRT) sequencing technology that can directly detect geometric isomers in DNA and RNA. Their approach uses engineered polymerases with high sensitivity to subtle structural differences between isomers[4]. The company has also created specialized nanopore-based sensors that can discriminate between geometric isomers based on their unique electrical signatures as they pass through the pore[5]. This technology enables high-throughput screening of isomer-specific enzyme interactions in complex biological samples.
Strengths: Proprietary single-molecule detection technologies. Strong focus on commercialization and practical applications. Weaknesses: Limited to nucleic acid-based applications. High cost of instrumentation may limit accessibility.
Innovations in Enzyme-Isomer Interactions
Machine learning for the discovery of nanomaterial-based molecular recognition
PatentPendingUS20240412824A1
Innovation
- The use of computational machine learning models to predict the likelihood of molecular recognition between candidate nanomaterial binders and analytes, allowing for the selection of promising binders without exhaustive physical testing, leveraging AI and laboratory test results to improve design efficiency.
Molecular recognition systems with pyrimidine analog pairing
PatentActiveUS10059737B1
Innovation
- The development of oligonucleotide compositions that form extended duplex regions by pairing small pyrimidine analogs with other small heterocycles, utilizing specific hydrogen bonding patterns such as donor-acceptor-donor, donor-donor-acceptor, and acceptor-donor-donor patterns, which maintain antiparallel orientation and stability despite violating size complementarity rules.
Regulatory Framework for Engineered Enzymes
The regulatory framework for engineered enzymes is a critical aspect of their development and application, particularly in the context of molecular recognition of geometric isomers. As enzyme engineering advances, regulatory bodies worldwide have established guidelines to ensure the safety, efficacy, and ethical use of these modified biological catalysts.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating engineered enzymes, especially those intended for use in food, pharmaceuticals, and medical applications. The FDA's approach is based on a case-by-case evaluation, considering the specific modifications made to the enzyme and its intended use. The agency typically requires extensive data on the enzyme's structure, function, and potential impacts on human health and the environment.
The European Union has implemented a comprehensive regulatory framework through the European Food Safety Authority (EFSA) and the European Medicines Agency (EMA). These bodies have established stringent guidelines for the assessment and approval of engineered enzymes. The EU's approach emphasizes the precautionary principle, requiring thorough risk assessments and post-market monitoring.
In Japan, the Ministry of Health, Labour and Welfare (MHLW) oversees the regulation of engineered enzymes, particularly those used in food and pharmaceutical applications. The Japanese regulatory framework focuses on the safety and efficacy of the enzymes, with specific attention to their potential allergenicity and environmental impact.
Globally, the Organisation for Economic Co-operation and Development (OECD) has developed guidelines for the safety assessment of recombinant-DNA organisms, which include engineered enzymes. These guidelines provide a harmonized approach to risk assessment and have been adopted by many countries worldwide.
For enzymes engineered specifically for molecular recognition of geometric isomers, regulatory bodies often require additional data on the specificity and selectivity of the enzyme. This includes detailed information on the enzyme's ability to distinguish between different isomers and the potential implications of this selectivity in various applications.
Environmental regulations also play a crucial role in the framework for engineered enzymes. Many countries have implemented strict guidelines for the containment and disposal of genetically modified organisms, including engineered enzymes, to prevent unintended release into the environment.
As the field of enzyme engineering continues to evolve, regulatory frameworks are adapting to keep pace with technological advancements. There is an increasing focus on developing international standards and harmonizing regulations across different countries to facilitate global research and commercialization of engineered enzymes while maintaining rigorous safety and ethical standards.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating engineered enzymes, especially those intended for use in food, pharmaceuticals, and medical applications. The FDA's approach is based on a case-by-case evaluation, considering the specific modifications made to the enzyme and its intended use. The agency typically requires extensive data on the enzyme's structure, function, and potential impacts on human health and the environment.
The European Union has implemented a comprehensive regulatory framework through the European Food Safety Authority (EFSA) and the European Medicines Agency (EMA). These bodies have established stringent guidelines for the assessment and approval of engineered enzymes. The EU's approach emphasizes the precautionary principle, requiring thorough risk assessments and post-market monitoring.
In Japan, the Ministry of Health, Labour and Welfare (MHLW) oversees the regulation of engineered enzymes, particularly those used in food and pharmaceutical applications. The Japanese regulatory framework focuses on the safety and efficacy of the enzymes, with specific attention to their potential allergenicity and environmental impact.
Globally, the Organisation for Economic Co-operation and Development (OECD) has developed guidelines for the safety assessment of recombinant-DNA organisms, which include engineered enzymes. These guidelines provide a harmonized approach to risk assessment and have been adopted by many countries worldwide.
For enzymes engineered specifically for molecular recognition of geometric isomers, regulatory bodies often require additional data on the specificity and selectivity of the enzyme. This includes detailed information on the enzyme's ability to distinguish between different isomers and the potential implications of this selectivity in various applications.
Environmental regulations also play a crucial role in the framework for engineered enzymes. Many countries have implemented strict guidelines for the containment and disposal of genetically modified organisms, including engineered enzymes, to prevent unintended release into the environment.
As the field of enzyme engineering continues to evolve, regulatory frameworks are adapting to keep pace with technological advancements. There is an increasing focus on developing international standards and harmonizing regulations across different countries to facilitate global research and commercialization of engineered enzymes while maintaining rigorous safety and ethical standards.
Environmental Impact of Isomer-Specific Catalysis
The environmental impact of isomer-specific catalysis in enzyme engineering is a critical consideration in the development and application of molecular recognition technologies. This approach offers significant potential for reducing the environmental footprint of chemical processes by enhancing reaction specificity and efficiency.
Isomer-specific catalysis enables the selective production of desired geometric isomers, minimizing the generation of unwanted by-products. This selectivity leads to a substantial reduction in waste production, as fewer side reactions occur and less material is discarded. Consequently, the overall resource consumption in chemical processes can be significantly decreased, contributing to more sustainable industrial practices.
The energy efficiency of chemical reactions is another area where isomer-specific catalysis demonstrates environmental benefits. By targeting specific isomers, these catalytic processes often require lower activation energies and can operate under milder conditions. This reduction in energy requirements translates to lower greenhouse gas emissions associated with power generation, aligning with global efforts to combat climate change.
Water usage and pollution are also positively impacted by the implementation of isomer-specific catalysis. The increased reaction specificity often results in cleaner product streams, reducing the need for extensive purification processes. This, in turn, leads to decreased water consumption and a lower volume of contaminated wastewater requiring treatment.
The application of isomer-specific catalysis in pharmaceutical manufacturing presents a particularly promising avenue for environmental improvement. Traditional synthetic routes often produce mixtures of isomers, necessitating complex separation processes and generating substantial waste. By contrast, enzyme-engineered catalysts capable of recognizing and selectively producing specific geometric isomers can dramatically streamline these processes, reducing both environmental impact and production costs.
Biodegradability of products and by-products is another environmental aspect influenced by isomer-specific catalysis. The ability to control isomer formation can lead to the production of compounds that are more readily biodegradable or less persistent in the environment. This characteristic is especially valuable in the development of agrochemicals and other products that may be released into ecosystems.
However, it is important to consider the potential environmental trade-offs associated with the development and production of engineered enzymes for isomer-specific catalysis. The resources and energy required for enzyme production and purification must be weighed against the environmental benefits gained in the catalytic processes. Life cycle assessments are crucial in evaluating the net environmental impact of these technologies.
In conclusion, the environmental impact of isomer-specific catalysis in enzyme engineering is predominantly positive, offering significant potential for reducing waste, energy consumption, and resource use in chemical processes. As this field continues to advance, it promises to play a key role in the development of more sustainable and environmentally friendly industrial practices across various sectors.
Isomer-specific catalysis enables the selective production of desired geometric isomers, minimizing the generation of unwanted by-products. This selectivity leads to a substantial reduction in waste production, as fewer side reactions occur and less material is discarded. Consequently, the overall resource consumption in chemical processes can be significantly decreased, contributing to more sustainable industrial practices.
The energy efficiency of chemical reactions is another area where isomer-specific catalysis demonstrates environmental benefits. By targeting specific isomers, these catalytic processes often require lower activation energies and can operate under milder conditions. This reduction in energy requirements translates to lower greenhouse gas emissions associated with power generation, aligning with global efforts to combat climate change.
Water usage and pollution are also positively impacted by the implementation of isomer-specific catalysis. The increased reaction specificity often results in cleaner product streams, reducing the need for extensive purification processes. This, in turn, leads to decreased water consumption and a lower volume of contaminated wastewater requiring treatment.
The application of isomer-specific catalysis in pharmaceutical manufacturing presents a particularly promising avenue for environmental improvement. Traditional synthetic routes often produce mixtures of isomers, necessitating complex separation processes and generating substantial waste. By contrast, enzyme-engineered catalysts capable of recognizing and selectively producing specific geometric isomers can dramatically streamline these processes, reducing both environmental impact and production costs.
Biodegradability of products and by-products is another environmental aspect influenced by isomer-specific catalysis. The ability to control isomer formation can lead to the production of compounds that are more readily biodegradable or less persistent in the environment. This characteristic is especially valuable in the development of agrochemicals and other products that may be released into ecosystems.
However, it is important to consider the potential environmental trade-offs associated with the development and production of engineered enzymes for isomer-specific catalysis. The resources and energy required for enzyme production and purification must be weighed against the environmental benefits gained in the catalytic processes. Life cycle assessments are crucial in evaluating the net environmental impact of these technologies.
In conclusion, the environmental impact of isomer-specific catalysis in enzyme engineering is predominantly positive, offering significant potential for reducing waste, energy consumption, and resource use in chemical processes. As this field continues to advance, it promises to play a key role in the development of more sustainable and environmentally friendly industrial practices across various sectors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!