Solid Electrolyte Interphase (SEI) Engineering for Silicon Anodes: Additives, Formation Protocols & Tests
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SEI Engineering Background and Objectives
Solid Electrolyte Interphase (SEI) engineering for silicon anodes has emerged as a critical area of research in the field of lithium-ion batteries. The development of this technology has been driven by the increasing demand for high-capacity energy storage systems in various applications, from portable electronics to electric vehicles. Silicon anodes have garnered significant attention due to their theoretical capacity, which is nearly ten times that of conventional graphite anodes.
The primary objective of SEI engineering for silicon anodes is to address the challenges associated with the material's inherent properties. Silicon undergoes substantial volume changes during lithiation and delithiation cycles, leading to mechanical stress and degradation of the electrode structure. This volume expansion can cause the SEI layer to crack and reform repeatedly, resulting in continuous electrolyte decomposition and capacity fade.
The evolution of SEI engineering has been marked by several key milestones. Initially, research focused on understanding the composition and formation mechanisms of the SEI layer on silicon surfaces. This led to the development of various electrolyte additives aimed at stabilizing the SEI and mitigating silicon's volume expansion effects. Concurrently, efforts were made to optimize the silicon anode structure, including the use of nanostructured silicon and silicon-carbon composites.
Recent advancements in SEI engineering have shifted towards more sophisticated approaches. These include the development of artificial SEI layers, pre-lithiation techniques, and the use of advanced characterization methods to gain deeper insights into SEI formation and evolution. The goal is to create a stable, flexible, and ion-conductive SEI layer that can accommodate silicon's volume changes while preventing continuous electrolyte decomposition.
The technological trajectory in this field is moving towards the integration of multiple strategies. This includes combining optimized electrolyte formulations with tailored formation protocols and advanced silicon anode architectures. The ultimate aim is to achieve long-cycle life, high capacity, and improved safety in silicon-based lithium-ion batteries.
As research progresses, the focus is increasingly on developing scalable and commercially viable solutions. This involves not only improving the performance of silicon anodes but also ensuring that the SEI engineering techniques are compatible with existing battery manufacturing processes. The successful implementation of these technologies could potentially revolutionize the energy storage landscape, enabling the widespread adoption of high-capacity lithium-ion batteries across various sectors.
The primary objective of SEI engineering for silicon anodes is to address the challenges associated with the material's inherent properties. Silicon undergoes substantial volume changes during lithiation and delithiation cycles, leading to mechanical stress and degradation of the electrode structure. This volume expansion can cause the SEI layer to crack and reform repeatedly, resulting in continuous electrolyte decomposition and capacity fade.
The evolution of SEI engineering has been marked by several key milestones. Initially, research focused on understanding the composition and formation mechanisms of the SEI layer on silicon surfaces. This led to the development of various electrolyte additives aimed at stabilizing the SEI and mitigating silicon's volume expansion effects. Concurrently, efforts were made to optimize the silicon anode structure, including the use of nanostructured silicon and silicon-carbon composites.
Recent advancements in SEI engineering have shifted towards more sophisticated approaches. These include the development of artificial SEI layers, pre-lithiation techniques, and the use of advanced characterization methods to gain deeper insights into SEI formation and evolution. The goal is to create a stable, flexible, and ion-conductive SEI layer that can accommodate silicon's volume changes while preventing continuous electrolyte decomposition.
The technological trajectory in this field is moving towards the integration of multiple strategies. This includes combining optimized electrolyte formulations with tailored formation protocols and advanced silicon anode architectures. The ultimate aim is to achieve long-cycle life, high capacity, and improved safety in silicon-based lithium-ion batteries.
As research progresses, the focus is increasingly on developing scalable and commercially viable solutions. This involves not only improving the performance of silicon anodes but also ensuring that the SEI engineering techniques are compatible with existing battery manufacturing processes. The successful implementation of these technologies could potentially revolutionize the energy storage landscape, enabling the widespread adoption of high-capacity lithium-ion batteries across various sectors.
Market Analysis for Si Anode Batteries
The market for silicon anode batteries is experiencing rapid growth, driven by the increasing demand for high-performance energy storage solutions in various sectors. Silicon anodes offer significant advantages over traditional graphite anodes, including higher energy density and improved cycle life. This has led to a surge in research and development activities focused on overcoming the challenges associated with silicon anodes, particularly in the area of Solid Electrolyte Interphase (SEI) engineering.
The global market for silicon anode batteries is projected to expand substantially in the coming years. The automotive industry is a key driver of this growth, as electric vehicle manufacturers seek to enhance battery performance and extend driving ranges. Consumer electronics, another major market segment, is also fueling demand for silicon anode batteries due to the need for longer-lasting portable devices.
Several factors are contributing to the market's positive outlook. Firstly, ongoing advancements in SEI engineering techniques, including the development of novel additives and formation protocols, are addressing the volume expansion issues associated with silicon anodes. This is improving the overall stability and longevity of silicon-based batteries, making them more attractive to potential adopters.
Additionally, government initiatives and regulations promoting clean energy and electric vehicles are indirectly boosting the silicon anode battery market. Many countries have set ambitious targets for reducing carbon emissions, which is accelerating the transition to electric mobility and renewable energy storage solutions.
The market landscape is characterized by intense competition among established battery manufacturers and emerging startups. Major players are investing heavily in research and development to gain a competitive edge in silicon anode technology. Collaborations between battery makers, automotive companies, and research institutions are becoming increasingly common, fostering innovation and accelerating commercialization efforts.
Despite the promising outlook, challenges remain in the widespread adoption of silicon anode batteries. Cost considerations, scalability issues, and the need for further improvements in cycle life are among the factors that could impact market growth. However, ongoing research in SEI engineering and other related areas is expected to address these challenges over time.
In conclusion, the market for silicon anode batteries shows strong growth potential, driven by technological advancements, increasing demand from key industries, and supportive regulatory environments. As SEI engineering continues to evolve, addressing the unique challenges of silicon anodes, the market is likely to see further expansion and innovation in the coming years.
The global market for silicon anode batteries is projected to expand substantially in the coming years. The automotive industry is a key driver of this growth, as electric vehicle manufacturers seek to enhance battery performance and extend driving ranges. Consumer electronics, another major market segment, is also fueling demand for silicon anode batteries due to the need for longer-lasting portable devices.
Several factors are contributing to the market's positive outlook. Firstly, ongoing advancements in SEI engineering techniques, including the development of novel additives and formation protocols, are addressing the volume expansion issues associated with silicon anodes. This is improving the overall stability and longevity of silicon-based batteries, making them more attractive to potential adopters.
Additionally, government initiatives and regulations promoting clean energy and electric vehicles are indirectly boosting the silicon anode battery market. Many countries have set ambitious targets for reducing carbon emissions, which is accelerating the transition to electric mobility and renewable energy storage solutions.
The market landscape is characterized by intense competition among established battery manufacturers and emerging startups. Major players are investing heavily in research and development to gain a competitive edge in silicon anode technology. Collaborations between battery makers, automotive companies, and research institutions are becoming increasingly common, fostering innovation and accelerating commercialization efforts.
Despite the promising outlook, challenges remain in the widespread adoption of silicon anode batteries. Cost considerations, scalability issues, and the need for further improvements in cycle life are among the factors that could impact market growth. However, ongoing research in SEI engineering and other related areas is expected to address these challenges over time.
In conclusion, the market for silicon anode batteries shows strong growth potential, driven by technological advancements, increasing demand from key industries, and supportive regulatory environments. As SEI engineering continues to evolve, addressing the unique challenges of silicon anodes, the market is likely to see further expansion and innovation in the coming years.
SEI Challenges on Si Anodes
Silicon anodes in lithium-ion batteries face significant challenges related to the formation and stability of the Solid Electrolyte Interphase (SEI). The primary issue stems from the substantial volume changes silicon undergoes during lithiation and delithiation cycles, which can reach up to 300%. This extreme volume fluctuation leads to continuous breaking and reforming of the SEI layer, resulting in accelerated electrolyte consumption and capacity fade.
The dynamic nature of silicon's surface during cycling poses a unique challenge for SEI formation. Unlike graphite anodes, where a stable SEI forms relatively quickly, silicon anodes require careful engineering to develop a robust and flexible SEI that can accommodate the volume changes. The SEI on silicon tends to be thicker and more complex in composition compared to that on graphite, making it more susceptible to cracking and delamination.
Another critical challenge is the reactivity of silicon with the electrolyte. Fresh silicon surfaces exposed during cycling are highly reactive, leading to continuous SEI growth and electrolyte decomposition. This ongoing process not only consumes active lithium but also increases the internal resistance of the battery, further degrading its performance over time.
The composition of the SEI on silicon anodes is also a concern. It typically contains a higher proportion of inorganic components, such as lithium silicates and fluorides, which are less elastic than the organic components found in graphite SEI. This inorganic-rich composition contributes to the brittleness of the SEI, making it more prone to fracture during volume changes.
Furthermore, the SEI on silicon anodes often suffers from non-uniform growth and distribution. This inhomogeneity can lead to localized areas of high current density, accelerating degradation in specific regions of the electrode. The uneven SEI also contributes to the formation of isolated silicon particles, which become electrochemically inactive over time, reducing the overall capacity of the anode.
The stability of the SEI under different operating conditions presents another challenge. Temperature fluctuations, high charge/discharge rates, and deep discharge cycles can all exacerbate the degradation of the SEI on silicon anodes. These factors can lead to accelerated SEI growth, increased impedance, and faster capacity fade, limiting the practical application of silicon anodes in various environments.
Addressing these challenges requires a multifaceted approach, including the development of novel electrolyte additives, optimized formation protocols, and advanced characterization techniques to understand and control SEI formation and evolution on silicon anodes.
The dynamic nature of silicon's surface during cycling poses a unique challenge for SEI formation. Unlike graphite anodes, where a stable SEI forms relatively quickly, silicon anodes require careful engineering to develop a robust and flexible SEI that can accommodate the volume changes. The SEI on silicon tends to be thicker and more complex in composition compared to that on graphite, making it more susceptible to cracking and delamination.
Another critical challenge is the reactivity of silicon with the electrolyte. Fresh silicon surfaces exposed during cycling are highly reactive, leading to continuous SEI growth and electrolyte decomposition. This ongoing process not only consumes active lithium but also increases the internal resistance of the battery, further degrading its performance over time.
The composition of the SEI on silicon anodes is also a concern. It typically contains a higher proportion of inorganic components, such as lithium silicates and fluorides, which are less elastic than the organic components found in graphite SEI. This inorganic-rich composition contributes to the brittleness of the SEI, making it more prone to fracture during volume changes.
Furthermore, the SEI on silicon anodes often suffers from non-uniform growth and distribution. This inhomogeneity can lead to localized areas of high current density, accelerating degradation in specific regions of the electrode. The uneven SEI also contributes to the formation of isolated silicon particles, which become electrochemically inactive over time, reducing the overall capacity of the anode.
The stability of the SEI under different operating conditions presents another challenge. Temperature fluctuations, high charge/discharge rates, and deep discharge cycles can all exacerbate the degradation of the SEI on silicon anodes. These factors can lead to accelerated SEI growth, increased impedance, and faster capacity fade, limiting the practical application of silicon anodes in various environments.
Addressing these challenges requires a multifaceted approach, including the development of novel electrolyte additives, optimized formation protocols, and advanced characterization techniques to understand and control SEI formation and evolution on silicon anodes.
Current SEI Engineering Solutions
01 SEI formation techniques for silicon anodes
Various techniques are employed to form stable SEI layers on silicon anodes. These include pre-lithiation, surface coatings, and electrolyte additives. These methods aim to create a protective layer that prevents continuous electrolyte decomposition and silicon degradation, thereby enhancing the overall stability and performance of the battery.- SEI formation techniques for silicon anodes: Various techniques are employed to form stable SEI layers on silicon anodes. These include pre-lithiation, surface coatings, and electrolyte additives. These methods aim to create a protective layer that prevents continuous electrolyte decomposition and silicon electrode degradation, thereby enhancing the overall stability and performance of the battery.
- Electrolyte composition for improved SEI stability: The composition of the electrolyte plays a crucial role in SEI formation and stability. Researchers are developing novel electrolyte formulations that include specific additives or solvents to promote the formation of a more stable and flexible SEI layer on silicon anodes. These tailored electrolytes aim to accommodate the volume changes of silicon during cycling and maintain SEI integrity.
- Nanostructured silicon anodes for enhanced SEI stability: Nanostructured silicon anodes, such as silicon nanowires, nanoparticles, or porous structures, are being explored to improve SEI stability. These nanostructures provide better accommodation for volume changes and offer increased surface area for SEI formation, potentially leading to more stable and uniform SEI layers.
- In-situ characterization of SEI formation and evolution: Advanced in-situ characterization techniques are being developed to study SEI formation and evolution on silicon anodes in real-time. These methods provide insights into the dynamic processes occurring at the electrode-electrolyte interface, helping researchers understand SEI growth mechanisms and design strategies for improved stability.
- Artificial SEI layers for silicon anodes: Researchers are exploring the use of artificial SEI layers deposited on silicon anodes prior to battery assembly. These pre-formed layers aim to provide a stable interface from the outset, reducing initial capacity loss and improving long-term cycling stability. Various materials and deposition techniques are being investigated for this purpose.
02 Electrolyte composition for improved SEI stability
The composition of the electrolyte plays a crucial role in SEI formation and stability. Researchers are developing novel electrolyte formulations that include specific additives or solvents to promote the formation of a more stable and flexible SEI layer on silicon anodes. These tailored electrolytes aim to accommodate the volume changes of silicon during cycling.Expand Specific Solutions03 Nanostructured silicon anodes for enhanced SEI stability
Nanostructured silicon anodes, such as silicon nanowires, nanoparticles, or porous structures, are being developed to improve SEI stability. These nanostructures provide better accommodation for volume changes and offer increased surface area for SEI formation, leading to more stable interfaces and improved cycling performance.Expand Specific Solutions04 Artificial SEI layers for silicon anodes
Researchers are exploring the use of artificial SEI layers deposited on silicon anodes before battery assembly. These pre-formed layers, often composed of inorganic or organic materials, aim to provide a stable interface from the outset, reducing initial capacity loss and improving long-term cycling stability.Expand Specific Solutions05 In-situ characterization of SEI formation and evolution
Advanced characterization techniques are being developed to study SEI formation and evolution on silicon anodes in real-time. These in-situ methods, including spectroscopic and microscopic techniques, provide valuable insights into the dynamic processes of SEI growth, composition changes, and degradation mechanisms, enabling the design of more effective SEI stabilization strategies.Expand Specific Solutions
Key Players in Si Anode Battery Industry
The Solid Electrolyte Interphase (SEI) engineering for silicon anodes is in a rapidly evolving phase, with significant market potential in the electric vehicle and energy storage sectors. The global market for advanced battery technologies is expanding, driven by increasing demand for high-performance energy storage solutions. Key players like LG Energy Solution, Contemporary Amperex Technology, and Enevate are at the forefront of this technology, investing heavily in R&D to overcome challenges associated with silicon anodes. The technology is approaching maturity, with several companies demonstrating promising results in lab settings and moving towards commercialization. Universities and research institutions, such as MIT and the University of Oslo, are also contributing significantly to advancing the fundamental understanding of SEI formation and optimization.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a novel approach to SEI engineering for silicon anodes, focusing on a combination of electrolyte additives and formation protocols. Their method involves using fluoroethylene carbonate (FEC) and vinylene carbonate (VC) as key additives to form a stable SEI layer[1]. The company has also implemented a multi-step formation protocol that includes a pre-lithiation step to minimize initial capacity loss[2]. This process involves carefully controlled charge-discharge cycles at varying temperatures to optimize SEI formation. Additionally, LG has developed advanced characterization techniques, including in-situ electrochemical impedance spectroscopy (EIS) and X-ray photoelectron spectroscopy (XPS), to monitor and analyze the SEI formation process in real-time[3].
Strengths: Improved cycle life and capacity retention of silicon anodes. Advanced characterization techniques for real-time SEI analysis. Weaknesses: Potentially higher production costs due to complex formation protocols. May require specialized equipment for implementation.
GM Global Technology Operations LLC
Technical Solution: GM has developed a comprehensive approach to SEI engineering for silicon anodes, focusing on silicon-graphene composite materials and advanced electrolyte formulations. Their technology utilizes a three-dimensional graphene network to encapsulate silicon nanoparticles, providing mechanical support and improved electrical conductivity[10]. GM's electrolyte additives include a proprietary blend of organic and inorganic compounds, designed to form a stable and flexible SEI layer that can accommodate the volume changes of silicon during cycling[11]. The company has also implemented a multi-step formation protocol that incorporates both constant current and constant voltage stages at varying temperatures to optimize SEI formation and minimize initial capacity loss. GM's testing methods include advanced in-operando neutron diffraction techniques to study structural changes in the silicon anode and SEI layer during cycling, as well as glow discharge optical emission spectroscopy (GD-OES) for depth profiling of the SEI composition[12].
Strengths: Silicon-graphene composite for enhanced stability and conductivity. Advanced in-operando characterization techniques. Weaknesses: Potential high costs associated with graphene production. Complexity in optimizing the silicon-graphene composite structure.
Core Innovations in SEI Additives
Solid electrolyte interphase (SEI) and a method for its preparation
PatentActiveIN202141055967A
Innovation
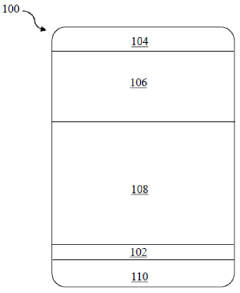
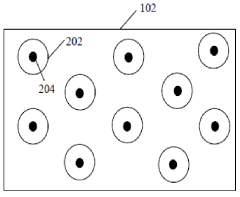
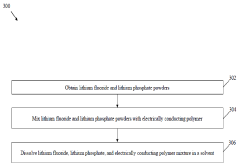
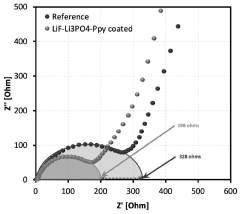
- A solid electrolyte interphase (SEI) comprising a porous polymer matrix embedded with metal precursors like lithium fluoride and lithium phosphate, coated on the current collector to prevent dendrite formation and enhance battery performance and safety, prepared through a method involving mixing metal precursors with an electrically conducting polymer and solvent, followed by solvent removal.
Environmental Impact of SEI Additives
The environmental impact of SEI additives in silicon anode batteries is a critical consideration as the demand for high-performance energy storage solutions continues to grow. These additives play a crucial role in enhancing battery performance and longevity, but their potential environmental consequences must be carefully evaluated.
One of the primary environmental concerns associated with SEI additives is their production process. Many additives are synthesized using complex chemical reactions that may involve toxic precursors or generate hazardous by-products. The manufacturing of these compounds often requires significant energy input and can result in greenhouse gas emissions, contributing to climate change.
Furthermore, the disposal of batteries containing SEI additives presents another environmental challenge. As these batteries reach the end of their life cycle, proper recycling and waste management become essential to prevent the release of potentially harmful substances into the environment. Some additives may persist in the environment or undergo transformations that could impact ecosystems and human health.
Water pollution is another potential concern related to SEI additives. If not properly contained during production or disposal, these chemicals could leach into groundwater or surface water systems, potentially affecting aquatic life and drinking water sources. The long-term effects of these additives on water quality and aquatic ecosystems are still being studied.
On the other hand, the use of SEI additives can have indirect positive environmental impacts. By improving battery performance and lifespan, these additives can reduce the overall number of batteries needed, potentially decreasing the environmental footprint associated with battery production and disposal. Additionally, enhanced battery efficiency can lead to more widespread adoption of electric vehicles and renewable energy storage systems, contributing to reduced carbon emissions in the transportation and energy sectors.
Researchers are actively exploring more environmentally friendly SEI additives, focusing on bio-based and naturally derived compounds. These alternatives aim to minimize the environmental impact while maintaining or improving battery performance. Efforts are also being made to develop additives that are easier to recycle or that degrade into harmless substances at the end of the battery's life.
As the battery industry continues to evolve, it is crucial to conduct comprehensive life cycle assessments of SEI additives. These assessments should consider the entire lifecycle of the additives, from raw material extraction to production, use, and disposal. Such evaluations will help identify areas for improvement and guide the development of more sustainable battery technologies.
One of the primary environmental concerns associated with SEI additives is their production process. Many additives are synthesized using complex chemical reactions that may involve toxic precursors or generate hazardous by-products. The manufacturing of these compounds often requires significant energy input and can result in greenhouse gas emissions, contributing to climate change.
Furthermore, the disposal of batteries containing SEI additives presents another environmental challenge. As these batteries reach the end of their life cycle, proper recycling and waste management become essential to prevent the release of potentially harmful substances into the environment. Some additives may persist in the environment or undergo transformations that could impact ecosystems and human health.
Water pollution is another potential concern related to SEI additives. If not properly contained during production or disposal, these chemicals could leach into groundwater or surface water systems, potentially affecting aquatic life and drinking water sources. The long-term effects of these additives on water quality and aquatic ecosystems are still being studied.
On the other hand, the use of SEI additives can have indirect positive environmental impacts. By improving battery performance and lifespan, these additives can reduce the overall number of batteries needed, potentially decreasing the environmental footprint associated with battery production and disposal. Additionally, enhanced battery efficiency can lead to more widespread adoption of electric vehicles and renewable energy storage systems, contributing to reduced carbon emissions in the transportation and energy sectors.
Researchers are actively exploring more environmentally friendly SEI additives, focusing on bio-based and naturally derived compounds. These alternatives aim to minimize the environmental impact while maintaining or improving battery performance. Efforts are also being made to develop additives that are easier to recycle or that degrade into harmless substances at the end of the battery's life.
As the battery industry continues to evolve, it is crucial to conduct comprehensive life cycle assessments of SEI additives. These assessments should consider the entire lifecycle of the additives, from raw material extraction to production, use, and disposal. Such evaluations will help identify areas for improvement and guide the development of more sustainable battery technologies.
Safety Considerations for Si Anodes
Safety considerations for silicon anodes in lithium-ion batteries are paramount due to their unique characteristics and potential risks. The high volume expansion of silicon during lithiation can lead to mechanical stress and structural instability, potentially causing electrode pulverization and capacity fade. This expansion may also lead to the formation of cracks in the SEI layer, exposing fresh silicon surfaces to the electrolyte and causing continuous SEI growth.
The continuous SEI formation consumes electrolyte and lithium ions, leading to capacity loss and increased internal resistance. Moreover, the unstable SEI can result in the formation of lithium dendrites, which may penetrate the separator and cause short circuits, posing severe safety hazards. The high reactivity of silicon with the electrolyte can also generate heat, potentially triggering thermal runaway in extreme cases.
To mitigate these safety concerns, several strategies have been developed. One approach involves the use of electrolyte additives that promote the formation of a more stable and flexible SEI layer. Fluoroethylene carbonate (FEC) and vinylene carbonate (VC) are commonly used additives that have shown promising results in enhancing the stability of the SEI on silicon anodes.
Another safety consideration is the design of silicon-based composite materials that can better accommodate volume changes. Silicon-carbon composites, for instance, can help buffer the volume expansion and maintain structural integrity. Additionally, nanostructured silicon materials, such as silicon nanowires or porous silicon, can provide better stress relaxation during cycling.
Proper formation protocols are crucial for ensuring the safety of silicon anodes. Slow initial charging rates and carefully controlled voltage windows during the first few cycles can help establish a more stable SEI layer. This initial conditioning process is critical for long-term cycling stability and safety.
Advanced characterization techniques, such as in-situ TEM and synchrotron-based X-ray techniques, are essential for monitoring the evolution of the SEI layer and detecting potential safety issues. These methods can provide valuable insights into the morphological and chemical changes occurring at the electrode-electrolyte interface during cycling.
Furthermore, the development of advanced battery management systems (BMS) is crucial for ensuring the safe operation of batteries with silicon anodes. These systems can monitor cell voltage, temperature, and other parameters in real-time, allowing for early detection of potential safety issues and implementation of appropriate safety measures.
The continuous SEI formation consumes electrolyte and lithium ions, leading to capacity loss and increased internal resistance. Moreover, the unstable SEI can result in the formation of lithium dendrites, which may penetrate the separator and cause short circuits, posing severe safety hazards. The high reactivity of silicon with the electrolyte can also generate heat, potentially triggering thermal runaway in extreme cases.
To mitigate these safety concerns, several strategies have been developed. One approach involves the use of electrolyte additives that promote the formation of a more stable and flexible SEI layer. Fluoroethylene carbonate (FEC) and vinylene carbonate (VC) are commonly used additives that have shown promising results in enhancing the stability of the SEI on silicon anodes.
Another safety consideration is the design of silicon-based composite materials that can better accommodate volume changes. Silicon-carbon composites, for instance, can help buffer the volume expansion and maintain structural integrity. Additionally, nanostructured silicon materials, such as silicon nanowires or porous silicon, can provide better stress relaxation during cycling.
Proper formation protocols are crucial for ensuring the safety of silicon anodes. Slow initial charging rates and carefully controlled voltage windows during the first few cycles can help establish a more stable SEI layer. This initial conditioning process is critical for long-term cycling stability and safety.
Advanced characterization techniques, such as in-situ TEM and synchrotron-based X-ray techniques, are essential for monitoring the evolution of the SEI layer and detecting potential safety issues. These methods can provide valuable insights into the morphological and chemical changes occurring at the electrode-electrolyte interface during cycling.
Furthermore, the development of advanced battery management systems (BMS) is crucial for ensuring the safe operation of batteries with silicon anodes. These systems can monitor cell voltage, temperature, and other parameters in real-time, allowing for early detection of potential safety issues and implementation of appropriate safety measures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!