Structural Properties of Geometric Isomers on Micelle Formation
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Geometric Isomers and Micelle Formation Background
Geometric isomers are molecules with the same molecular formula but different spatial arrangements of atoms. In the context of micelle formation, these structural variations can significantly influence the self-assembly process and the resulting micelle properties. The study of geometric isomers in micelle formation has gained considerable attention due to its implications in various fields, including drug delivery, nanotechnology, and materials science.
Micelles are self-assembled structures formed by amphiphilic molecules in aqueous solutions. These molecules typically consist of a hydrophilic head group and a hydrophobic tail. When the concentration of these molecules exceeds the critical micelle concentration (CMC), they spontaneously aggregate to form spherical or cylindrical structures with the hydrophobic tails oriented towards the center and the hydrophilic heads facing the aqueous environment.
The structural properties of geometric isomers play a crucial role in determining the characteristics of micelle formation. Cis-trans isomerism, for instance, can significantly affect the packing of molecules within the micelle structure. Cis isomers often result in more compact and stable micelles due to their bent shape, which allows for tighter packing. In contrast, trans isomers tend to form less stable micelles with larger sizes due to their linear structure.
The impact of geometric isomerism on micelle formation extends beyond simple structural differences. It can influence various properties such as the critical micelle concentration, micelle size, shape, and stability. These factors, in turn, affect the micelle's ability to solubilize hydrophobic compounds, its interaction with biological membranes, and its potential applications in drug delivery systems.
Research in this field has evolved from basic studies of micelle formation to more complex investigations of how geometric isomers can be utilized to fine-tune micelle properties for specific applications. For example, the use of photoswitchable geometric isomers has opened up possibilities for creating stimuli-responsive micelles that can change their properties upon exposure to light.
Understanding the relationship between geometric isomerism and micelle formation is not only of fundamental scientific interest but also has practical implications. It provides insights into the design of more efficient surfactants, the development of novel drug delivery systems, and the creation of advanced materials with tailored properties. As research in this area continues to advance, it promises to unlock new possibilities in fields ranging from pharmaceuticals to environmental remediation.
Micelles are self-assembled structures formed by amphiphilic molecules in aqueous solutions. These molecules typically consist of a hydrophilic head group and a hydrophobic tail. When the concentration of these molecules exceeds the critical micelle concentration (CMC), they spontaneously aggregate to form spherical or cylindrical structures with the hydrophobic tails oriented towards the center and the hydrophilic heads facing the aqueous environment.
The structural properties of geometric isomers play a crucial role in determining the characteristics of micelle formation. Cis-trans isomerism, for instance, can significantly affect the packing of molecules within the micelle structure. Cis isomers often result in more compact and stable micelles due to their bent shape, which allows for tighter packing. In contrast, trans isomers tend to form less stable micelles with larger sizes due to their linear structure.
The impact of geometric isomerism on micelle formation extends beyond simple structural differences. It can influence various properties such as the critical micelle concentration, micelle size, shape, and stability. These factors, in turn, affect the micelle's ability to solubilize hydrophobic compounds, its interaction with biological membranes, and its potential applications in drug delivery systems.
Research in this field has evolved from basic studies of micelle formation to more complex investigations of how geometric isomers can be utilized to fine-tune micelle properties for specific applications. For example, the use of photoswitchable geometric isomers has opened up possibilities for creating stimuli-responsive micelles that can change their properties upon exposure to light.
Understanding the relationship between geometric isomerism and micelle formation is not only of fundamental scientific interest but also has practical implications. It provides insights into the design of more efficient surfactants, the development of novel drug delivery systems, and the creation of advanced materials with tailored properties. As research in this area continues to advance, it promises to unlock new possibilities in fields ranging from pharmaceuticals to environmental remediation.
Market Analysis for Micelle-Based Products
The market for micelle-based products has experienced significant growth in recent years, driven by increasing applications in pharmaceuticals, cosmetics, and food industries. Micelles, formed by the self-assembly of amphiphilic molecules, offer unique properties that make them valuable in various product formulations. In the pharmaceutical sector, micelles are utilized for drug delivery systems, enhancing the solubility and bioavailability of poorly water-soluble drugs. This application has led to the development of novel therapeutic approaches and improved treatment efficacy.
The cosmetics industry has also embraced micelle technology, particularly in skincare and cleansing products. Micellar water, a gentle yet effective cleansing solution, has gained popularity among consumers seeking alternatives to traditional cleansers. The ability of micelles to encapsulate both hydrophilic and hydrophobic substances has enabled the creation of more stable and effective cosmetic formulations, driving market growth in this sector.
In the food industry, micelles are employed as emulsifiers and stabilizers, improving the texture and shelf life of various products. This application has opened new possibilities for food manufacturers to develop innovative products with enhanced nutritional profiles and sensory characteristics. The increasing consumer demand for clean-label and natural ingredients has further boosted the adoption of micelle-based solutions in food formulations.
The global market for micelle-based products is expected to continue its upward trajectory, with a compound annual growth rate projected to remain strong over the next five years. Factors contributing to this growth include ongoing research and development efforts, expanding applications across industries, and growing consumer awareness of the benefits of micelle-based products.
Geographically, North America and Europe currently dominate the market, owing to their advanced healthcare and personal care industries. However, the Asia-Pacific region is emerging as a lucrative market, driven by rapid industrialization, increasing disposable incomes, and growing awareness of personal care and health products. This regional shift is likely to create new opportunities for market players and reshape the competitive landscape.
Key challenges facing the micelle-based product market include regulatory hurdles, particularly in pharmaceutical applications, and the need for continuous innovation to differentiate products in an increasingly crowded market. Additionally, the cost of research and development for new micelle formulations may pose barriers to entry for smaller companies.
The cosmetics industry has also embraced micelle technology, particularly in skincare and cleansing products. Micellar water, a gentle yet effective cleansing solution, has gained popularity among consumers seeking alternatives to traditional cleansers. The ability of micelles to encapsulate both hydrophilic and hydrophobic substances has enabled the creation of more stable and effective cosmetic formulations, driving market growth in this sector.
In the food industry, micelles are employed as emulsifiers and stabilizers, improving the texture and shelf life of various products. This application has opened new possibilities for food manufacturers to develop innovative products with enhanced nutritional profiles and sensory characteristics. The increasing consumer demand for clean-label and natural ingredients has further boosted the adoption of micelle-based solutions in food formulations.
The global market for micelle-based products is expected to continue its upward trajectory, with a compound annual growth rate projected to remain strong over the next five years. Factors contributing to this growth include ongoing research and development efforts, expanding applications across industries, and growing consumer awareness of the benefits of micelle-based products.
Geographically, North America and Europe currently dominate the market, owing to their advanced healthcare and personal care industries. However, the Asia-Pacific region is emerging as a lucrative market, driven by rapid industrialization, increasing disposable incomes, and growing awareness of personal care and health products. This regional shift is likely to create new opportunities for market players and reshape the competitive landscape.
Key challenges facing the micelle-based product market include regulatory hurdles, particularly in pharmaceutical applications, and the need for continuous innovation to differentiate products in an increasingly crowded market. Additionally, the cost of research and development for new micelle formulations may pose barriers to entry for smaller companies.
Current Challenges in Geometric Isomer Research
Despite significant advancements in geometric isomer research, several challenges persist in understanding their structural properties and their impact on micelle formation. One of the primary obstacles is the complexity of molecular interactions in micellar systems, which makes it difficult to isolate and study the specific effects of geometric isomers. The dynamic nature of micelles further complicates the analysis, as the structures are constantly in flux, making it challenging to capture and characterize their properties accurately.
Another significant challenge lies in the limitations of current analytical techniques. While advanced spectroscopic methods have improved our ability to study molecular structures, they often struggle to provide real-time, high-resolution data on the behavior of geometric isomers within micelles. This gap in observational capabilities hinders our understanding of the precise mechanisms by which isomers influence micelle formation and stability.
The diversity of geometric isomers and their potential combinations in micellar systems also presents a formidable challenge. Each isomer may interact differently with the surrounding environment and other molecules, leading to a vast array of possible structural configurations. This complexity makes it difficult to develop comprehensive models that can predict micelle behavior across various isomeric compositions.
Furthermore, the influence of external factors such as temperature, pH, and ionic strength on the behavior of geometric isomers in micelles remains poorly understood. These environmental variables can significantly alter the properties of both the isomers and the resulting micelles, adding another layer of complexity to the research.
The interdisciplinary nature of this field also poses challenges in terms of integrating knowledge from various domains. Bridging the gap between theoretical predictions and experimental observations often requires expertise in physical chemistry, molecular biology, and advanced computational modeling. The lack of standardized protocols for studying geometric isomers in micellar systems further hampers progress and makes it difficult to compare results across different studies.
Lastly, the translation of fundamental research findings into practical applications faces significant hurdles. While the potential applications of geometric isomer-influenced micelles in drug delivery, cosmetics, and material science are promising, scaling up laboratory discoveries to industrial processes remains challenging. This gap between basic research and applied technology slows down the development of novel products and materials based on the unique properties of geometric isomers in micellar systems.
Another significant challenge lies in the limitations of current analytical techniques. While advanced spectroscopic methods have improved our ability to study molecular structures, they often struggle to provide real-time, high-resolution data on the behavior of geometric isomers within micelles. This gap in observational capabilities hinders our understanding of the precise mechanisms by which isomers influence micelle formation and stability.
The diversity of geometric isomers and their potential combinations in micellar systems also presents a formidable challenge. Each isomer may interact differently with the surrounding environment and other molecules, leading to a vast array of possible structural configurations. This complexity makes it difficult to develop comprehensive models that can predict micelle behavior across various isomeric compositions.
Furthermore, the influence of external factors such as temperature, pH, and ionic strength on the behavior of geometric isomers in micelles remains poorly understood. These environmental variables can significantly alter the properties of both the isomers and the resulting micelles, adding another layer of complexity to the research.
The interdisciplinary nature of this field also poses challenges in terms of integrating knowledge from various domains. Bridging the gap between theoretical predictions and experimental observations often requires expertise in physical chemistry, molecular biology, and advanced computational modeling. The lack of standardized protocols for studying geometric isomers in micellar systems further hampers progress and makes it difficult to compare results across different studies.
Lastly, the translation of fundamental research findings into practical applications faces significant hurdles. While the potential applications of geometric isomer-influenced micelles in drug delivery, cosmetics, and material science are promising, scaling up laboratory discoveries to industrial processes remains challenging. This gap between basic research and applied technology slows down the development of novel products and materials based on the unique properties of geometric isomers in micellar systems.
Existing Methodologies for Isomer Structure Analysis
01 Structural differences between geometric isomers
Geometric isomers have the same molecular formula but different spatial arrangements of atoms. These structural differences can lead to variations in physical and chemical properties, such as melting point, boiling point, and reactivity. The spatial arrangement of atoms in geometric isomers affects their interactions with other molecules and their behavior in chemical reactions.- Structural differences between geometric isomers: Geometric isomers have the same molecular formula but different spatial arrangements of atoms. These structural differences can lead to variations in physical and chemical properties, such as melting point, boiling point, and reactivity. The spatial arrangement of atoms in geometric isomers affects their interactions with other molecules and their behavior in chemical reactions.
- Analytical methods for identifying geometric isomers: Various analytical techniques are used to identify and characterize geometric isomers. These methods include spectroscopic techniques such as NMR spectroscopy, infrared spectroscopy, and X-ray crystallography. These techniques can provide information about the spatial arrangement of atoms in the molecule, allowing researchers to distinguish between different geometric isomers and determine their structural properties.
- Computational modeling of geometric isomers: Computational methods are employed to model and predict the structural properties of geometric isomers. These techniques involve using advanced algorithms and software to simulate the molecular structure and behavior of different isomers. Computational modeling can help researchers understand the energetics, stability, and reactivity of geometric isomers, as well as predict their physical and chemical properties.
- Interconversion between geometric isomers: Geometric isomers can sometimes interconvert between different forms under specific conditions. This process, known as isomerization, can be induced by factors such as heat, light, or catalysts. Understanding the mechanisms and conditions for interconversion is crucial for controlling the production and stability of specific geometric isomers in various applications, including pharmaceuticals and materials science.
- Applications of geometric isomers in various fields: The unique structural properties of geometric isomers find applications in diverse fields. In pharmaceuticals, different geometric isomers of a drug molecule can have varying biological activities. In materials science, geometric isomers can be used to create materials with specific optical or electronic properties. The ability to control and manipulate geometric isomers is crucial for developing new products and technologies in these fields.
02 Identification and characterization of geometric isomers
Various analytical techniques are used to identify and characterize geometric isomers. These methods include spectroscopic techniques such as NMR, IR, and UV-Vis spectroscopy, as well as X-ray crystallography. These techniques help determine the spatial arrangement of atoms and distinguish between different geometric isomers of the same compound.Expand Specific Solutions03 Interconversion between geometric isomers
Geometric isomers can sometimes interconvert between different forms under specific conditions. This process, known as isomerization, can be induced by heat, light, or catalysts. Understanding the conditions and mechanisms of interconversion is crucial for controlling the isomeric composition of compounds in various applications, such as in the pharmaceutical and chemical industries.Expand Specific Solutions04 Applications of geometric isomers in materials science
The unique structural properties of geometric isomers are exploited in various materials science applications. These include the development of advanced polymers, liquid crystals, and molecular machines. The ability to control and manipulate the spatial arrangement of atoms in geometric isomers allows for the creation of materials with tailored properties and functionalities.Expand Specific Solutions05 Computational modeling of geometric isomers
Computational methods are employed to study and predict the structural properties of geometric isomers. These include molecular dynamics simulations, quantum mechanical calculations, and machine learning approaches. Such computational tools aid in understanding the relationship between molecular structure and properties, as well as in designing new compounds with desired characteristics.Expand Specific Solutions
Key Players in Surfactant Industry
The research on structural properties of geometric isomers in micelle formation is in a developing stage, with growing market potential due to its applications in drug delivery and nanotechnology. The field is characterized by a mix of academic institutions and pharmaceutical companies, indicating a transition from basic research to applied science. Key players like University of Washington, Pfizer, and MIT are driving innovation, while specialized firms such as PhaseRx and Amicrobe are focusing on niche applications. The technology's maturity varies, with established companies like Bayer HealthCare leveraging their resources for advanced development, while startups and research institutions continue to explore fundamental aspects and novel applications.
University of Washington
Technical Solution: The University of Washington has developed a novel approach to studying geometric isomers and their effects on micelle formation using advanced fluorescence spectroscopy techniques[4]. Their method employs time-resolved fluorescence anisotropy to probe the local environment and dynamics of isomeric molecules within micelles[5]. Additionally, they have implemented a unique combination of circular dichroism and dynamic light scattering to investigate how isomeric structures influence the size, shape, and chirality of resulting micelles[6]. The university's research also focuses on developing predictive models that correlate isomeric structural properties with micelle characteristics, enabling more efficient design of surfactant systems for various applications.
Strengths: Innovative spectroscopic techniques, focus on structure-property relationships. Weaknesses: Limited to fluorescence-active compounds, potential interference from complex mixtures.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced techniques for studying geometric isomers and their impact on micelle formation. They utilize high-resolution nuclear magnetic resonance (NMR) spectroscopy to analyze the structural properties of isomers in solution[1]. Their approach combines experimental methods with molecular dynamics simulations to provide a comprehensive understanding of how isomeric structures influence micelle formation and stability[2]. MIT researchers have also pioneered the use of small-angle neutron scattering (SANS) to investigate the shape and size of micelles formed by different geometric isomers[3]. This multi-faceted approach allows for a detailed characterization of isomer-induced changes in micelle morphology and dynamics.
Strengths: Cutting-edge analytical techniques, integration of experimental and computational methods. Weaknesses: High equipment costs, complexity in data interpretation.
Core Innovations in Micelle Characterization
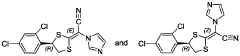
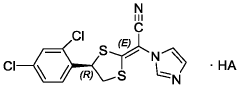
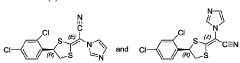
Compounds of r-(-)-(e)-[4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene]-1 -imidazolylacetonitrile-ha (luliconazole-ha) as antifungals
PatentWO2017108972A1
Innovation
- A process involving treating a mixture of geometrical isomers of R-(-)-(E)-[4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene]-1-imidazolylacetonitrile with an acid in the presence of an organic solvent to achieve a mixture with a higher E-isomer content, followed by conventional isolation techniques, resulting in high yield and purity of the E-isomer suitable for pharmaceutical formulations.
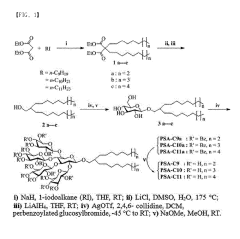
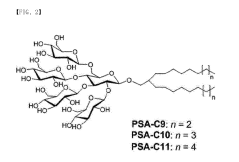
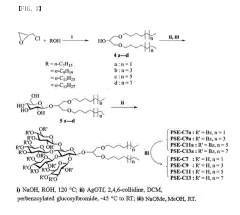
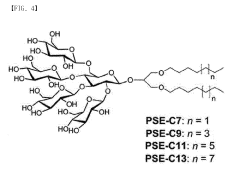
Amphipathic compound having novel penta-saccharide hydrophilic group and use thereof
PatentInactiveUS20190169218A1
Innovation
- Development of novel amphipathic compounds with a glucose-centered high-density penta-saccharide hydrophilic group that forms small complexes with membrane proteins, optimizing hydrophilic-lipophilic balance and enhancing stability and solubilization.
Environmental Impact of Surfactants
The environmental impact of surfactants used in micelle formation is a critical consideration in the study of geometric isomers and their structural properties. Surfactants, essential components in micelle formation, can have significant effects on aquatic ecosystems and soil environments when released into nature. These compounds can alter the surface tension of water, potentially disrupting the delicate balance of aquatic life and interfering with the natural processes of various organisms.
One of the primary concerns is the potential for surfactants to accumulate in water bodies, leading to foam formation on the surface of rivers, lakes, and oceans. This foam can impede oxygen transfer between the air and water, negatively affecting aquatic life and potentially leading to eutrophication. Furthermore, some surfactants have been found to be toxic to certain aquatic species, particularly fish and invertebrates, even at low concentrations.
The biodegradability of surfactants is a crucial factor in assessing their environmental impact. While many modern surfactants are designed to be biodegradable, the rate of degradation can vary significantly depending on the specific molecular structure. Geometric isomers of surfactants may exhibit different biodegradation rates, potentially leading to varying levels of environmental persistence and impact.
Soil ecosystems can also be affected by surfactants, particularly when they are present in wastewater used for irrigation or when they leach into groundwater. These compounds can alter soil structure and permeability, potentially affecting plant growth and soil microbial communities. The adsorption of surfactants to soil particles may also influence the mobility and bioavailability of other pollutants, such as heavy metals and organic contaminants.
The production and disposal of surfactants contribute to their environmental footprint. Manufacturing processes often require significant energy inputs and may generate by-products that need careful management. End-of-life considerations, such as the proper treatment of surfactant-containing wastewater, are essential to minimize environmental impact.
Research into the structural properties of geometric isomers in micelle formation can contribute to the development of more environmentally friendly surfactants. By understanding how molecular structure influences micelle formation and stability, it may be possible to design surfactants that maintain their desired functionality while minimizing negative environmental impacts. This could include developing surfactants with improved biodegradability, reduced toxicity, or enhanced efficiency, allowing for lower usage concentrations.
One of the primary concerns is the potential for surfactants to accumulate in water bodies, leading to foam formation on the surface of rivers, lakes, and oceans. This foam can impede oxygen transfer between the air and water, negatively affecting aquatic life and potentially leading to eutrophication. Furthermore, some surfactants have been found to be toxic to certain aquatic species, particularly fish and invertebrates, even at low concentrations.
The biodegradability of surfactants is a crucial factor in assessing their environmental impact. While many modern surfactants are designed to be biodegradable, the rate of degradation can vary significantly depending on the specific molecular structure. Geometric isomers of surfactants may exhibit different biodegradation rates, potentially leading to varying levels of environmental persistence and impact.
Soil ecosystems can also be affected by surfactants, particularly when they are present in wastewater used for irrigation or when they leach into groundwater. These compounds can alter soil structure and permeability, potentially affecting plant growth and soil microbial communities. The adsorption of surfactants to soil particles may also influence the mobility and bioavailability of other pollutants, such as heavy metals and organic contaminants.
The production and disposal of surfactants contribute to their environmental footprint. Manufacturing processes often require significant energy inputs and may generate by-products that need careful management. End-of-life considerations, such as the proper treatment of surfactant-containing wastewater, are essential to minimize environmental impact.
Research into the structural properties of geometric isomers in micelle formation can contribute to the development of more environmentally friendly surfactants. By understanding how molecular structure influences micelle formation and stability, it may be possible to design surfactants that maintain their desired functionality while minimizing negative environmental impacts. This could include developing surfactants with improved biodegradability, reduced toxicity, or enhanced efficiency, allowing for lower usage concentrations.
Applications in Pharmaceutical Delivery Systems
The application of geometric isomers in micelle formation has significant implications for pharmaceutical delivery systems. Micelles, self-assembling structures formed by amphiphilic molecules, have emerged as versatile carriers for drug delivery. The structural properties of geometric isomers play a crucial role in determining the characteristics and performance of these micelle-based delivery systems.
Geometric isomers, with their distinct spatial arrangements of atoms, can influence the packing and organization of molecules within micelles. This, in turn, affects the size, shape, and stability of the resulting structures. For instance, cis-trans isomerism in fatty acids can lead to differences in micelle formation, with trans isomers often forming more stable and compact structures compared to their cis counterparts.
In the context of pharmaceutical delivery, these structural variations can be exploited to optimize drug encapsulation, release kinetics, and targeting efficiency. Micelles formed from geometric isomers with specific configurations may exhibit enhanced drug loading capacity or improved penetration through biological barriers. For example, micelles composed of trans-isomers might provide a more rigid core, potentially increasing drug retention and prolonging circulation time in the bloodstream.
The impact of geometric isomerism extends to the interaction between micelles and biological membranes. The spatial arrangement of hydrophilic and hydrophobic regions in isomeric surfactants can influence the membrane permeability and cellular uptake of micelle-encapsulated drugs. This property can be leveraged to design delivery systems with improved bioavailability or targeted delivery to specific tissues or organs.
Furthermore, the use of stimuli-responsive geometric isomers in micelle formation opens up possibilities for controlled drug release. Environmental triggers such as pH, temperature, or light can induce conformational changes in these isomers, altering the micelle structure and facilitating on-demand drug release at specific sites within the body.
The research on geometric isomers in micelle formation also contributes to the development of multi-functional delivery systems. By incorporating different isomeric forms of amphiphilic molecules, it is possible to create micelles with compartmentalized structures, enabling the co-delivery of multiple therapeutic agents or the combination of diagnostic and therapeutic functionalities in a single nanocarrier.
As the field of nanomedicine continues to advance, the understanding and manipulation of geometric isomers in micelle formation will likely play an increasingly important role in the design of next-generation pharmaceutical delivery systems. This research area holds promise for addressing challenges in drug solubility, stability, and targeted delivery, ultimately leading to more effective and personalized therapeutic approaches.
Geometric isomers, with their distinct spatial arrangements of atoms, can influence the packing and organization of molecules within micelles. This, in turn, affects the size, shape, and stability of the resulting structures. For instance, cis-trans isomerism in fatty acids can lead to differences in micelle formation, with trans isomers often forming more stable and compact structures compared to their cis counterparts.
In the context of pharmaceutical delivery, these structural variations can be exploited to optimize drug encapsulation, release kinetics, and targeting efficiency. Micelles formed from geometric isomers with specific configurations may exhibit enhanced drug loading capacity or improved penetration through biological barriers. For example, micelles composed of trans-isomers might provide a more rigid core, potentially increasing drug retention and prolonging circulation time in the bloodstream.
The impact of geometric isomerism extends to the interaction between micelles and biological membranes. The spatial arrangement of hydrophilic and hydrophobic regions in isomeric surfactants can influence the membrane permeability and cellular uptake of micelle-encapsulated drugs. This property can be leveraged to design delivery systems with improved bioavailability or targeted delivery to specific tissues or organs.
Furthermore, the use of stimuli-responsive geometric isomers in micelle formation opens up possibilities for controlled drug release. Environmental triggers such as pH, temperature, or light can induce conformational changes in these isomers, altering the micelle structure and facilitating on-demand drug release at specific sites within the body.
The research on geometric isomers in micelle formation also contributes to the development of multi-functional delivery systems. By incorporating different isomeric forms of amphiphilic molecules, it is possible to create micelles with compartmentalized structures, enabling the co-delivery of multiple therapeutic agents or the combination of diagnostic and therapeutic functionalities in a single nanocarrier.
As the field of nanomedicine continues to advance, the understanding and manipulation of geometric isomers in micelle formation will likely play an increasingly important role in the design of next-generation pharmaceutical delivery systems. This research area holds promise for addressing challenges in drug solubility, stability, and targeted delivery, ultimately leading to more effective and personalized therapeutic approaches.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!