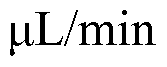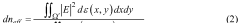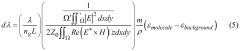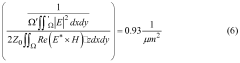Role of silicon photonics in non-invasive medical testing.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Silicon Photonics in Medical Testing: Background and Objectives
Silicon photonics has emerged as a transformative technology in the field of non-invasive medical testing, offering unprecedented opportunities for miniaturization, integration, and enhanced performance of diagnostic devices. The evolution of this technology can be traced back to the early 2000s when researchers began exploring the potential of integrating photonic components on silicon substrates. Since then, silicon photonics has experienced rapid advancements, driven by the synergy between the mature silicon semiconductor industry and the growing demand for high-performance optical systems.
The primary objective of silicon photonics in medical testing is to leverage the unique properties of light interaction with biological samples to develop highly sensitive, compact, and cost-effective diagnostic tools. By integrating multiple optical functions on a single chip, silicon photonics aims to revolutionize point-of-care diagnostics, enabling real-time, non-invasive monitoring of various health parameters. This technology holds the promise of bringing laboratory-grade analytical capabilities to portable devices, potentially transforming healthcare delivery and patient monitoring.
The technological trajectory of silicon photonics in medical testing is closely aligned with broader trends in healthcare, such as personalized medicine and remote patient monitoring. As the global population ages and healthcare systems face increasing pressure, there is a growing need for accessible, accurate, and rapid diagnostic solutions. Silicon photonics is poised to address these challenges by enabling the development of compact, multi-functional biosensors capable of detecting a wide range of biomarkers with high sensitivity and specificity.
Key technological goals in this field include improving the integration density of photonic components, enhancing the sensitivity and specificity of optical sensing mechanisms, and developing robust, scalable manufacturing processes. Researchers are also focusing on overcoming challenges related to light coupling, thermal management, and packaging to ensure the reliability and practicality of silicon photonic devices in real-world medical applications.
The convergence of silicon photonics with other emerging technologies, such as artificial intelligence and microfluidics, is expected to further accelerate innovation in non-invasive medical testing. This synergy could lead to the development of intelligent diagnostic platforms capable of performing complex analyses on minute sample volumes, potentially enabling early disease detection and personalized treatment strategies.
As the field progresses, it is anticipated that silicon photonics will play a crucial role in addressing global health challenges, particularly in resource-limited settings where access to advanced diagnostic tools is limited. The ability to produce low-cost, high-performance diagnostic devices at scale could significantly impact public health initiatives and contribute to more equitable healthcare access worldwide.
The primary objective of silicon photonics in medical testing is to leverage the unique properties of light interaction with biological samples to develop highly sensitive, compact, and cost-effective diagnostic tools. By integrating multiple optical functions on a single chip, silicon photonics aims to revolutionize point-of-care diagnostics, enabling real-time, non-invasive monitoring of various health parameters. This technology holds the promise of bringing laboratory-grade analytical capabilities to portable devices, potentially transforming healthcare delivery and patient monitoring.
The technological trajectory of silicon photonics in medical testing is closely aligned with broader trends in healthcare, such as personalized medicine and remote patient monitoring. As the global population ages and healthcare systems face increasing pressure, there is a growing need for accessible, accurate, and rapid diagnostic solutions. Silicon photonics is poised to address these challenges by enabling the development of compact, multi-functional biosensors capable of detecting a wide range of biomarkers with high sensitivity and specificity.
Key technological goals in this field include improving the integration density of photonic components, enhancing the sensitivity and specificity of optical sensing mechanisms, and developing robust, scalable manufacturing processes. Researchers are also focusing on overcoming challenges related to light coupling, thermal management, and packaging to ensure the reliability and practicality of silicon photonic devices in real-world medical applications.
The convergence of silicon photonics with other emerging technologies, such as artificial intelligence and microfluidics, is expected to further accelerate innovation in non-invasive medical testing. This synergy could lead to the development of intelligent diagnostic platforms capable of performing complex analyses on minute sample volumes, potentially enabling early disease detection and personalized treatment strategies.
As the field progresses, it is anticipated that silicon photonics will play a crucial role in addressing global health challenges, particularly in resource-limited settings where access to advanced diagnostic tools is limited. The ability to produce low-cost, high-performance diagnostic devices at scale could significantly impact public health initiatives and contribute to more equitable healthcare access worldwide.
Market Analysis for Non-Invasive Diagnostic Technologies
The non-invasive diagnostic technologies market has experienced significant growth in recent years, driven by increasing demand for early disease detection, patient comfort, and cost-effective healthcare solutions. This market segment encompasses a wide range of technologies, including imaging systems, wearable devices, and biomarker analysis tools. The global non-invasive diagnostic market was valued at approximately $12 billion in 2020 and is projected to reach $21 billion by 2025, with a compound annual growth rate (CAGR) of 11.8%.
Silicon photonics, a rapidly evolving technology, is poised to play a crucial role in advancing non-invasive medical testing. The integration of silicon photonics in diagnostic devices offers several advantages, such as miniaturization, improved sensitivity, and reduced costs. These benefits align well with the market's demand for portable, accurate, and affordable diagnostic solutions.
The market for non-invasive diagnostic technologies is segmented based on technology type, application area, and geography. Key technology segments include optical imaging, electromagnetic imaging, and molecular diagnostics. Silicon photonics has the potential to impact all these segments, particularly in areas such as spectroscopy-based diagnostics and lab-on-a-chip devices.
Geographically, North America currently dominates the non-invasive diagnostic market, followed by Europe and Asia-Pacific. However, emerging economies in Asia-Pacific and Latin America are expected to witness the highest growth rates in the coming years, driven by improving healthcare infrastructure and increasing awareness of preventive healthcare.
The adoption of silicon photonics in non-invasive medical testing is influenced by several market drivers and challenges. Key drivers include the growing prevalence of chronic diseases, increasing healthcare expenditure, and technological advancements in photonics. However, challenges such as high initial investment costs and regulatory hurdles may impact market growth.
Looking ahead, the integration of silicon photonics with other emerging technologies like artificial intelligence and the Internet of Things is expected to create new opportunities in the non-invasive diagnostic market. This convergence could lead to the development of smart, connected diagnostic devices capable of real-time health monitoring and personalized medicine applications.
Silicon photonics, a rapidly evolving technology, is poised to play a crucial role in advancing non-invasive medical testing. The integration of silicon photonics in diagnostic devices offers several advantages, such as miniaturization, improved sensitivity, and reduced costs. These benefits align well with the market's demand for portable, accurate, and affordable diagnostic solutions.
The market for non-invasive diagnostic technologies is segmented based on technology type, application area, and geography. Key technology segments include optical imaging, electromagnetic imaging, and molecular diagnostics. Silicon photonics has the potential to impact all these segments, particularly in areas such as spectroscopy-based diagnostics and lab-on-a-chip devices.
Geographically, North America currently dominates the non-invasive diagnostic market, followed by Europe and Asia-Pacific. However, emerging economies in Asia-Pacific and Latin America are expected to witness the highest growth rates in the coming years, driven by improving healthcare infrastructure and increasing awareness of preventive healthcare.
The adoption of silicon photonics in non-invasive medical testing is influenced by several market drivers and challenges. Key drivers include the growing prevalence of chronic diseases, increasing healthcare expenditure, and technological advancements in photonics. However, challenges such as high initial investment costs and regulatory hurdles may impact market growth.
Looking ahead, the integration of silicon photonics with other emerging technologies like artificial intelligence and the Internet of Things is expected to create new opportunities in the non-invasive diagnostic market. This convergence could lead to the development of smart, connected diagnostic devices capable of real-time health monitoring and personalized medicine applications.
Current State and Challenges in Silicon Photonics for Medical Applications
Silicon photonics has emerged as a promising technology for non-invasive medical testing, offering significant advancements in diagnostic capabilities. Currently, the field is experiencing rapid growth, with numerous research institutions and companies actively developing silicon photonic devices for medical applications. The integration of photonic components on silicon chips has enabled the miniaturization of complex optical systems, leading to more compact and cost-effective medical devices.
One of the primary areas of focus in silicon photonics for medical applications is the development of biosensors. These sensors utilize the interaction between light and biological molecules to detect and quantify various biomarkers. The high sensitivity and specificity of silicon photonic biosensors have shown great potential for early disease detection and continuous health monitoring. However, challenges remain in achieving consistent performance across different manufacturing batches and ensuring long-term stability in real-world clinical environments.
Another significant application of silicon photonics in medical testing is optical coherence tomography (OCT). Silicon photonic OCT systems offer high-resolution, three-dimensional imaging of biological tissues, enabling non-invasive diagnostics for various medical conditions. The integration of OCT components on silicon chips has led to more compact and portable devices, expanding their use in point-of-care settings. Nevertheless, improving the imaging depth and speed while maintaining high resolution remains a technical challenge.
The development of on-chip spectrometers using silicon photonics has also gained traction in medical applications. These devices enable the analysis of complex biological samples by measuring their spectral properties. While silicon photonic spectrometers offer advantages in terms of size and cost, enhancing their spectral range and resolution to match the performance of traditional benchtop instruments is an ongoing challenge.
One of the major hurdles in advancing silicon photonics for medical applications is the integration of active components, such as light sources and detectors, directly on silicon chips. While progress has been made in this area, achieving efficient and reliable on-chip light generation and detection remains a significant technical challenge. Overcoming this limitation would enable fully integrated, single-chip photonic solutions for medical testing.
The biocompatibility and long-term stability of silicon photonic devices in biological environments present another set of challenges. Ensuring that these devices can withstand exposure to various bodily fluids and maintain their performance over extended periods is crucial for their adoption in clinical settings. Research efforts are ongoing to develop suitable packaging and surface functionalization techniques to address these issues.
One of the primary areas of focus in silicon photonics for medical applications is the development of biosensors. These sensors utilize the interaction between light and biological molecules to detect and quantify various biomarkers. The high sensitivity and specificity of silicon photonic biosensors have shown great potential for early disease detection and continuous health monitoring. However, challenges remain in achieving consistent performance across different manufacturing batches and ensuring long-term stability in real-world clinical environments.
Another significant application of silicon photonics in medical testing is optical coherence tomography (OCT). Silicon photonic OCT systems offer high-resolution, three-dimensional imaging of biological tissues, enabling non-invasive diagnostics for various medical conditions. The integration of OCT components on silicon chips has led to more compact and portable devices, expanding their use in point-of-care settings. Nevertheless, improving the imaging depth and speed while maintaining high resolution remains a technical challenge.
The development of on-chip spectrometers using silicon photonics has also gained traction in medical applications. These devices enable the analysis of complex biological samples by measuring their spectral properties. While silicon photonic spectrometers offer advantages in terms of size and cost, enhancing their spectral range and resolution to match the performance of traditional benchtop instruments is an ongoing challenge.
One of the major hurdles in advancing silicon photonics for medical applications is the integration of active components, such as light sources and detectors, directly on silicon chips. While progress has been made in this area, achieving efficient and reliable on-chip light generation and detection remains a significant technical challenge. Overcoming this limitation would enable fully integrated, single-chip photonic solutions for medical testing.
The biocompatibility and long-term stability of silicon photonic devices in biological environments present another set of challenges. Ensuring that these devices can withstand exposure to various bodily fluids and maintain their performance over extended periods is crucial for their adoption in clinical settings. Research efforts are ongoing to develop suitable packaging and surface functionalization techniques to address these issues.
Existing Silicon Photonics Solutions for Non-Invasive Testing
01 Integrated photonic devices
Silicon photonics technology enables the integration of various optical components on a single chip. This includes waveguides, modulators, detectors, and other photonic elements, allowing for compact and efficient optical systems. The integration of these components facilitates high-speed data transmission and processing in optical communication networks and computing applications.- Integrated photonic devices: Silicon photonics technology enables the integration of various optical components on a single chip. This includes waveguides, modulators, detectors, and other photonic elements, allowing for compact and efficient optical systems. The integration of these components facilitates high-speed data transmission and processing in a small form factor.
- Optical communication systems: Silicon photonics is extensively used in optical communication systems to enhance data transmission capabilities. This technology enables the development of high-bandwidth, low-latency communication links for applications such as data centers, telecommunications, and long-distance fiber optic networks. It offers advantages in terms of power efficiency and scalability compared to traditional electronic systems.
- Photonic integrated circuits (PICs): Silicon photonics allows for the creation of complex photonic integrated circuits that combine multiple optical functions on a single chip. These PICs can include components such as lasers, modulators, multiplexers, and photodetectors, enabling advanced functionalities in areas like optical computing, sensing, and signal processing.
- Silicon-based light sources and detectors: Advancements in silicon photonics have led to the development of efficient light sources and detectors integrated on silicon substrates. This includes the creation of silicon-based lasers, LEDs, and photodetectors, which are crucial components for various photonic applications. These developments overcome the limitations of silicon's indirect bandgap and enable fully integrated photonic systems.
- Photonic-electronic integration: Silicon photonics facilitates the integration of photonic and electronic components on the same chip or package. This hybrid integration allows for seamless interaction between optical and electrical signals, enabling high-performance computing systems, optical interconnects, and novel architectures for data processing and communication.
02 Silicon-based optical modulators
Advanced modulators are developed using silicon photonics technology to manipulate light signals efficiently. These modulators can achieve high-speed operation and low power consumption, making them suitable for data centers and telecommunications applications. Various modulation techniques are employed to encode information onto optical carriers.Expand Specific Solutions03 Photonic integrated circuits for quantum computing
Silicon photonics is applied in the development of quantum computing hardware. Photonic integrated circuits are designed to manipulate and process quantum information using light. These circuits can include components for generating, manipulating, and detecting single photons, enabling the implementation of quantum algorithms and quantum communication protocols.Expand Specific Solutions04 Silicon photonics in optical interconnects
Silicon photonics technology is utilized to create high-bandwidth optical interconnects for chip-to-chip and intra-chip communication. These interconnects offer advantages over traditional electrical interconnects in terms of speed, power efficiency, and bandwidth. The integration of optical components with electronic circuits enables seamless communication between different parts of a computing system.Expand Specific Solutions05 Hybrid integration of III-V materials with silicon photonics
Researchers are exploring the integration of III-V semiconductor materials with silicon photonics platforms to overcome some limitations of pure silicon-based devices. This hybrid approach combines the light-emitting properties of III-V materials with the processing advantages of silicon, enabling the development of on-chip lasers and high-performance photodetectors for silicon photonic circuits.Expand Specific Solutions
Key Players in Silicon Photonics and Medical Diagnostics
The role of silicon photonics in non-invasive medical testing is gaining momentum in a rapidly evolving market. The industry is in its growth phase, with increasing adoption across healthcare applications. The global silicon photonics market is projected to expand significantly, driven by demand for advanced medical diagnostics. Technologically, silicon photonics is maturing, with key players like Huawei, Toshiba, and Texas Instruments advancing its capabilities. Universities such as MIT and the University of Washington are also contributing to research and development. While still emerging, the technology shows promise for enabling more accurate, efficient, and cost-effective non-invasive medical tests, positioning it as a potentially disruptive force in healthcare diagnostics.
Toshiba Corp.
Technical Solution: Toshiba has developed silicon photonics technology for non-invasive medical testing, focusing on compact, high-performance optical sensors. Their approach utilizes integrated photonic circuits on silicon chips to detect biomarkers in bodily fluids with high sensitivity and specificity. Toshiba's platform employs advanced waveguide structures and resonators to enhance light-matter interactions, improving detection limits for various biomolecules. The company has also explored the use of silicon photonics for spectroscopic analysis, enabling label-free detection of multiple analytes simultaneously[6]. Toshiba's technology aims to enable rapid, on-site testing for various medical conditions, potentially reducing the need for invasive procedures and improving patient outcomes[7].
Strengths: High integration potential with existing semiconductor manufacturing processes; potential for multiplexed sensing. Weaknesses: May require specialized equipment for sample preparation and analysis; limited to optical detection methods.
Huawei Technologies Co., Ltd.
Technical Solution: Huawei has developed silicon photonics technology for non-invasive medical testing, focusing on high-speed, low-power optical sensing platforms. Their approach utilizes integrated photonic circuits on silicon chips to detect biomarkers in bodily fluids with high sensitivity and rapid response times. Huawei's platform employs advanced modulation techniques and signal processing algorithms to enhance the detection of weak optical signals from biomolecule interactions. The company has also explored the use of artificial intelligence and machine learning to improve the accuracy and reliability of their diagnostic systems[8]. Huawei's technology aims to enable real-time, continuous monitoring of various health parameters, potentially revolutionizing personalized medicine and remote patient care[9].
Strengths: Advanced signal processing and AI integration for improved accuracy; potential for real-time, continuous monitoring. Weaknesses: May face regulatory challenges in some markets due to data privacy concerns; limited clinical validation compared to established medical device manufacturers.
Core Innovations in Silicon Photonics for Medical Diagnostics
Photonic pathogen detection
PatentActiveUS20200408785A1
Innovation
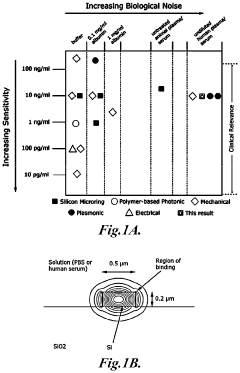
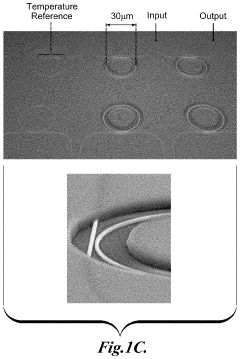
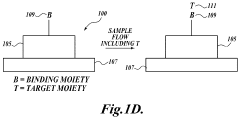
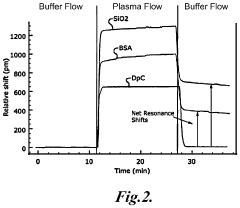
- The use of zwitterionic polymer-based surface chemistry on silicon microring resonators reduces non-specific protein adsorption, enabling label-free biosensing with clinically relevant sensitivity in undiluted human serum by configuring the binding coating to bind pathogen-associated moieties or antibodies indicative of immune responses, allowing for the detection of pathogens and immune responses in complex biological samples.
Photonic blood typing
PatentWO2013013220A2
Innovation
- The development of a photonic device with a zwitterionic polymer-based surface chemistry on silicon microring resonators that reduces non-specific protein adsorption, enabling label-free biosensing with clinically relevant sensitivity in undiluted human serum by extending the evanescent field to detect target moieties indicative of blood type.
Regulatory Framework for Silicon Photonics in Healthcare
The regulatory framework for silicon photonics in healthcare is a complex and evolving landscape that plays a crucial role in shaping the development and adoption of this technology in medical applications. As silicon photonics continues to advance in non-invasive medical testing, regulatory bodies worldwide are working to establish guidelines and standards to ensure patient safety, device efficacy, and data privacy.
In the United States, the Food and Drug Administration (FDA) is the primary regulatory agency overseeing medical devices, including those incorporating silicon photonics technology. The FDA has established a risk-based classification system for medical devices, with Class I, II, and III designations. Silicon photonics-based devices for non-invasive medical testing typically fall under Class II, requiring premarket notification (510(k)) or de novo classification, depending on their novelty and risk profile.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which came into full effect in 2021 and 2022, respectively. These regulations provide a comprehensive framework for the assessment and approval of medical devices, including those utilizing silicon photonics. The CE marking process ensures that devices meet essential safety and performance requirements before entering the European market.
In Asia, countries like Japan and China have their own regulatory frameworks. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) oversees the approval process for medical devices, while China's National Medical Products Administration (NMPA) regulates medical devices through a classification system similar to that of the FDA.
One of the key challenges in regulating silicon photonics in healthcare is the rapid pace of technological advancement. Regulatory agencies must strike a balance between ensuring patient safety and fostering innovation. To address this, many agencies have implemented expedited review processes for breakthrough technologies, allowing for faster market entry while maintaining rigorous safety standards.
Data privacy and security regulations also play a significant role in the regulatory framework for silicon photonics in healthcare. As these devices often collect and transmit sensitive patient data, compliance with regulations such as the General Data Protection Regulation (GDPR) in the EU and the Health Insurance Portability and Accountability Act (HIPAA) in the US is essential.
Standardization efforts are underway to establish common protocols and specifications for silicon photonics in medical applications. Organizations such as the International Electrotechnical Commission (IEC) and the Institute of Electrical and Electronics Engineers (IEEE) are working on developing standards that will facilitate interoperability and ensure consistent performance across different devices and manufacturers.
In the United States, the Food and Drug Administration (FDA) is the primary regulatory agency overseeing medical devices, including those incorporating silicon photonics technology. The FDA has established a risk-based classification system for medical devices, with Class I, II, and III designations. Silicon photonics-based devices for non-invasive medical testing typically fall under Class II, requiring premarket notification (510(k)) or de novo classification, depending on their novelty and risk profile.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which came into full effect in 2021 and 2022, respectively. These regulations provide a comprehensive framework for the assessment and approval of medical devices, including those utilizing silicon photonics. The CE marking process ensures that devices meet essential safety and performance requirements before entering the European market.
In Asia, countries like Japan and China have their own regulatory frameworks. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) oversees the approval process for medical devices, while China's National Medical Products Administration (NMPA) regulates medical devices through a classification system similar to that of the FDA.
One of the key challenges in regulating silicon photonics in healthcare is the rapid pace of technological advancement. Regulatory agencies must strike a balance between ensuring patient safety and fostering innovation. To address this, many agencies have implemented expedited review processes for breakthrough technologies, allowing for faster market entry while maintaining rigorous safety standards.
Data privacy and security regulations also play a significant role in the regulatory framework for silicon photonics in healthcare. As these devices often collect and transmit sensitive patient data, compliance with regulations such as the General Data Protection Regulation (GDPR) in the EU and the Health Insurance Portability and Accountability Act (HIPAA) in the US is essential.
Standardization efforts are underway to establish common protocols and specifications for silicon photonics in medical applications. Organizations such as the International Electrotechnical Commission (IEC) and the Institute of Electrical and Electronics Engineers (IEEE) are working on developing standards that will facilitate interoperability and ensure consistent performance across different devices and manufacturers.
Ethical Implications of Non-Invasive Testing Technologies
The integration of silicon photonics in non-invasive medical testing brings forth a range of ethical considerations that must be carefully addressed. As this technology advances, it offers unprecedented capabilities for early disease detection and continuous health monitoring, potentially revolutionizing preventive healthcare. However, these advancements also raise significant ethical concerns that require thorough examination and proactive management.
One primary ethical consideration is the potential for unintended consequences in patient care. While non-invasive testing using silicon photonics promises to reduce physical discomfort and risks associated with traditional invasive procedures, it may lead to overdiagnosis or unnecessary treatments. The high sensitivity of these devices could detect minor abnormalities that may never develop into clinically significant conditions, potentially causing undue stress and leading to overtreatment.
Privacy and data security present another critical ethical challenge. The vast amount of personal health data generated by silicon photonics-based devices necessitates robust safeguards to protect patient confidentiality. There is a risk that this sensitive information could be vulnerable to breaches or misuse, potentially leading to discrimination in employment, insurance, or other areas of life. Ensuring proper data governance and establishing clear guidelines for data ownership and usage are essential to maintain public trust in these technologies.
The issue of equitable access to advanced non-invasive testing technologies is also a significant ethical concern. As silicon photonics-based devices become more sophisticated and potentially more expensive, there is a risk of exacerbating existing healthcare disparities. Ensuring that these technologies are accessible to all segments of society, regardless of socioeconomic status, is crucial to prevent the widening of health inequalities.
Furthermore, the rapid advancement of non-invasive testing technologies raises questions about informed consent and patient autonomy. As these tests become more comprehensive and capable of detecting a wide range of conditions, patients may face complex decisions about what information they want to know about their health. Balancing the right to know with the right not to know becomes increasingly challenging, particularly when dealing with predictive or probabilistic health information.
Lastly, the potential for these technologies to be used for purposes beyond their intended medical applications raises ethical concerns. For instance, the use of non-invasive testing for employee screening or insurance risk assessment could lead to discrimination and infringement of personal liberties. Establishing clear regulatory frameworks and ethical guidelines for the development and application of silicon photonics in non-invasive testing is crucial to ensure that these technologies are used responsibly and in ways that benefit society as a whole.
One primary ethical consideration is the potential for unintended consequences in patient care. While non-invasive testing using silicon photonics promises to reduce physical discomfort and risks associated with traditional invasive procedures, it may lead to overdiagnosis or unnecessary treatments. The high sensitivity of these devices could detect minor abnormalities that may never develop into clinically significant conditions, potentially causing undue stress and leading to overtreatment.
Privacy and data security present another critical ethical challenge. The vast amount of personal health data generated by silicon photonics-based devices necessitates robust safeguards to protect patient confidentiality. There is a risk that this sensitive information could be vulnerable to breaches or misuse, potentially leading to discrimination in employment, insurance, or other areas of life. Ensuring proper data governance and establishing clear guidelines for data ownership and usage are essential to maintain public trust in these technologies.
The issue of equitable access to advanced non-invasive testing technologies is also a significant ethical concern. As silicon photonics-based devices become more sophisticated and potentially more expensive, there is a risk of exacerbating existing healthcare disparities. Ensuring that these technologies are accessible to all segments of society, regardless of socioeconomic status, is crucial to prevent the widening of health inequalities.
Furthermore, the rapid advancement of non-invasive testing technologies raises questions about informed consent and patient autonomy. As these tests become more comprehensive and capable of detecting a wide range of conditions, patients may face complex decisions about what information they want to know about their health. Balancing the right to know with the right not to know becomes increasingly challenging, particularly when dealing with predictive or probabilistic health information.
Lastly, the potential for these technologies to be used for purposes beyond their intended medical applications raises ethical concerns. For instance, the use of non-invasive testing for employee screening or insurance risk assessment could lead to discrimination and infringement of personal liberties. Establishing clear regulatory frameworks and ethical guidelines for the development and application of silicon photonics in non-invasive testing is crucial to ensure that these technologies are used responsibly and in ways that benefit society as a whole.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!