Silicon photonics and its role in personalized medicine developments.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Silicon Photonics Evolution and Objectives
Silicon photonics has emerged as a transformative technology in the field of integrated optics, with its evolution closely tied to the advancements in semiconductor manufacturing processes. The journey of silicon photonics began in the late 1980s when researchers first explored the possibility of using silicon as a platform for optical devices. Over the past three decades, this technology has progressed from a conceptual idea to a commercially viable solution, addressing critical challenges in data communication, sensing, and now, personalized medicine.
The primary objective of silicon photonics in the context of personalized medicine is to develop miniaturized, highly integrated optical systems capable of performing complex diagnostic and therapeutic functions. These systems aim to leverage the unique properties of silicon, such as its high refractive index and compatibility with CMOS fabrication processes, to create compact, cost-effective, and high-performance photonic devices.
One of the key goals is to enable rapid, accurate, and non-invasive diagnostic tools that can detect biomarkers, analyze genetic material, and monitor physiological parameters in real-time. This capability is crucial for early disease detection, treatment monitoring, and tailoring medical interventions to individual patients' needs. Silicon photonics offers the potential to integrate multiple optical functions on a single chip, including light sources, modulators, detectors, and waveguides, thereby facilitating the development of lab-on-a-chip devices for point-of-care diagnostics.
Another significant objective is to enhance the precision and efficacy of therapeutic interventions. Silicon photonic devices can be designed to deliver targeted light-based treatments, such as photodynamic therapy or optogenetic stimulation, with unprecedented spatial and temporal control. This level of precision is essential for minimizing side effects and maximizing treatment outcomes in personalized medicine approaches.
The evolution of silicon photonics in personalized medicine is also driven by the need for scalable and cost-effective solutions. As the technology matures, there is a growing focus on developing manufacturing processes that can produce high-volume, low-cost photonic integrated circuits (PICs) suitable for widespread adoption in healthcare settings. This includes efforts to improve the integration of electronic and photonic components, enhance the performance of key photonic elements, and develop standardized design and fabrication methodologies.
Looking ahead, the objectives for silicon photonics in personalized medicine include pushing the boundaries of integration density, improving sensitivity and specificity of diagnostic tools, and expanding the range of biomedical applications. Researchers are exploring novel materials and structures, such as hybrid silicon-organic photonics, to overcome current limitations and unlock new functionalities. The ultimate goal is to create a versatile photonic platform that can adapt to the diverse and evolving needs of personalized medicine, from early disease detection to precision treatment delivery and long-term health monitoring.
The primary objective of silicon photonics in the context of personalized medicine is to develop miniaturized, highly integrated optical systems capable of performing complex diagnostic and therapeutic functions. These systems aim to leverage the unique properties of silicon, such as its high refractive index and compatibility with CMOS fabrication processes, to create compact, cost-effective, and high-performance photonic devices.
One of the key goals is to enable rapid, accurate, and non-invasive diagnostic tools that can detect biomarkers, analyze genetic material, and monitor physiological parameters in real-time. This capability is crucial for early disease detection, treatment monitoring, and tailoring medical interventions to individual patients' needs. Silicon photonics offers the potential to integrate multiple optical functions on a single chip, including light sources, modulators, detectors, and waveguides, thereby facilitating the development of lab-on-a-chip devices for point-of-care diagnostics.
Another significant objective is to enhance the precision and efficacy of therapeutic interventions. Silicon photonic devices can be designed to deliver targeted light-based treatments, such as photodynamic therapy or optogenetic stimulation, with unprecedented spatial and temporal control. This level of precision is essential for minimizing side effects and maximizing treatment outcomes in personalized medicine approaches.
The evolution of silicon photonics in personalized medicine is also driven by the need for scalable and cost-effective solutions. As the technology matures, there is a growing focus on developing manufacturing processes that can produce high-volume, low-cost photonic integrated circuits (PICs) suitable for widespread adoption in healthcare settings. This includes efforts to improve the integration of electronic and photonic components, enhance the performance of key photonic elements, and develop standardized design and fabrication methodologies.
Looking ahead, the objectives for silicon photonics in personalized medicine include pushing the boundaries of integration density, improving sensitivity and specificity of diagnostic tools, and expanding the range of biomedical applications. Researchers are exploring novel materials and structures, such as hybrid silicon-organic photonics, to overcome current limitations and unlock new functionalities. The ultimate goal is to create a versatile photonic platform that can adapt to the diverse and evolving needs of personalized medicine, from early disease detection to precision treatment delivery and long-term health monitoring.
Personalized Medicine Market Analysis
The personalized medicine market has been experiencing significant growth in recent years, driven by advancements in genomics, data analytics, and precision diagnostics. This market segment is expected to continue its upward trajectory, with silicon photonics playing an increasingly crucial role in its development.
The global personalized medicine market size was valued at approximately $1.57 trillion in 2020 and is projected to reach $3.18 trillion by 2028, growing at a CAGR of 9.2% during the forecast period. This substantial growth is attributed to the increasing adoption of targeted therapies, the rising prevalence of chronic diseases, and the growing demand for more effective and tailored treatment options.
Within the personalized medicine market, several key segments are emerging as particularly promising areas for growth. These include genomics, pharmacogenomics, companion diagnostics, and biomarkers. The genomics segment, which includes technologies for DNA sequencing and analysis, is expected to witness the highest growth rate due to its critical role in identifying genetic variations that influence disease risk and treatment response.
Silicon photonics is poised to play a significant role in advancing personalized medicine, particularly in the areas of diagnostics and drug discovery. The integration of silicon photonics into diagnostic devices enables faster, more accurate, and cost-effective analysis of biological samples. This technology is particularly valuable in applications such as point-of-care testing and rapid DNA sequencing, which are essential components of personalized medicine approaches.
The adoption of personalized medicine is not uniform across all regions. North America currently holds the largest market share, driven by advanced healthcare infrastructure, significant investments in research and development, and favorable regulatory policies. However, the Asia-Pacific region is expected to witness the fastest growth rate in the coming years, fueled by increasing healthcare expenditure, growing awareness of personalized medicine, and improving access to advanced diagnostic technologies.
Key market players in the personalized medicine space include pharmaceutical giants like Pfizer, Novartis, and Roche, as well as specialized biotech companies and diagnostic equipment manufacturers. These companies are increasingly investing in silicon photonics-based technologies to enhance their product offerings and maintain a competitive edge in the rapidly evolving market.
Despite the promising outlook, the personalized medicine market faces several challenges. These include the high cost of developing and implementing personalized treatments, regulatory hurdles, and the need for extensive clinical validation. Additionally, concerns about data privacy and the ethical implications of genetic testing continue to be important considerations for market growth and adoption.
The global personalized medicine market size was valued at approximately $1.57 trillion in 2020 and is projected to reach $3.18 trillion by 2028, growing at a CAGR of 9.2% during the forecast period. This substantial growth is attributed to the increasing adoption of targeted therapies, the rising prevalence of chronic diseases, and the growing demand for more effective and tailored treatment options.
Within the personalized medicine market, several key segments are emerging as particularly promising areas for growth. These include genomics, pharmacogenomics, companion diagnostics, and biomarkers. The genomics segment, which includes technologies for DNA sequencing and analysis, is expected to witness the highest growth rate due to its critical role in identifying genetic variations that influence disease risk and treatment response.
Silicon photonics is poised to play a significant role in advancing personalized medicine, particularly in the areas of diagnostics and drug discovery. The integration of silicon photonics into diagnostic devices enables faster, more accurate, and cost-effective analysis of biological samples. This technology is particularly valuable in applications such as point-of-care testing and rapid DNA sequencing, which are essential components of personalized medicine approaches.
The adoption of personalized medicine is not uniform across all regions. North America currently holds the largest market share, driven by advanced healthcare infrastructure, significant investments in research and development, and favorable regulatory policies. However, the Asia-Pacific region is expected to witness the fastest growth rate in the coming years, fueled by increasing healthcare expenditure, growing awareness of personalized medicine, and improving access to advanced diagnostic technologies.
Key market players in the personalized medicine space include pharmaceutical giants like Pfizer, Novartis, and Roche, as well as specialized biotech companies and diagnostic equipment manufacturers. These companies are increasingly investing in silicon photonics-based technologies to enhance their product offerings and maintain a competitive edge in the rapidly evolving market.
Despite the promising outlook, the personalized medicine market faces several challenges. These include the high cost of developing and implementing personalized treatments, regulatory hurdles, and the need for extensive clinical validation. Additionally, concerns about data privacy and the ethical implications of genetic testing continue to be important considerations for market growth and adoption.
Silicon Photonics: Current State and Challenges
Silicon photonics has emerged as a transformative technology in the field of integrated photonics, offering unprecedented potential for miniaturization, scalability, and cost-effectiveness. However, its application in personalized medicine developments faces several challenges that need to be addressed for widespread adoption.
The current state of silicon photonics in personalized medicine is characterized by rapid advancements in biosensing, lab-on-a-chip devices, and point-of-care diagnostics. These applications leverage the unique properties of silicon, such as its high refractive index and compatibility with CMOS fabrication processes, to create highly sensitive and integrated photonic devices.
One of the primary challenges in this field is the integration of multiple functionalities on a single chip. While silicon photonics excels in light manipulation and routing, it lacks efficient light emission and detection capabilities. This limitation necessitates the development of hybrid integration techniques, combining silicon with III-V semiconductors or other materials to achieve full functionality.
Another significant challenge is the need for improved sensitivity and specificity in biosensing applications. Current silicon photonic biosensors struggle to detect low concentrations of biomarkers, which is crucial for early disease detection and personalized treatment monitoring. Researchers are exploring various approaches, including surface functionalization and novel resonator designs, to enhance sensor performance.
The miniaturization of silicon photonic devices for portable and wearable medical applications presents another hurdle. While silicon photonics offers inherent advantages in size reduction, further optimization is required to create truly compact and energy-efficient devices suitable for continuous health monitoring.
Data processing and analysis pose additional challenges in the context of personalized medicine. The vast amount of data generated by silicon photonic sensors necessitates the development of advanced algorithms and machine learning techniques to extract meaningful insights and enable real-time decision-making.
Manufacturing scalability and cost-effectiveness remain ongoing concerns. While silicon photonics leverages existing semiconductor fabrication infrastructure, the production of high-quality photonic devices with consistent performance across large volumes is still challenging. Addressing these manufacturing issues is crucial for the widespread adoption of silicon photonic technologies in personalized medicine.
Lastly, regulatory hurdles and standardization efforts present significant challenges. The integration of silicon photonic devices into medical applications requires rigorous testing and validation to ensure safety and efficacy. Establishing industry-wide standards for device performance, reliability, and interoperability is essential for accelerating the adoption of these technologies in clinical settings.
The current state of silicon photonics in personalized medicine is characterized by rapid advancements in biosensing, lab-on-a-chip devices, and point-of-care diagnostics. These applications leverage the unique properties of silicon, such as its high refractive index and compatibility with CMOS fabrication processes, to create highly sensitive and integrated photonic devices.
One of the primary challenges in this field is the integration of multiple functionalities on a single chip. While silicon photonics excels in light manipulation and routing, it lacks efficient light emission and detection capabilities. This limitation necessitates the development of hybrid integration techniques, combining silicon with III-V semiconductors or other materials to achieve full functionality.
Another significant challenge is the need for improved sensitivity and specificity in biosensing applications. Current silicon photonic biosensors struggle to detect low concentrations of biomarkers, which is crucial for early disease detection and personalized treatment monitoring. Researchers are exploring various approaches, including surface functionalization and novel resonator designs, to enhance sensor performance.
The miniaturization of silicon photonic devices for portable and wearable medical applications presents another hurdle. While silicon photonics offers inherent advantages in size reduction, further optimization is required to create truly compact and energy-efficient devices suitable for continuous health monitoring.
Data processing and analysis pose additional challenges in the context of personalized medicine. The vast amount of data generated by silicon photonic sensors necessitates the development of advanced algorithms and machine learning techniques to extract meaningful insights and enable real-time decision-making.
Manufacturing scalability and cost-effectiveness remain ongoing concerns. While silicon photonics leverages existing semiconductor fabrication infrastructure, the production of high-quality photonic devices with consistent performance across large volumes is still challenging. Addressing these manufacturing issues is crucial for the widespread adoption of silicon photonic technologies in personalized medicine.
Lastly, regulatory hurdles and standardization efforts present significant challenges. The integration of silicon photonic devices into medical applications requires rigorous testing and validation to ensure safety and efficacy. Establishing industry-wide standards for device performance, reliability, and interoperability is essential for accelerating the adoption of these technologies in clinical settings.
Silicon Photonics Solutions in Personalized Medicine
01 Integrated photonic devices
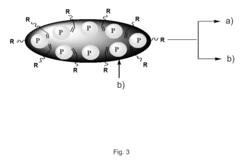
Silicon photonics technology enables the integration of various optical components on a single chip. This includes waveguides, modulators, detectors, and other photonic elements, allowing for compact and efficient optical systems. The integration of these components facilitates high-speed data transmission and processing in a small form factor.- Optical interconnects and communication systems: Silicon photonics technology is utilized in developing high-speed optical interconnects and communication systems. These systems integrate optical components on silicon chips, enabling efficient data transmission and processing in various applications, including data centers and telecommunications networks.
- Integration of photonic and electronic components: Silicon photonics allows for the integration of photonic and electronic components on a single chip. This integration enables the development of compact, energy-efficient devices that combine the benefits of both optical and electronic technologies, leading to improved performance in various applications.
- Waveguide structures and optical modulators: Advanced waveguide structures and optical modulators are key components in silicon photonics. These elements are designed to efficiently guide and manipulate light on silicon chips, enabling the development of high-performance optical devices for various applications, including sensing and signal processing.
- Silicon photonic sensors and detectors: Silicon photonics technology is employed in the development of highly sensitive optical sensors and detectors. These devices leverage the unique properties of silicon to create compact, efficient, and cost-effective sensing solutions for applications in healthcare, environmental monitoring, and industrial processes.
- Quantum photonics and integrated quantum devices: Silicon photonics is being explored for quantum applications, including the development of integrated quantum devices. This emerging field combines the advantages of silicon photonics with quantum technologies to create novel solutions for quantum computing, quantum communication, and quantum sensing.
02 Optical communication systems
Silicon photonics is extensively used in optical communication systems, enabling high-bandwidth data transmission. These systems incorporate silicon-based photonic components for signal generation, modulation, and detection, allowing for efficient and cost-effective communication networks. The technology supports various applications, including data centers and telecommunications.Expand Specific Solutions03 Photonic integrated circuits (PICs)
Photonic integrated circuits are a key application of silicon photonics, combining multiple optical functions on a single chip. These circuits can include lasers, modulators, multiplexers, and detectors, all integrated on a silicon substrate. PICs offer advantages in terms of size, power consumption, and performance for various applications in optical computing and sensing.Expand Specific Solutions04 Silicon-based optical interconnects
Silicon photonics enables the development of optical interconnects for chip-to-chip and intra-chip communication. These interconnects use silicon-based waveguides and other photonic components to transmit data optically, offering higher bandwidth and lower power consumption compared to traditional electrical interconnects. This technology is crucial for addressing communication bottlenecks in high-performance computing systems.Expand Specific Solutions05 Silicon photonic sensors
Silicon photonics technology is utilized in the development of various optical sensors. These sensors leverage the unique properties of silicon-based photonic structures to detect and measure physical, chemical, or biological parameters. Applications include environmental monitoring, biomedical sensing, and industrial process control, offering high sensitivity and integration capabilities.Expand Specific Solutions
Key Players in Silicon Photonics and Personalized Medicine
Silicon photonics is emerging as a key technology in personalized medicine, with the market currently in its growth phase. The global silicon photonics market is expected to reach $4.6 billion by 2027, driven by increasing demand for high-speed data transmission and sensing applications in healthcare. While the technology is maturing, it is not yet fully commercialized. Companies like Intel, Huawei, and Lumentum are leading the development of silicon photonics platforms, with Intel particularly focused on integrating photonics with electronic circuits. Research institutions such as the Shanghai Institute of Microsystem & Information Technology and the Electronics & Telecommunications Research Institute are also contributing to advancements in this field. The convergence of silicon photonics with AI and machine learning is expected to further accelerate its adoption in personalized medicine applications.
Intel Corp.
Technical Solution: Intel's silicon photonics technology for personalized medicine focuses on developing integrated photonic circuits for biosensing and diagnostic applications. Their platform combines multiple photonic functions on a single chip, enabling high-sensitivity, multiplexed detection of biomarkers. Intel has demonstrated a silicon photonic biosensor capable of detecting protein biomarkers at concentrations as low as 0.1 pg/mL, which is 1000 times more sensitive than conventional ELISA tests [1]. The company is also working on integrating microfluidics with silicon photonics to create lab-on-a-chip devices for point-of-care diagnostics. Intel's recent advancements include developing wavelength division multiplexing (WDM) technology for simultaneous detection of multiple biomarkers, potentially revolutionizing early disease detection and treatment monitoring [2].
Strengths: Advanced manufacturing capabilities, extensive R&D resources, and established partnerships with healthcare institutions. Weaknesses: Relatively new entrant in the medical diagnostics field, facing competition from specialized biotech companies.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche is leveraging silicon photonics in personalized medicine through its development of advanced diagnostic platforms. The company's silicon photonics-based biosensors are designed for rapid, sensitive, and multiplexed detection of disease biomarkers. Roche has developed a silicon photonic ring resonator array capable of simultaneously detecting multiple cancer biomarkers with a limit of detection in the femtomolar range [3]. Their technology integrates seamlessly with existing laboratory automation systems, enabling high-throughput screening for personalized medicine applications. Roche is also exploring the use of silicon photonics in portable diagnostic devices for point-of-care testing, aiming to bring laboratory-grade diagnostics closer to patients. Recent developments include the integration of machine learning algorithms with silicon photonic sensors to improve diagnostic accuracy and predict treatment responses [4].
Strengths: Extensive experience in diagnostics and pharmaceuticals, global distribution network, and strong regulatory expertise. Weaknesses: High development costs and potential challenges in scaling up production of silicon photonic devices.
Breakthrough Silicon Photonics Technologies
Silicon photonics integration with optical fibers
PatentPendingIN202311039438A
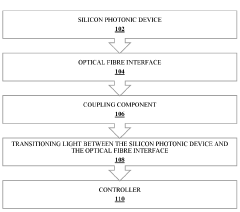
Innovation
- A system and method that includes a silicon photonic device, an optical fiber interface with a coupling component, and a controller for efficient light signal transmission and regulation, integrated onto a single photonic integrated circuit (PIC) with a silicon-on-insulator (SOI) platform, utilizing edge or grating couplers and thermal tuning to minimize losses and adapt to thermal variations.
Silica nanoparticles postloaded with photosensitizers for drug delivery in photodynamic therapy
PatentInactiveUS20110288234A1
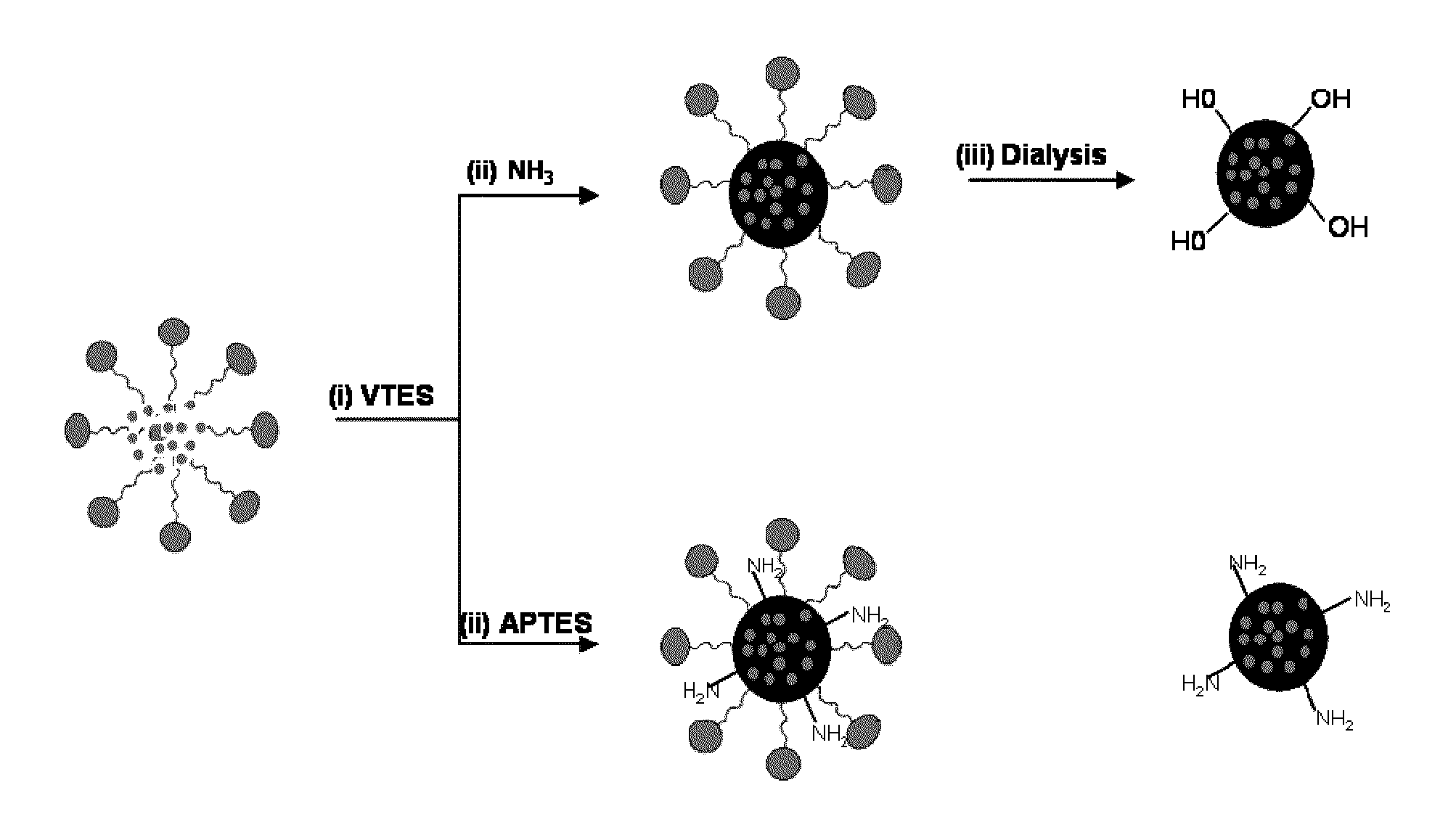
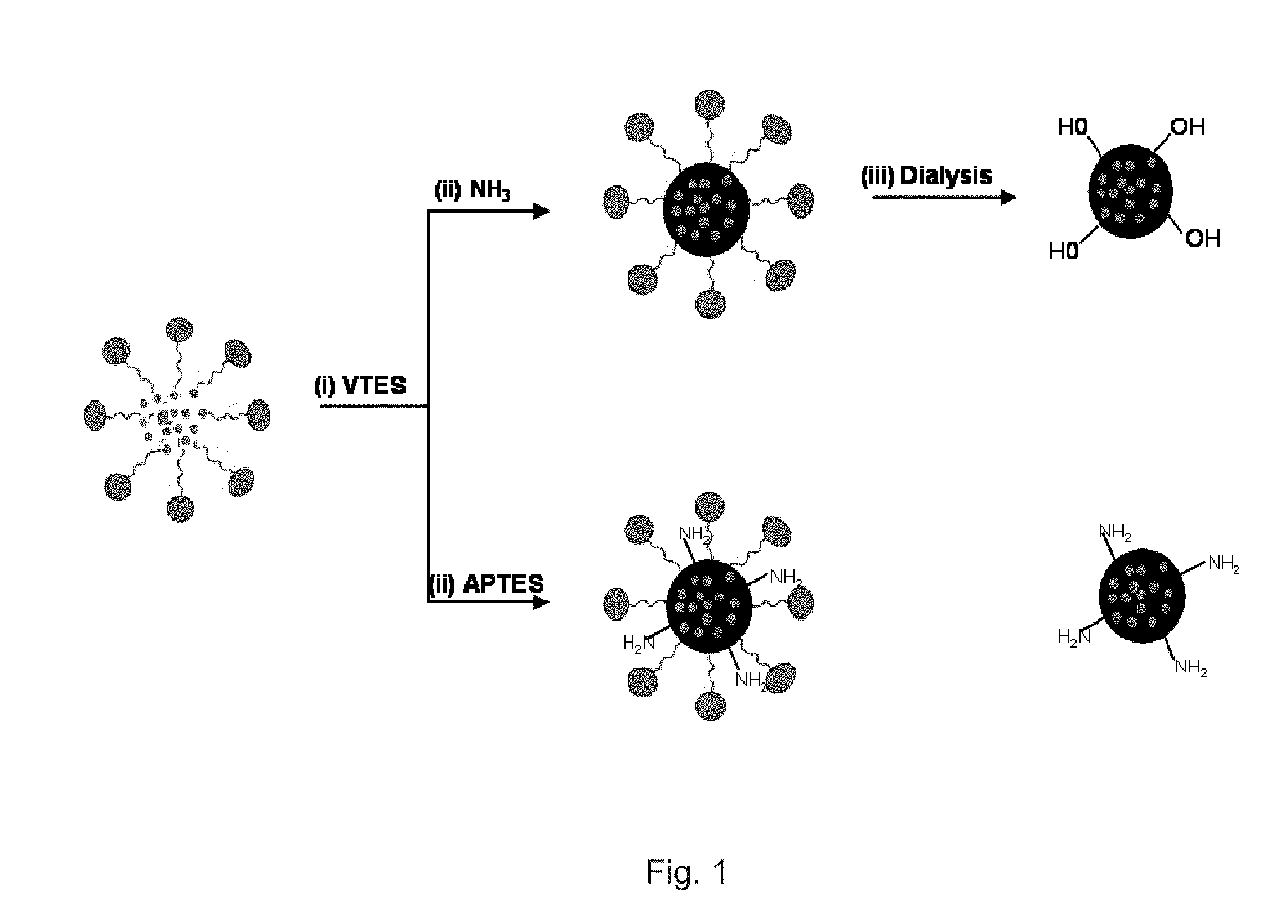
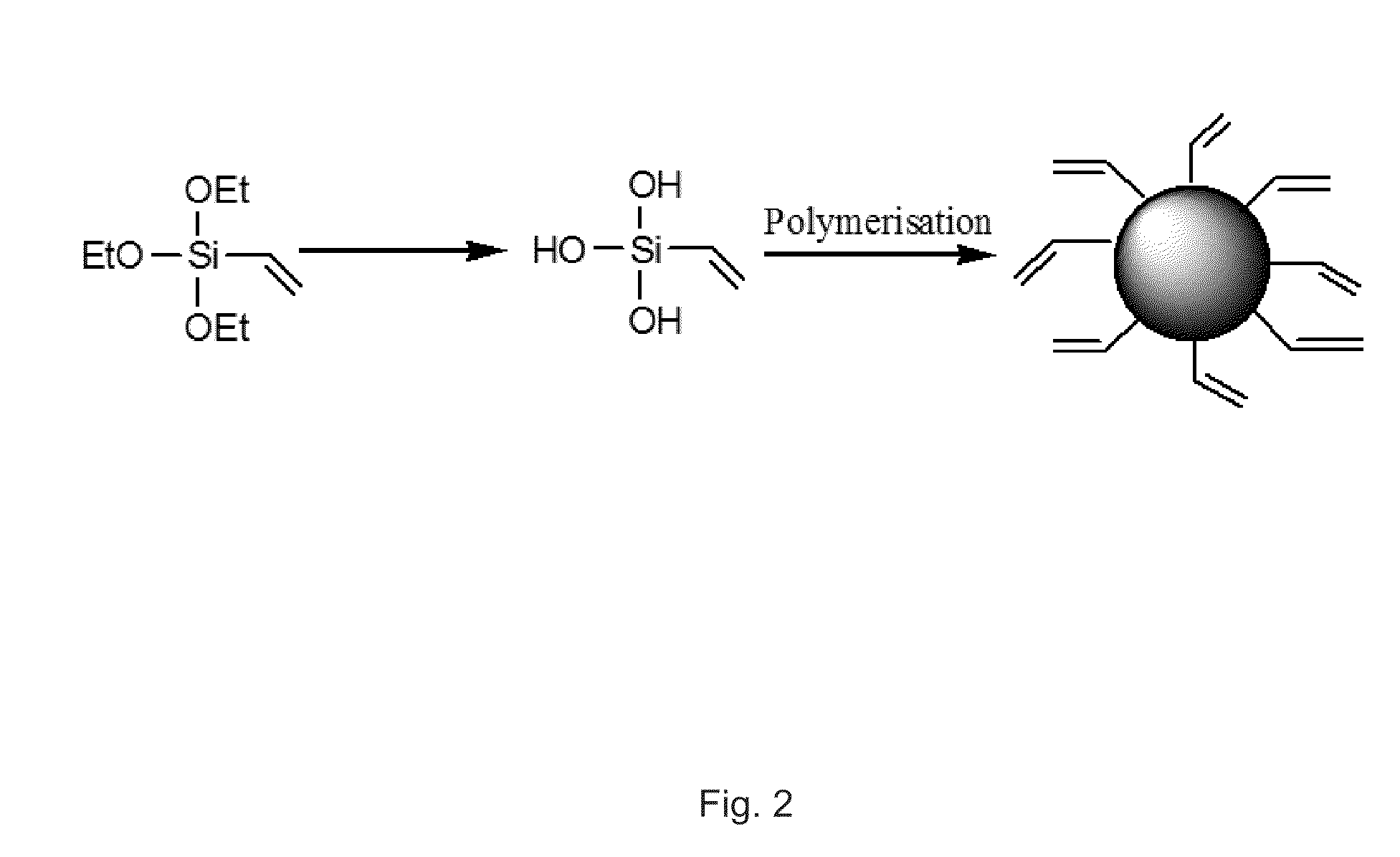
Innovation
- Development of organically modified silica (ORMOSIL) nanoparticles post-loaded with tetrapyrrole-based photosensitizers and imaging agents, which are designed to prevent premature release and enhance tumor selectivity through surface functionalization and encapsulation, allowing for targeted delivery and imaging.
Regulatory Framework for Silicon Photonics in Healthcare
The regulatory framework for silicon photonics in healthcare is a complex and evolving landscape that plays a crucial role in shaping the development and adoption of this technology in personalized medicine. As silicon photonics continues to advance and demonstrate its potential in medical applications, regulatory bodies worldwide are working to establish guidelines that ensure patient safety, device efficacy, and data privacy.
In the United States, the Food and Drug Administration (FDA) is the primary regulatory agency overseeing medical devices, including those incorporating silicon photonics technology. The FDA has established a risk-based classification system for medical devices, with Class I, II, and III designations. Silicon photonics-based devices may fall into different categories depending on their intended use and potential risks.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which came into effect in 2021 and 2022, respectively. These regulations provide a comprehensive framework for the approval and monitoring of medical devices, including those utilizing silicon photonics technology. The MDR and IVDR emphasize post-market surveillance and clinical evidence requirements, which may impact the development and commercialization of silicon photonics-based medical devices.
In Asia, countries like Japan and China have their own regulatory frameworks for medical devices. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established guidelines for the approval and registration of medical devices, which would apply to silicon photonics-based technologies used in healthcare applications.
One of the key challenges in regulating silicon photonics in healthcare is the rapid pace of technological advancement. Regulatory bodies must strike a balance between ensuring patient safety and fostering innovation. To address this, some agencies have implemented expedited review processes for breakthrough technologies, which may benefit silicon photonics-based devices that demonstrate significant potential in personalized medicine.
Data privacy and security regulations also play a crucial role in the adoption of silicon photonics in healthcare. As these devices often involve the collection and processing of sensitive patient data, compliance with regulations such as the General Data Protection Regulation (GDPR) in the EU and the Health Insurance Portability and Accountability Act (HIPAA) in the US is essential.
Standardization efforts are underway to facilitate the integration of silicon photonics in healthcare. Organizations such as the International Electrotechnical Commission (IEC) and the Institute of Electrical and Electronics Engineers (IEEE) are working on developing standards for silicon photonics components and systems, which may help streamline the regulatory approval process for medical devices incorporating this technology.
In the United States, the Food and Drug Administration (FDA) is the primary regulatory agency overseeing medical devices, including those incorporating silicon photonics technology. The FDA has established a risk-based classification system for medical devices, with Class I, II, and III designations. Silicon photonics-based devices may fall into different categories depending on their intended use and potential risks.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which came into effect in 2021 and 2022, respectively. These regulations provide a comprehensive framework for the approval and monitoring of medical devices, including those utilizing silicon photonics technology. The MDR and IVDR emphasize post-market surveillance and clinical evidence requirements, which may impact the development and commercialization of silicon photonics-based medical devices.
In Asia, countries like Japan and China have their own regulatory frameworks for medical devices. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established guidelines for the approval and registration of medical devices, which would apply to silicon photonics-based technologies used in healthcare applications.
One of the key challenges in regulating silicon photonics in healthcare is the rapid pace of technological advancement. Regulatory bodies must strike a balance between ensuring patient safety and fostering innovation. To address this, some agencies have implemented expedited review processes for breakthrough technologies, which may benefit silicon photonics-based devices that demonstrate significant potential in personalized medicine.
Data privacy and security regulations also play a crucial role in the adoption of silicon photonics in healthcare. As these devices often involve the collection and processing of sensitive patient data, compliance with regulations such as the General Data Protection Regulation (GDPR) in the EU and the Health Insurance Portability and Accountability Act (HIPAA) in the US is essential.
Standardization efforts are underway to facilitate the integration of silicon photonics in healthcare. Organizations such as the International Electrotechnical Commission (IEC) and the Institute of Electrical and Electronics Engineers (IEEE) are working on developing standards for silicon photonics components and systems, which may help streamline the regulatory approval process for medical devices incorporating this technology.
Ethical Implications of Personalized Medicine Technologies
The integration of silicon photonics in personalized medicine developments raises significant ethical considerations that must be carefully addressed. As these technologies advance, they offer unprecedented capabilities for tailoring medical treatments to individual genetic profiles, potentially revolutionizing healthcare outcomes. However, this progress also brings forth complex ethical dilemmas.
One primary concern is the potential for genetic discrimination. As silicon photonics enables more accurate and accessible genetic testing, there is a risk that individuals could face prejudice or unfair treatment based on their genetic predispositions. This could impact various aspects of life, including employment opportunities and insurance coverage, necessitating robust legal and ethical frameworks to protect individuals' rights and privacy.
The issue of data privacy and security is another critical ethical consideration. Silicon photonics-based diagnostic tools generate vast amounts of sensitive personal health information. Ensuring the confidentiality and secure storage of this data is paramount to maintain patient trust and prevent misuse. Striking a balance between data accessibility for research purposes and individual privacy rights presents an ongoing challenge.
Equitable access to personalized medicine technologies is a further ethical imperative. The advanced nature of silicon photonics-based diagnostics and treatments may lead to high costs, potentially creating a divide in healthcare quality between those who can afford these cutting-edge solutions and those who cannot. This raises questions about healthcare equity and the responsibility of society to ensure fair access to life-changing medical innovations.
The concept of informed consent becomes more complex in the context of personalized medicine. Patients may struggle to fully comprehend the implications of genetic testing results or the nuances of tailored treatments. Healthcare providers must develop effective communication strategies to ensure patients can make truly informed decisions about their care.
Lastly, the potential for unintended consequences in genetic manipulation enabled by advanced diagnostic tools presents ethical challenges. While the ability to identify and potentially modify genetic predispositions offers immense medical benefits, it also raises concerns about the limits of human intervention in natural genetic processes. Establishing ethical guidelines for the application of these technologies is crucial to prevent misuse and uphold societal values.
As silicon photonics continues to advance personalized medicine, ongoing dialogue between scientists, ethicists, policymakers, and the public is essential to navigate these complex ethical landscapes and ensure that technological progress aligns with societal values and ethical standards.
One primary concern is the potential for genetic discrimination. As silicon photonics enables more accurate and accessible genetic testing, there is a risk that individuals could face prejudice or unfair treatment based on their genetic predispositions. This could impact various aspects of life, including employment opportunities and insurance coverage, necessitating robust legal and ethical frameworks to protect individuals' rights and privacy.
The issue of data privacy and security is another critical ethical consideration. Silicon photonics-based diagnostic tools generate vast amounts of sensitive personal health information. Ensuring the confidentiality and secure storage of this data is paramount to maintain patient trust and prevent misuse. Striking a balance between data accessibility for research purposes and individual privacy rights presents an ongoing challenge.
Equitable access to personalized medicine technologies is a further ethical imperative. The advanced nature of silicon photonics-based diagnostics and treatments may lead to high costs, potentially creating a divide in healthcare quality between those who can afford these cutting-edge solutions and those who cannot. This raises questions about healthcare equity and the responsibility of society to ensure fair access to life-changing medical innovations.
The concept of informed consent becomes more complex in the context of personalized medicine. Patients may struggle to fully comprehend the implications of genetic testing results or the nuances of tailored treatments. Healthcare providers must develop effective communication strategies to ensure patients can make truly informed decisions about their care.
Lastly, the potential for unintended consequences in genetic manipulation enabled by advanced diagnostic tools presents ethical challenges. While the ability to identify and potentially modify genetic predispositions offers immense medical benefits, it also raises concerns about the limits of human intervention in natural genetic processes. Establishing ethical guidelines for the application of these technologies is crucial to prevent misuse and uphold societal values.
As silicon photonics continues to advance personalized medicine, ongoing dialogue between scientists, ethicists, policymakers, and the public is essential to navigate these complex ethical landscapes and ensure that technological progress aligns with societal values and ethical standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!