Separation Techniques for Identifying Geometric Isomers in Organic Mixtures
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Geometric Isomer Separation: Background and Objectives
Geometric isomers, a subset of stereoisomers, have identical molecular formulas and bonding sequences but differ in the spatial arrangement of their atoms. The study of these compounds has been a crucial aspect of organic chemistry for over a century, with significant implications in various fields, including pharmaceuticals, materials science, and biochemistry.
The development of separation techniques for identifying geometric isomers in organic mixtures has evolved alongside advancements in analytical chemistry. Early methods relied on physical properties such as melting point and boiling point, which often proved inadequate for complex mixtures. The advent of chromatographic techniques in the mid-20th century marked a turning point in isomer separation, enabling more precise and efficient identification.
In recent decades, the field has witnessed remarkable progress, driven by the increasing demand for pure isomeric compounds in drug development and the need for accurate analysis in environmental monitoring. High-performance liquid chromatography (HPLC), gas chromatography (GC), and their variants have become cornerstone techniques in isomer separation. These methods exploit subtle differences in the physical and chemical properties of geometric isomers to achieve separation.
The objectives of current research in geometric isomer separation are multifaceted. Primarily, there is a push towards developing more sensitive and selective separation methods capable of resolving complex mixtures with minimal sample preparation. This includes the refinement of existing chromatographic techniques and the exploration of novel approaches such as supercritical fluid chromatography and capillary electrophoresis.
Another key goal is the miniaturization and automation of separation processes, aiming to reduce analysis time, sample volume requirements, and overall costs. This trend aligns with the broader movement towards lab-on-a-chip technologies and high-throughput screening in pharmaceutical research.
Furthermore, there is a growing emphasis on developing green separation techniques that minimize the use of harmful solvents and reduce environmental impact. This objective reflects the increasing awareness of sustainability in scientific research and industrial applications.
The integration of separation techniques with advanced detection methods, such as mass spectrometry and nuclear magnetic resonance spectroscopy, represents another critical area of development. These hyphenated techniques aim to provide not only separation but also structural elucidation of geometric isomers in a single analytical run.
As we look to the future, the field of geometric isomer separation is poised for further innovation. Emerging technologies, including machine learning algorithms for method optimization and predictive modeling of separation behavior, are expected to play a significant role in advancing the field. The ultimate aim is to develop robust, versatile, and efficient separation techniques that can meet the diverse needs of researchers and industries dealing with complex organic mixtures.
The development of separation techniques for identifying geometric isomers in organic mixtures has evolved alongside advancements in analytical chemistry. Early methods relied on physical properties such as melting point and boiling point, which often proved inadequate for complex mixtures. The advent of chromatographic techniques in the mid-20th century marked a turning point in isomer separation, enabling more precise and efficient identification.
In recent decades, the field has witnessed remarkable progress, driven by the increasing demand for pure isomeric compounds in drug development and the need for accurate analysis in environmental monitoring. High-performance liquid chromatography (HPLC), gas chromatography (GC), and their variants have become cornerstone techniques in isomer separation. These methods exploit subtle differences in the physical and chemical properties of geometric isomers to achieve separation.
The objectives of current research in geometric isomer separation are multifaceted. Primarily, there is a push towards developing more sensitive and selective separation methods capable of resolving complex mixtures with minimal sample preparation. This includes the refinement of existing chromatographic techniques and the exploration of novel approaches such as supercritical fluid chromatography and capillary electrophoresis.
Another key goal is the miniaturization and automation of separation processes, aiming to reduce analysis time, sample volume requirements, and overall costs. This trend aligns with the broader movement towards lab-on-a-chip technologies and high-throughput screening in pharmaceutical research.
Furthermore, there is a growing emphasis on developing green separation techniques that minimize the use of harmful solvents and reduce environmental impact. This objective reflects the increasing awareness of sustainability in scientific research and industrial applications.
The integration of separation techniques with advanced detection methods, such as mass spectrometry and nuclear magnetic resonance spectroscopy, represents another critical area of development. These hyphenated techniques aim to provide not only separation but also structural elucidation of geometric isomers in a single analytical run.
As we look to the future, the field of geometric isomer separation is poised for further innovation. Emerging technologies, including machine learning algorithms for method optimization and predictive modeling of separation behavior, are expected to play a significant role in advancing the field. The ultimate aim is to develop robust, versatile, and efficient separation techniques that can meet the diverse needs of researchers and industries dealing with complex organic mixtures.
Market Analysis for Isomer Separation Technologies
The market for isomer separation technologies has experienced significant growth in recent years, driven by increasing demand across various industries, particularly in pharmaceuticals, petrochemicals, and fine chemicals. The global market for chromatography instruments, a key technology for isomer separation, was valued at $8.6 billion in 2020 and is projected to reach $11.9 billion by 2025, growing at a CAGR of 6.7%.
Pharmaceutical companies are the largest consumers of isomer separation technologies, accounting for approximately 40% of the market share. The growing emphasis on chiral drugs and the need for high-purity compounds have fueled the demand for advanced separation techniques. The pharmaceutical industry's focus on developing single-enantiomer drugs has further boosted the market for chiral separation technologies.
In the petrochemical sector, the demand for isomer separation technologies is driven by the need to produce high-quality fuels and lubricants. The increasing regulations on fuel quality and environmental concerns have led to a greater emphasis on efficient separation processes. This sector represents about 25% of the market for isomer separation technologies.
The fine chemicals industry, including flavors and fragrances, agrochemicals, and specialty chemicals, accounts for approximately 20% of the market. The need for pure isomers in these applications has led to increased adoption of advanced separation techniques.
Geographically, North America dominates the market with a share of around 35%, followed by Europe at 30% and Asia-Pacific at 25%. The Asia-Pacific region is expected to witness the highest growth rate in the coming years due to rapid industrialization and increasing investments in research and development.
Key players in the isomer separation technology market include Agilent Technologies, Thermo Fisher Scientific, Waters Corporation, and Shimadzu Corporation. These companies are focusing on developing innovative technologies and expanding their product portfolios to maintain their market positions.
The market is characterized by a high degree of competition and technological advancements. Companies are investing heavily in research and development to improve separation efficiency, reduce processing times, and lower operational costs. There is a growing trend towards the development of miniaturized and automated separation systems, which offer advantages in terms of speed, sample size requirements, and ease of use.
Pharmaceutical companies are the largest consumers of isomer separation technologies, accounting for approximately 40% of the market share. The growing emphasis on chiral drugs and the need for high-purity compounds have fueled the demand for advanced separation techniques. The pharmaceutical industry's focus on developing single-enantiomer drugs has further boosted the market for chiral separation technologies.
In the petrochemical sector, the demand for isomer separation technologies is driven by the need to produce high-quality fuels and lubricants. The increasing regulations on fuel quality and environmental concerns have led to a greater emphasis on efficient separation processes. This sector represents about 25% of the market for isomer separation technologies.
The fine chemicals industry, including flavors and fragrances, agrochemicals, and specialty chemicals, accounts for approximately 20% of the market. The need for pure isomers in these applications has led to increased adoption of advanced separation techniques.
Geographically, North America dominates the market with a share of around 35%, followed by Europe at 30% and Asia-Pacific at 25%. The Asia-Pacific region is expected to witness the highest growth rate in the coming years due to rapid industrialization and increasing investments in research and development.
Key players in the isomer separation technology market include Agilent Technologies, Thermo Fisher Scientific, Waters Corporation, and Shimadzu Corporation. These companies are focusing on developing innovative technologies and expanding their product portfolios to maintain their market positions.
The market is characterized by a high degree of competition and technological advancements. Companies are investing heavily in research and development to improve separation efficiency, reduce processing times, and lower operational costs. There is a growing trend towards the development of miniaturized and automated separation systems, which offer advantages in terms of speed, sample size requirements, and ease of use.
Current Challenges in Geometric Isomer Identification
The identification of geometric isomers in organic mixtures presents several significant challenges that continue to perplex researchers and analytical chemists. One of the primary difficulties lies in the structural similarity between geometric isomers, which often results in nearly identical physical and chemical properties. This similarity makes traditional separation techniques, such as distillation or crystallization, largely ineffective for isolating and identifying these compounds.
Chromatographic methods, while widely used, face limitations when dealing with geometric isomers. Gas chromatography (GC) and high-performance liquid chromatography (HPLC) often struggle to achieve sufficient resolution between isomeric pairs, especially when dealing with complex mixtures. The selection of appropriate stationary phases and optimization of mobile phase compositions remain challenging, requiring extensive method development and validation processes.
Spectroscopic techniques, such as nuclear magnetic resonance (NMR) spectroscopy, also encounter obstacles in distinguishing geometric isomers. While NMR can provide valuable structural information, the spectral differences between geometric isomers can be subtle and difficult to interpret, particularly in mixtures where signal overlap is common. This challenge is exacerbated when dealing with low-concentration isomers or in the presence of interfering compounds.
Mass spectrometry, another powerful analytical tool, faces its own set of challenges in geometric isomer identification. The identical molecular masses and similar fragmentation patterns of geometric isomers often result in indistinguishable mass spectra, making definitive identification problematic. Advanced techniques like ion mobility spectrometry-mass spectrometry (IMS-MS) show promise but are still in the early stages of development for routine isomer analysis.
The development of chiral separation techniques has made significant strides in recent years, but their application to geometric isomer separation remains limited. Chiral stationary phases and chiral derivatization agents, while effective for enantiomers, often lack the specificity required to differentiate between geometric isomers.
Another challenge lies in the analysis of geometric isomers in complex biological matrices. The presence of numerous interfering compounds and the potential for isomerization during sample preparation and analysis further complicate the identification process. This is particularly problematic in fields such as metabolomics and natural product chemistry, where accurate isomer identification is crucial for understanding biological processes and developing new therapeutic agents.
Lastly, the lack of comprehensive databases and reference standards for geometric isomers hinders the development and validation of new analytical methods. The synthesis and characterization of pure geometric isomer standards can be time-consuming and costly, limiting the availability of reliable reference materials for method development and confirmation of analytical results.
Chromatographic methods, while widely used, face limitations when dealing with geometric isomers. Gas chromatography (GC) and high-performance liquid chromatography (HPLC) often struggle to achieve sufficient resolution between isomeric pairs, especially when dealing with complex mixtures. The selection of appropriate stationary phases and optimization of mobile phase compositions remain challenging, requiring extensive method development and validation processes.
Spectroscopic techniques, such as nuclear magnetic resonance (NMR) spectroscopy, also encounter obstacles in distinguishing geometric isomers. While NMR can provide valuable structural information, the spectral differences between geometric isomers can be subtle and difficult to interpret, particularly in mixtures where signal overlap is common. This challenge is exacerbated when dealing with low-concentration isomers or in the presence of interfering compounds.
Mass spectrometry, another powerful analytical tool, faces its own set of challenges in geometric isomer identification. The identical molecular masses and similar fragmentation patterns of geometric isomers often result in indistinguishable mass spectra, making definitive identification problematic. Advanced techniques like ion mobility spectrometry-mass spectrometry (IMS-MS) show promise but are still in the early stages of development for routine isomer analysis.
The development of chiral separation techniques has made significant strides in recent years, but their application to geometric isomer separation remains limited. Chiral stationary phases and chiral derivatization agents, while effective for enantiomers, often lack the specificity required to differentiate between geometric isomers.
Another challenge lies in the analysis of geometric isomers in complex biological matrices. The presence of numerous interfering compounds and the potential for isomerization during sample preparation and analysis further complicate the identification process. This is particularly problematic in fields such as metabolomics and natural product chemistry, where accurate isomer identification is crucial for understanding biological processes and developing new therapeutic agents.
Lastly, the lack of comprehensive databases and reference standards for geometric isomers hinders the development and validation of new analytical methods. The synthesis and characterization of pure geometric isomer standards can be time-consuming and costly, limiting the availability of reliable reference materials for method development and confirmation of analytical results.
Existing Methods for Geometric Isomer Separation
01 Chromatographic separation techniques
Chromatographic methods are widely used for separation and identification of compounds. These techniques involve the distribution of components between a stationary phase and a mobile phase. Various types of chromatography, such as liquid chromatography, gas chromatography, and thin-layer chromatography, can be employed depending on the nature of the sample and the desired separation.- Chromatographic separation techniques: Chromatographic methods are widely used for separating and identifying various compounds. These techniques involve the distribution of components between a stationary phase and a mobile phase. Different types of chromatography, such as gas chromatography, liquid chromatography, and thin-layer chromatography, can be employed depending on the nature of the sample and the desired separation.
- Spectroscopic identification methods: Spectroscopic techniques are powerful tools for identifying and characterizing substances based on their interaction with electromagnetic radiation. These methods include UV-visible spectroscopy, infrared spectroscopy, nuclear magnetic resonance spectroscopy, and mass spectrometry. Each technique provides unique information about the molecular structure and composition of the analyte.
- Electrophoretic separation techniques: Electrophoresis is a separation method that utilizes the differential migration of charged particles in an electric field. This technique is particularly useful for separating and identifying proteins, nucleic acids, and other biomolecules. Various forms of electrophoresis, such as gel electrophoresis and capillary electrophoresis, can be employed depending on the specific application.
- Membrane-based separation methods: Membrane-based separation techniques utilize selective permeability to separate components based on size, charge, or other physicochemical properties. These methods include ultrafiltration, nanofiltration, and reverse osmosis. Membrane separations are widely used in various industries for purification, concentration, and fractionation of complex mixtures.
- Automated identification systems: Advanced automated systems have been developed to streamline the process of separation and identification. These systems often integrate multiple analytical techniques and utilize sophisticated software algorithms for data analysis and interpretation. Such automated platforms can significantly enhance the efficiency and accuracy of compound identification in complex matrices.
02 Spectroscopic identification methods
Spectroscopic techniques are essential for identifying and characterizing separated compounds. These methods include UV-visible spectroscopy, infrared spectroscopy, nuclear magnetic resonance spectroscopy, and mass spectrometry. Each technique provides unique information about the molecular structure and composition of the analytes, allowing for accurate identification.Expand Specific Solutions03 Electrophoretic separation techniques
Electrophoresis is a separation technique based on the differential migration of charged particles in an electric field. Various forms of electrophoresis, such as gel electrophoresis, capillary electrophoresis, and isoelectric focusing, are used for separating and identifying biomolecules like proteins and nucleic acids.Expand Specific Solutions04 Membrane-based separation methods
Membrane-based separation techniques utilize selective permeability to separate mixtures. These methods include ultrafiltration, nanofiltration, and reverse osmosis. They are particularly useful for separating particles and molecules based on size, charge, or other physicochemical properties.Expand Specific Solutions05 Data analysis and pattern recognition for identification
Advanced data analysis techniques and pattern recognition algorithms are employed to process and interpret the data obtained from various separation and analytical methods. These computational approaches enhance the accuracy and efficiency of compound identification, especially in complex mixtures or when dealing with large datasets.Expand Specific Solutions
Key Players in Analytical Chemistry and Separation Industry
The field of separation techniques for identifying geometric isomers in organic mixtures is in a mature stage of development, with a well-established market and robust technological foundation. The global market for chromatography and spectroscopy techniques, key methods in this area, is substantial and growing steadily. Companies like Daicel Corp. and China Petroleum & Chemical Corp. are major players, leveraging their expertise in chemical separations. Pharmaceutical firms such as Astellas Pharma, Inc. and Pfizer Inc. are also significant contributors, applying these techniques in drug development. Academic institutions like Cornell University and research organizations like The Broad Institute, Inc. continue to drive innovation in this field, pushing the boundaries of separation technology and its applications in organic chemistry and pharmaceutical research.
Daicel Corp.
Technical Solution: Daicel Corp. has pioneered the development of chiral stationary phases for the separation of geometric isomers. Their CHIRALPAK and CHIRALCEL series of columns are widely used in the pharmaceutical and chemical industries for the separation of geometric isomers[4]. Daicel's technology is based on polysaccharide derivatives that offer unique selectivity for geometric isomers. The company has also developed immobilized chiral stationary phases that provide enhanced stability and broader solvent compatibility[5]. Recently, Daicel has introduced new mixed-mode chiral stationary phases that combine multiple separation mechanisms, allowing for improved resolution of complex mixtures containing geometric isomers[6].
Strengths: Extensive range of specialized chiral columns, high selectivity for geometric isomers, and broad industry adoption. Weaknesses: Focus primarily on chromatographic methods may limit applicability in non-chromatographic separation techniques.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed innovative separation techniques for geometric isomers in petroleum-based organic mixtures. Their approach combines advanced distillation techniques with membrane separation technology. Sinopec has implemented a novel extractive distillation process using ionic liquids as entrainers, which significantly enhances the separation of geometric isomers in hydrocarbon mixtures[7]. The company has also developed a series of composite membranes with tailored pore sizes and surface chemistries that allow for selective permeation of specific geometric isomers[8]. Additionally, Sinopec has integrated molecular simulation techniques to optimize the design of separation processes for complex mixtures containing geometric isomers[9].
Strengths: Specialized techniques for petroleum-based mixtures, integration of multiple separation technologies, and use of advanced materials. Weaknesses: Techniques may be less applicable outside the petrochemical industry.
Innovative Approaches in Isomer Identification
Separation of olefinic isomers
PatentInactiveUS6861512B2
Innovation
- The method involves using a mobile phase with an aliphatic hydrocarbon that interacts with an organosilane stationary phase containing a pendant aliphatic functional group, allowing for the separation of cis and trans isomers by flowing through a column, where the aliphatic hydrocarbon preferentially interacts with the isomers to resolve the mixture, enabling the collection of each isomer as a distinct effluent stream.
Compositions and methods for the liquid-phase separation of isomers of aromatic molecules
PatentActiveUS20170354902A1
Innovation
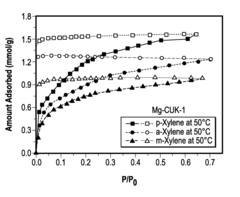
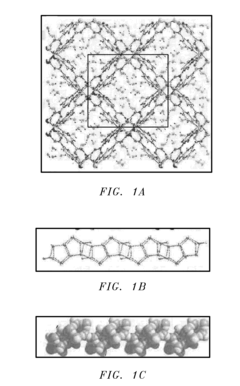
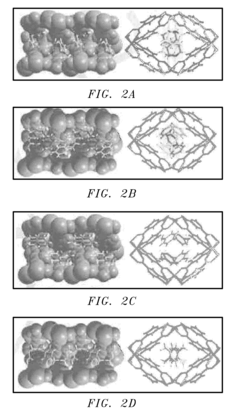
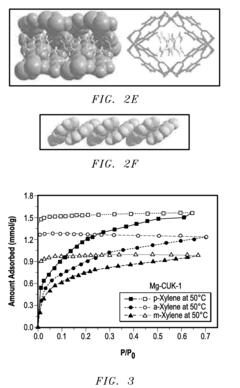
- A porous coordination polymer material based on Mg(II) and 2,4-pyridinedicarboxylic acid with a 1-D pore structure, synthesized using an aqueous microwave-assisted method, selectively adsorbs p-isomers of DVB at room temperature, allowing for the separation of p-DVB from crude mixtures by preferential binding and subsequent desorption.
Environmental Impact of Separation Techniques
The environmental impact of separation techniques used for identifying geometric isomers in organic mixtures is a critical consideration in modern analytical chemistry. These techniques, while essential for scientific research and industrial applications, can have significant environmental implications that must be carefully evaluated and mitigated.
One of the primary environmental concerns associated with separation techniques is the use of organic solvents. Many chromatographic methods, such as high-performance liquid chromatography (HPLC) and gas chromatography (GC), rely heavily on these solvents for mobile phases and sample preparation. The production, use, and disposal of organic solvents contribute to air pollution, water contamination, and greenhouse gas emissions. Volatile organic compounds (VOCs) released during these processes can lead to the formation of ground-level ozone and photochemical smog, negatively impacting air quality and human health.
Energy consumption is another significant environmental factor to consider. Separation techniques often require substantial amounts of energy for operation, particularly in the case of high-pressure systems and temperature-controlled environments. This energy demand contributes to increased carbon emissions and places a burden on power grids, especially when non-renewable energy sources are utilized.
Water usage is a concern in liquid chromatography techniques, where large volumes of water may be required for mobile phases and system cleaning. The contamination of this water with organic solvents and analytes necessitates proper treatment before disposal, adding to the environmental footprint of these techniques.
The generation of hazardous waste is an inevitable byproduct of many separation processes. Used solvents, contaminated samples, and spent chromatographic columns often require specialized disposal methods to prevent environmental contamination. Improper handling or disposal of these materials can lead to soil and groundwater pollution, posing risks to ecosystems and human health.
To address these environmental challenges, researchers and industry professionals are exploring more sustainable approaches to separation techniques. Green chemistry principles are being applied to develop environmentally friendly solvents, such as supercritical CO2 and ionic liquids, which can reduce the reliance on traditional organic solvents. Additionally, miniaturization of separation systems, such as micro-HPLC and capillary electrophoresis, is helping to decrease solvent consumption and energy requirements.
Recycling and recovery systems for solvents are becoming more prevalent in laboratory and industrial settings, reducing waste generation and the need for new solvent production. Furthermore, the development of solvent-free separation techniques, such as solid-phase microextraction (SPME) and thermal desorption methods, offers promising alternatives with minimal environmental impact.
As the field of analytical chemistry continues to evolve, it is crucial to balance the need for effective separation techniques with environmental responsibility. Ongoing research and innovation in this area will be essential to minimize the ecological footprint of these important analytical tools while maintaining their efficacy in identifying geometric isomers in organic mixtures.
One of the primary environmental concerns associated with separation techniques is the use of organic solvents. Many chromatographic methods, such as high-performance liquid chromatography (HPLC) and gas chromatography (GC), rely heavily on these solvents for mobile phases and sample preparation. The production, use, and disposal of organic solvents contribute to air pollution, water contamination, and greenhouse gas emissions. Volatile organic compounds (VOCs) released during these processes can lead to the formation of ground-level ozone and photochemical smog, negatively impacting air quality and human health.
Energy consumption is another significant environmental factor to consider. Separation techniques often require substantial amounts of energy for operation, particularly in the case of high-pressure systems and temperature-controlled environments. This energy demand contributes to increased carbon emissions and places a burden on power grids, especially when non-renewable energy sources are utilized.
Water usage is a concern in liquid chromatography techniques, where large volumes of water may be required for mobile phases and system cleaning. The contamination of this water with organic solvents and analytes necessitates proper treatment before disposal, adding to the environmental footprint of these techniques.
The generation of hazardous waste is an inevitable byproduct of many separation processes. Used solvents, contaminated samples, and spent chromatographic columns often require specialized disposal methods to prevent environmental contamination. Improper handling or disposal of these materials can lead to soil and groundwater pollution, posing risks to ecosystems and human health.
To address these environmental challenges, researchers and industry professionals are exploring more sustainable approaches to separation techniques. Green chemistry principles are being applied to develop environmentally friendly solvents, such as supercritical CO2 and ionic liquids, which can reduce the reliance on traditional organic solvents. Additionally, miniaturization of separation systems, such as micro-HPLC and capillary electrophoresis, is helping to decrease solvent consumption and energy requirements.
Recycling and recovery systems for solvents are becoming more prevalent in laboratory and industrial settings, reducing waste generation and the need for new solvent production. Furthermore, the development of solvent-free separation techniques, such as solid-phase microextraction (SPME) and thermal desorption methods, offers promising alternatives with minimal environmental impact.
As the field of analytical chemistry continues to evolve, it is crucial to balance the need for effective separation techniques with environmental responsibility. Ongoing research and innovation in this area will be essential to minimize the ecological footprint of these important analytical tools while maintaining their efficacy in identifying geometric isomers in organic mixtures.
Regulatory Framework for Analytical Methods in Chemistry
The regulatory framework for analytical methods in chemistry plays a crucial role in ensuring the reliability, accuracy, and consistency of separation techniques used for identifying geometric isomers in organic mixtures. Regulatory bodies such as the International Conference on Harmonisation (ICH), the United States Food and Drug Administration (FDA), and the European Medicines Agency (EMA) have established guidelines and standards for analytical method validation and quality control.
These regulatory frameworks typically require analytical methods to demonstrate specificity, accuracy, precision, linearity, range, and robustness. For separation techniques used in identifying geometric isomers, additional considerations may include resolution, peak symmetry, and selectivity. The ICH Q2(R1) guideline, for instance, provides detailed recommendations for the validation of analytical procedures, including those used for identification and quantification of isomers.
Regulatory bodies often require method validation protocols to be submitted as part of the drug approval process. These protocols must demonstrate that the chosen separation technique can reliably distinguish between geometric isomers and accurately quantify their relative proportions in complex organic mixtures. The FDA's Guidance for Industry on Analytical Procedures and Methods Validation for Drugs and Biologics emphasizes the importance of method suitability for its intended use and the need for ongoing method verification throughout the product lifecycle.
In the context of geometric isomer separation, regulatory frameworks may also address specific challenges such as the potential for interconversion between isomers during analysis. This requires validated stability-indicating methods and appropriate sample handling procedures. The European Pharmacopoeia and the United States Pharmacopeia provide monographs for many substances, including specific requirements for isomer separation and identification.
Compliance with Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) regulations is essential when developing and implementing separation techniques for geometric isomers. These regulations ensure that analytical methods are developed, validated, and executed in a controlled environment with proper documentation and traceability.
Regulatory bodies also emphasize the importance of using state-of-the-art analytical technologies. As separation techniques for geometric isomers evolve, regulatory frameworks are updated to incorporate new methodologies and quality standards. This includes guidance on the use of advanced chromatographic techniques, spectroscopic methods, and data analysis tools for isomer identification and quantification.
These regulatory frameworks typically require analytical methods to demonstrate specificity, accuracy, precision, linearity, range, and robustness. For separation techniques used in identifying geometric isomers, additional considerations may include resolution, peak symmetry, and selectivity. The ICH Q2(R1) guideline, for instance, provides detailed recommendations for the validation of analytical procedures, including those used for identification and quantification of isomers.
Regulatory bodies often require method validation protocols to be submitted as part of the drug approval process. These protocols must demonstrate that the chosen separation technique can reliably distinguish between geometric isomers and accurately quantify their relative proportions in complex organic mixtures. The FDA's Guidance for Industry on Analytical Procedures and Methods Validation for Drugs and Biologics emphasizes the importance of method suitability for its intended use and the need for ongoing method verification throughout the product lifecycle.
In the context of geometric isomer separation, regulatory frameworks may also address specific challenges such as the potential for interconversion between isomers during analysis. This requires validated stability-indicating methods and appropriate sample handling procedures. The European Pharmacopoeia and the United States Pharmacopeia provide monographs for many substances, including specific requirements for isomer separation and identification.
Compliance with Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) regulations is essential when developing and implementing separation techniques for geometric isomers. These regulations ensure that analytical methods are developed, validated, and executed in a controlled environment with proper documentation and traceability.
Regulatory bodies also emphasize the importance of using state-of-the-art analytical technologies. As separation techniques for geometric isomers evolve, regulatory frameworks are updated to incorporate new methodologies and quality standards. This includes guidance on the use of advanced chromatographic techniques, spectroscopic methods, and data analysis tools for isomer identification and quantification.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!