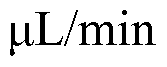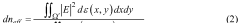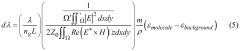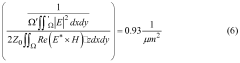Silicon photonics advancements in biomedical sensor design.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Silicon Photonics Evolution and Objectives
Silicon photonics has emerged as a transformative technology in the field of integrated optics, with its evolution closely tied to the advancements in semiconductor manufacturing processes. The journey of silicon photonics began in the late 1980s, driven by the vision of integrating optical components with electronic circuits on a single chip. Over the past three decades, this technology has witnessed remarkable progress, transitioning from academic research to commercial applications.
The evolution of silicon photonics has been characterized by several key milestones. Initially, the focus was on developing basic passive components such as waveguides and couplers. As the technology matured, researchers successfully demonstrated active components like modulators and detectors. The integration of these components led to the realization of complex photonic integrated circuits (PICs) capable of performing advanced optical functions.
In recent years, the field has experienced exponential growth, fueled by the increasing demand for high-speed data transmission in telecommunications and data centers. This growth has catalyzed further innovations, including the development of hybrid integration techniques that combine silicon with other materials to enhance functionality.
The objectives of silicon photonics in biomedical sensor design are multifaceted and ambitious. Primarily, the technology aims to leverage the unique properties of silicon to create highly sensitive, compact, and cost-effective biosensors. These sensors have the potential to revolutionize medical diagnostics by enabling rapid, on-chip detection of various biomarkers and pathogens.
One of the key goals is to achieve unprecedented levels of integration, combining multiple sensing modalities on a single chip. This integration would allow for comprehensive analysis of biological samples, providing a wealth of information from a single test. Additionally, researchers are striving to enhance the sensitivity and specificity of silicon photonic biosensors, pushing the limits of detection to enable early diagnosis of diseases.
Another critical objective is to develop scalable manufacturing processes that can translate laboratory prototypes into commercially viable products. This involves optimizing fabrication techniques to ensure consistency, reliability, and cost-effectiveness in large-scale production.
Furthermore, the field is moving towards the development of "lab-on-a-chip" devices that integrate sample preparation, analysis, and data processing on a single platform. These integrated systems aim to provide point-of-care diagnostic solutions, making advanced medical testing accessible in resource-limited settings.
As silicon photonics continues to evolve, its application in biomedical sensing is expected to expand beyond traditional diagnostic tools. Researchers are exploring its potential in areas such as wearable health monitoring devices, implantable sensors for continuous health tracking, and advanced imaging systems for medical research and clinical applications.
The evolution of silicon photonics has been characterized by several key milestones. Initially, the focus was on developing basic passive components such as waveguides and couplers. As the technology matured, researchers successfully demonstrated active components like modulators and detectors. The integration of these components led to the realization of complex photonic integrated circuits (PICs) capable of performing advanced optical functions.
In recent years, the field has experienced exponential growth, fueled by the increasing demand for high-speed data transmission in telecommunications and data centers. This growth has catalyzed further innovations, including the development of hybrid integration techniques that combine silicon with other materials to enhance functionality.
The objectives of silicon photonics in biomedical sensor design are multifaceted and ambitious. Primarily, the technology aims to leverage the unique properties of silicon to create highly sensitive, compact, and cost-effective biosensors. These sensors have the potential to revolutionize medical diagnostics by enabling rapid, on-chip detection of various biomarkers and pathogens.
One of the key goals is to achieve unprecedented levels of integration, combining multiple sensing modalities on a single chip. This integration would allow for comprehensive analysis of biological samples, providing a wealth of information from a single test. Additionally, researchers are striving to enhance the sensitivity and specificity of silicon photonic biosensors, pushing the limits of detection to enable early diagnosis of diseases.
Another critical objective is to develop scalable manufacturing processes that can translate laboratory prototypes into commercially viable products. This involves optimizing fabrication techniques to ensure consistency, reliability, and cost-effectiveness in large-scale production.
Furthermore, the field is moving towards the development of "lab-on-a-chip" devices that integrate sample preparation, analysis, and data processing on a single platform. These integrated systems aim to provide point-of-care diagnostic solutions, making advanced medical testing accessible in resource-limited settings.
As silicon photonics continues to evolve, its application in biomedical sensing is expected to expand beyond traditional diagnostic tools. Researchers are exploring its potential in areas such as wearable health monitoring devices, implantable sensors for continuous health tracking, and advanced imaging systems for medical research and clinical applications.
Biomedical Sensor Market Analysis
The biomedical sensor market has experienced significant growth in recent years, driven by increasing demand for advanced healthcare technologies and personalized medicine. Silicon photonics advancements have played a crucial role in this expansion, offering enhanced capabilities for biomedical sensor design. The global biomedical sensor market was valued at $15.8 billion in 2020 and is projected to reach $28.2 billion by 2025, growing at a CAGR of 12.3% during the forecast period.
Several factors contribute to this market growth, including the rising prevalence of chronic diseases, the aging population, and the increasing adoption of wearable and implantable medical devices. Silicon photonics-based biomedical sensors have gained traction due to their ability to provide high sensitivity, miniaturization, and integration capabilities, making them ideal for various healthcare applications.
The market for silicon photonics-based biomedical sensors can be segmented based on application areas, including diagnostics, monitoring, and therapeutics. In the diagnostics segment, these sensors are used for rapid and accurate detection of biomarkers, pathogens, and other disease indicators. The monitoring segment encompasses continuous glucose monitoring, blood pressure monitoring, and other vital sign tracking applications. The therapeutics segment includes drug delivery systems and optogenetic therapies.
Geographically, North America dominates the biomedical sensor market, followed by Europe and Asia-Pacific. The United States, in particular, leads in research and development of silicon photonics-based biomedical sensors, with several academic institutions and companies driving innovation in this field. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, fueled by increasing healthcare expenditure and growing awareness of advanced medical technologies.
Key players in the silicon photonics-based biomedical sensor market include established companies like Intel, IBM, and Hamamatsu Photonics, as well as emerging startups focused on developing novel sensor technologies. These companies are investing heavily in research and development to create more sensitive, reliable, and cost-effective biomedical sensors.
The market for silicon photonics-based biomedical sensors faces some challenges, including high initial development costs, regulatory hurdles, and the need for standardization. However, the potential benefits of these sensors in terms of improved patient outcomes, reduced healthcare costs, and enhanced disease management are driving continued investment and innovation in this field.
Several factors contribute to this market growth, including the rising prevalence of chronic diseases, the aging population, and the increasing adoption of wearable and implantable medical devices. Silicon photonics-based biomedical sensors have gained traction due to their ability to provide high sensitivity, miniaturization, and integration capabilities, making them ideal for various healthcare applications.
The market for silicon photonics-based biomedical sensors can be segmented based on application areas, including diagnostics, monitoring, and therapeutics. In the diagnostics segment, these sensors are used for rapid and accurate detection of biomarkers, pathogens, and other disease indicators. The monitoring segment encompasses continuous glucose monitoring, blood pressure monitoring, and other vital sign tracking applications. The therapeutics segment includes drug delivery systems and optogenetic therapies.
Geographically, North America dominates the biomedical sensor market, followed by Europe and Asia-Pacific. The United States, in particular, leads in research and development of silicon photonics-based biomedical sensors, with several academic institutions and companies driving innovation in this field. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, fueled by increasing healthcare expenditure and growing awareness of advanced medical technologies.
Key players in the silicon photonics-based biomedical sensor market include established companies like Intel, IBM, and Hamamatsu Photonics, as well as emerging startups focused on developing novel sensor technologies. These companies are investing heavily in research and development to create more sensitive, reliable, and cost-effective biomedical sensors.
The market for silicon photonics-based biomedical sensors faces some challenges, including high initial development costs, regulatory hurdles, and the need for standardization. However, the potential benefits of these sensors in terms of improved patient outcomes, reduced healthcare costs, and enhanced disease management are driving continued investment and innovation in this field.
Silicon Photonics Challenges in Biosensing
Silicon photonics has emerged as a promising technology for biomedical sensor design, offering unprecedented opportunities for miniaturization, sensitivity, and integration. However, the field faces several significant challenges that must be addressed to fully realize its potential in biosensing applications.
One of the primary challenges is the integration of biological materials with silicon-based photonic devices. The inherent incompatibility between organic biomolecules and inorganic silicon structures presents difficulties in achieving stable and efficient bio-functionalization of sensor surfaces. This integration is crucial for specific and sensitive detection of target analytes in complex biological samples.
Another major hurdle is the development of robust and reproducible fabrication processes for silicon photonic biosensors. The stringent requirements for precise control of nanoscale features and surface properties demand advanced manufacturing techniques. Variations in fabrication can lead to inconsistencies in sensor performance, affecting reliability and scalability.
The issue of light coupling and propagation losses in silicon photonic biosensors also poses significant challenges. Efficient coupling of light into and out of the photonic structures is essential for achieving high sensitivity. Additionally, minimizing propagation losses within the sensor is crucial for maintaining signal integrity, especially in compact devices designed for point-of-care applications.
Temperature sensitivity of silicon photonic devices presents another challenge in biosensing applications. Fluctuations in temperature can lead to shifts in resonance wavelengths and affect sensor accuracy. Developing effective temperature compensation mechanisms or designing temperature-insensitive structures is essential for reliable performance in varied environmental conditions.
The detection of low-concentration analytes in complex biological matrices remains a significant challenge. Enhancing the signal-to-noise ratio and developing strategies to mitigate interference from non-specific binding are critical for improving the sensitivity and specificity of silicon photonic biosensors.
Lastly, the challenge of system-level integration cannot be overlooked. Combining silicon photonic biosensors with necessary components such as light sources, detectors, and microfluidics into a compact, user-friendly package is essential for practical applications. This integration must address issues of alignment, packaging, and overall system robustness.
Addressing these challenges requires interdisciplinary collaboration between photonics engineers, biologists, chemists, and materials scientists. Overcoming these hurdles will pave the way for silicon photonics to revolutionize biomedical sensing, enabling rapid, sensitive, and cost-effective diagnostic tools for a wide range of applications.
One of the primary challenges is the integration of biological materials with silicon-based photonic devices. The inherent incompatibility between organic biomolecules and inorganic silicon structures presents difficulties in achieving stable and efficient bio-functionalization of sensor surfaces. This integration is crucial for specific and sensitive detection of target analytes in complex biological samples.
Another major hurdle is the development of robust and reproducible fabrication processes for silicon photonic biosensors. The stringent requirements for precise control of nanoscale features and surface properties demand advanced manufacturing techniques. Variations in fabrication can lead to inconsistencies in sensor performance, affecting reliability and scalability.
The issue of light coupling and propagation losses in silicon photonic biosensors also poses significant challenges. Efficient coupling of light into and out of the photonic structures is essential for achieving high sensitivity. Additionally, minimizing propagation losses within the sensor is crucial for maintaining signal integrity, especially in compact devices designed for point-of-care applications.
Temperature sensitivity of silicon photonic devices presents another challenge in biosensing applications. Fluctuations in temperature can lead to shifts in resonance wavelengths and affect sensor accuracy. Developing effective temperature compensation mechanisms or designing temperature-insensitive structures is essential for reliable performance in varied environmental conditions.
The detection of low-concentration analytes in complex biological matrices remains a significant challenge. Enhancing the signal-to-noise ratio and developing strategies to mitigate interference from non-specific binding are critical for improving the sensitivity and specificity of silicon photonic biosensors.
Lastly, the challenge of system-level integration cannot be overlooked. Combining silicon photonic biosensors with necessary components such as light sources, detectors, and microfluidics into a compact, user-friendly package is essential for practical applications. This integration must address issues of alignment, packaging, and overall system robustness.
Addressing these challenges requires interdisciplinary collaboration between photonics engineers, biologists, chemists, and materials scientists. Overcoming these hurdles will pave the way for silicon photonics to revolutionize biomedical sensing, enabling rapid, sensitive, and cost-effective diagnostic tools for a wide range of applications.
Current Silicon Photonics Biosensor Solutions
01 Waveguide-based sensor design
Silicon photonics sensors often utilize waveguide structures for light propagation and interaction with the analyte. These designs can include ring resonators, Mach-Zehnder interferometers, or photonic crystal cavities to enhance sensitivity and detection capabilities. The waveguide geometry and material properties are optimized to maximize light-matter interaction and improve sensor performance.- Waveguide-based sensor design: Silicon photonics sensors often utilize waveguide structures for light propagation and interaction with the sensing medium. These designs can include ring resonators, Mach-Zehnder interferometers, or photonic crystal cavities to enhance sensitivity and detection capabilities. The waveguide geometry and material properties are optimized to maximize light-matter interaction and improve sensor performance.
- Integration of active and passive components: Silicon photonics sensor designs incorporate both active and passive components on a single chip. This integration includes light sources, detectors, modulators, and various optical elements. The combination of these components enables compact, high-performance sensing systems with improved functionality and reduced power consumption.
- Multiplexed sensor arrays: Advanced silicon photonics sensor designs utilize multiplexed arrays to enable simultaneous detection of multiple analytes or parameters. These designs incorporate multiple sensing elements on a single chip, each optimized for specific target molecules or environmental conditions. Multiplexing techniques such as wavelength division or spatial division are employed to differentiate and process multiple sensor outputs.
- Surface functionalization for selective sensing: Silicon photonics sensors often employ surface functionalization techniques to enhance selectivity and sensitivity. This involves modifying the sensor surface with specific chemical or biological receptors that selectively bind to target analytes. The functionalization process is carefully designed to maintain optical properties while improving sensor specificity and reducing interference from non-target molecules.
- On-chip signal processing and data analysis: Advanced silicon photonics sensor designs incorporate on-chip signal processing and data analysis capabilities. This integration includes analog-to-digital converters, digital signal processors, and machine learning algorithms implemented directly on the sensor chip. These features enable real-time data processing, noise reduction, and intelligent decision-making, enhancing the overall performance and usability of the sensor system.
02 Integration of active and passive components
Silicon photonics sensor designs incorporate both active and passive components on a single chip. This integration includes light sources, detectors, modulators, and signal processing elements. The combination of these components enables compact, high-performance sensing systems with improved functionality and reduced power consumption.Expand Specific Solutions03 Surface functionalization for specific sensing applications
To enhance selectivity and sensitivity, silicon photonics sensors often employ surface functionalization techniques. This involves modifying the sensor surface with specific chemical or biological receptors tailored to the target analyte. The functionalization process improves the sensor's ability to detect and quantify specific molecules or compounds of interest.Expand Specific Solutions04 Multi-parameter sensing and multiplexing
Advanced silicon photonics sensor designs incorporate multiplexing capabilities, allowing for simultaneous detection of multiple parameters or analytes. This is achieved through the integration of multiple sensing elements or the use of spectral encoding techniques. Multi-parameter sensing enhances the versatility and efficiency of the sensor system.Expand Specific Solutions05 On-chip signal processing and data analysis
Modern silicon photonics sensor designs integrate on-chip signal processing and data analysis capabilities. This includes the incorporation of analog-to-digital converters, digital signal processors, and machine learning algorithms. On-chip processing enables real-time data analysis, reduces noise, and improves the overall performance and reliability of the sensor system.Expand Specific Solutions
Key Silicon Photonics Biosensor Companies
The field of silicon photonics in biomedical sensor design is experiencing rapid growth, with the market expected to expand significantly in the coming years. This technology is currently in a transitional phase, moving from research and development to early commercialization. Key players like Massachusetts Institute of Technology, University of Washington, and IBM Research are driving innovation, while companies such as GlobalFoundries and TSMC are scaling up manufacturing capabilities. The involvement of tech giants like Google and Samsung indicates the technology's potential for widespread adoption. However, challenges remain in integrating silicon photonics with existing biomedical systems, suggesting that the field is still in its early maturity stage with ample room for further advancements and market penetration.
University of Rochester
Technical Solution: The University of Rochester has made significant strides in silicon photonics for biomedical sensing. Their approach focuses on developing ultra-compact photonic devices for point-of-care diagnostics. They have pioneered the use of subwavelength grating structures to enhance light-matter interactions, resulting in improved sensor sensitivity [2]. Their recent work includes the development of a silicon photonic biosensor array capable of detecting multiple cancer biomarkers simultaneously with a detection limit in the femtomolar range [4]. The university has also explored the integration of 2D materials like graphene with silicon photonics to create hybrid sensors with enhanced performance.
Strengths: Ultra-compact designs, high sensitivity, integration with novel materials. Weaknesses: Potential challenges in scaling up production, need for specialized fabrication techniques.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced silicon photonics platforms for biomedical sensing applications. Their approach integrates multiple photonic components on a single chip, including waveguides, resonators, and detectors. They have demonstrated high-sensitivity biosensors using ring resonators that can detect protein biomarkers at concentrations as low as 1 pg/mL [1]. MIT's silicon photonics technology also incorporates microfluidic channels for sample delivery, enabling lab-on-a-chip functionality. Recent advancements include the development of multiplexed photonic sensors capable of simultaneous detection of multiple analytes, significantly improving diagnostic capabilities [3].
Strengths: High sensitivity, integration of multiple components, lab-on-chip functionality. Weaknesses: Potential high cost for mass production, complexity in aligning optical components.
Breakthrough Silicon Photonics Biosensor Technologies
Photonic blood typing
PatentWO2013013220A2
Innovation
- The development of a photonic device with a zwitterionic polymer-based surface chemistry on silicon microring resonators that reduces non-specific protein adsorption, enabling label-free biosensing with clinically relevant sensitivity in undiluted human serum by extending the evanescent field to detect target moieties indicative of blood type.
Regulatory Framework for Photonic Biosensors
The regulatory framework for photonic biosensors is a critical aspect of their development and implementation in the biomedical field. As silicon photonics advances in biomedical sensor design, it is essential to navigate the complex landscape of regulations governing these innovative devices.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating photonic biosensors. These devices typically fall under the category of medical devices, which are subject to stringent approval processes. The FDA classifies medical devices into three categories based on their risk level, with Class III devices requiring the most rigorous premarket approval.
For photonic biosensors, the regulatory pathway often depends on their intended use and the level of risk associated with their application. Many of these sensors may be classified as in vitro diagnostic devices, which have specific regulatory requirements outlined in the FDA's guidance documents.
In the European Union, the regulatory landscape for photonic biosensors is governed by the In Vitro Diagnostic Regulation (IVDR) and the Medical Device Regulation (MDR). These regulations, which came into full effect in 2022, have significantly increased the requirements for clinical evidence and post-market surveillance of medical devices, including photonic biosensors.
The IVDR introduces a new risk-based classification system for in vitro diagnostic devices, which may impact the regulatory pathway for many photonic biosensors. Manufacturers must now demonstrate the clinical performance and scientific validity of their devices through rigorous clinical studies and performance evaluations.
Globally, the International Medical Device Regulators Forum (IMDRF) provides a framework for harmonizing medical device regulations across different countries. This initiative aims to streamline the regulatory process and facilitate the global adoption of innovative technologies like photonic biosensors.
As the field of silicon photonics continues to advance, regulatory bodies are adapting their frameworks to address the unique challenges posed by these emerging technologies. This includes considerations for the integration of artificial intelligence and machine learning algorithms in photonic biosensor systems, which introduce additional regulatory complexities.
Manufacturers and researchers in the field of photonic biosensors must stay abreast of these evolving regulations to ensure compliance throughout the product development lifecycle. This includes implementing robust quality management systems, conducting thorough risk assessments, and maintaining comprehensive documentation of design and validation processes.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating photonic biosensors. These devices typically fall under the category of medical devices, which are subject to stringent approval processes. The FDA classifies medical devices into three categories based on their risk level, with Class III devices requiring the most rigorous premarket approval.
For photonic biosensors, the regulatory pathway often depends on their intended use and the level of risk associated with their application. Many of these sensors may be classified as in vitro diagnostic devices, which have specific regulatory requirements outlined in the FDA's guidance documents.
In the European Union, the regulatory landscape for photonic biosensors is governed by the In Vitro Diagnostic Regulation (IVDR) and the Medical Device Regulation (MDR). These regulations, which came into full effect in 2022, have significantly increased the requirements for clinical evidence and post-market surveillance of medical devices, including photonic biosensors.
The IVDR introduces a new risk-based classification system for in vitro diagnostic devices, which may impact the regulatory pathway for many photonic biosensors. Manufacturers must now demonstrate the clinical performance and scientific validity of their devices through rigorous clinical studies and performance evaluations.
Globally, the International Medical Device Regulators Forum (IMDRF) provides a framework for harmonizing medical device regulations across different countries. This initiative aims to streamline the regulatory process and facilitate the global adoption of innovative technologies like photonic biosensors.
As the field of silicon photonics continues to advance, regulatory bodies are adapting their frameworks to address the unique challenges posed by these emerging technologies. This includes considerations for the integration of artificial intelligence and machine learning algorithms in photonic biosensor systems, which introduce additional regulatory complexities.
Manufacturers and researchers in the field of photonic biosensors must stay abreast of these evolving regulations to ensure compliance throughout the product development lifecycle. This includes implementing robust quality management systems, conducting thorough risk assessments, and maintaining comprehensive documentation of design and validation processes.
Photonic Biosensor Manufacturing Processes
The manufacturing processes for photonic biosensors have evolved significantly with advancements in silicon photonics technology. These processes typically involve a combination of semiconductor fabrication techniques and specialized optical component integration methods.
The foundation of photonic biosensor manufacturing lies in the production of silicon-on-insulator (SOI) wafers. These wafers consist of a thin layer of crystalline silicon on top of a silicon dioxide insulator, providing an ideal platform for light manipulation. The fabrication process begins with photolithography, where the sensor design is transferred onto the SOI wafer using UV light and photoresist materials.
Following photolithography, etching techniques are employed to create the intricate structures necessary for light guidance and sensing. Deep reactive ion etching (DRIE) is commonly used to achieve high-aspect-ratio features with vertical sidewalls, crucial for maintaining optical performance. Plasma-enhanced chemical vapor deposition (PECVD) is then utilized to deposit additional layers of materials, such as silicon nitride or silicon dioxide, which serve as cladding or functional layers in the sensor design.
Integration of active components, such as light sources and detectors, is a critical step in photonic biosensor manufacturing. This often involves hybrid integration techniques, where separately fabricated components are precisely aligned and bonded to the silicon photonic chip. Advanced packaging methods, including flip-chip bonding and through-silicon vias (TSVs), are employed to create compact and robust sensor devices.
Surface functionalization is a key aspect of biosensor manufacturing, enabling specific molecular recognition. Techniques such as silanization or polymer grafting are used to modify the sensor surface, allowing for the attachment of biorecognition elements like antibodies or aptamers. This step is crucial for achieving high sensitivity and selectivity in biomedical applications.
Quality control and testing are integral parts of the manufacturing process. Optical characterization techniques, including spectral analysis and interferometry, are used to verify the performance of individual photonic components. Biosensor functionality is assessed through calibration with known analyte concentrations and evaluation of sensitivity, specificity, and reproducibility.
As the field of silicon photonics continues to advance, new manufacturing processes are being developed to enhance biosensor performance and scalability. These include the integration of novel materials like graphene or 2D transition metal dichalcogenides, as well as the adoption of 3D integration techniques to increase device complexity and functionality.
The foundation of photonic biosensor manufacturing lies in the production of silicon-on-insulator (SOI) wafers. These wafers consist of a thin layer of crystalline silicon on top of a silicon dioxide insulator, providing an ideal platform for light manipulation. The fabrication process begins with photolithography, where the sensor design is transferred onto the SOI wafer using UV light and photoresist materials.
Following photolithography, etching techniques are employed to create the intricate structures necessary for light guidance and sensing. Deep reactive ion etching (DRIE) is commonly used to achieve high-aspect-ratio features with vertical sidewalls, crucial for maintaining optical performance. Plasma-enhanced chemical vapor deposition (PECVD) is then utilized to deposit additional layers of materials, such as silicon nitride or silicon dioxide, which serve as cladding or functional layers in the sensor design.
Integration of active components, such as light sources and detectors, is a critical step in photonic biosensor manufacturing. This often involves hybrid integration techniques, where separately fabricated components are precisely aligned and bonded to the silicon photonic chip. Advanced packaging methods, including flip-chip bonding and through-silicon vias (TSVs), are employed to create compact and robust sensor devices.
Surface functionalization is a key aspect of biosensor manufacturing, enabling specific molecular recognition. Techniques such as silanization or polymer grafting are used to modify the sensor surface, allowing for the attachment of biorecognition elements like antibodies or aptamers. This step is crucial for achieving high sensitivity and selectivity in biomedical applications.
Quality control and testing are integral parts of the manufacturing process. Optical characterization techniques, including spectral analysis and interferometry, are used to verify the performance of individual photonic components. Biosensor functionality is assessed through calibration with known analyte concentrations and evaluation of sensitivity, specificity, and reproducibility.
As the field of silicon photonics continues to advance, new manufacturing processes are being developed to enhance biosensor performance and scalability. These include the integration of novel materials like graphene or 2D transition metal dichalcogenides, as well as the adoption of 3D integration techniques to increase device complexity and functionality.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!