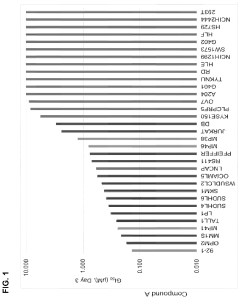
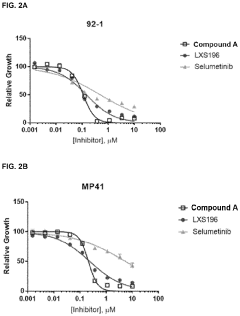
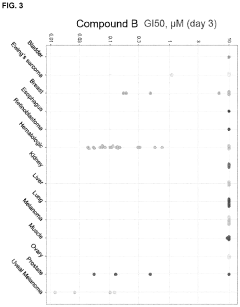
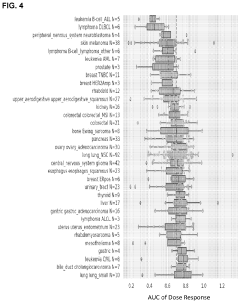
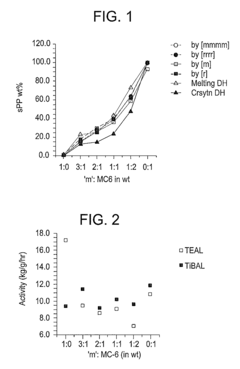
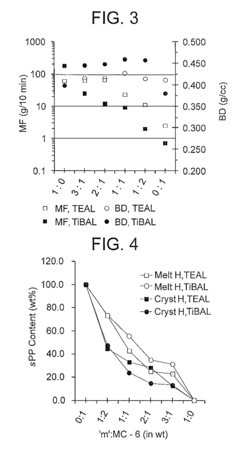
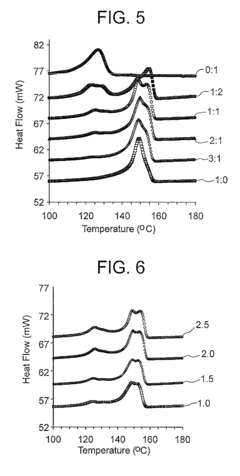
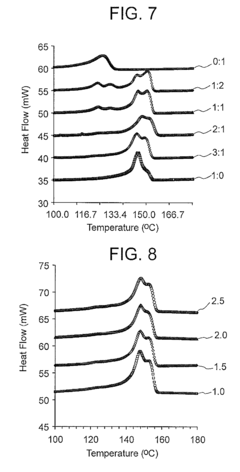
Stereochemical Effects of Geometric Isomers in Catalytic Processes
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Geometric Isomers in Catalysis: Background and Objectives
Geometric isomers, a subset of stereoisomers, have played a crucial role in the field of catalysis since the early days of organometallic chemistry. These compounds, which possess the same molecular formula but differ in the spatial arrangement of their atoms, have been the subject of extensive research due to their unique properties and potential applications in various catalytic processes.
The study of geometric isomers in catalysis can be traced back to the mid-20th century when scientists began to recognize the importance of molecular structure in determining catalytic activity. Early work focused on simple coordination compounds, such as square planar complexes of platinum and palladium, which exhibit cis-trans isomerism. These studies laid the foundation for understanding how subtle changes in molecular geometry can significantly impact catalytic performance.
As the field progressed, researchers expanded their investigations to include more complex systems, such as organometallic catalysts used in industrial processes. The discovery of Ziegler-Natta catalysts in the 1950s marked a significant milestone, demonstrating the profound influence of geometric isomerism on polymerization reactions. This breakthrough led to the development of stereospecific catalysts, capable of producing polymers with controlled tacticity and enhanced properties.
In recent decades, the focus has shifted towards understanding the stereochemical effects of geometric isomers in asymmetric catalysis. This area has gained tremendous importance due to its potential applications in the pharmaceutical and fine chemical industries, where the production of enantiomerically pure compounds is crucial. Researchers have explored various chiral ligands and metal complexes, investigating how the spatial arrangement of atoms in these catalysts can induce stereoselectivity in organic transformations.
The advent of advanced spectroscopic techniques and computational methods has greatly facilitated the study of geometric isomers in catalysis. These tools have enabled scientists to elucidate reaction mechanisms, predict catalytic activity, and design novel catalysts with improved performance. X-ray crystallography, NMR spectroscopy, and density functional theory (DFT) calculations have become indispensable in characterizing the structure and behavior of geometric isomers in catalytic systems.
The primary objective of current research in this field is to develop a comprehensive understanding of how the stereochemical properties of geometric isomers influence catalytic processes at the molecular level. This includes investigating the effects of isomerism on catalyst activation, substrate binding, transition state stabilization, and product release. By gaining insights into these fundamental aspects, researchers aim to establish structure-activity relationships that can guide the rational design of more efficient and selective catalysts.
Another important goal is to explore the potential of geometric isomers in emerging areas of catalysis, such as photocatalysis, electrocatalysis, and biocatalysis. These fields offer new opportunities for exploiting the unique properties of geometric isomers to achieve unprecedented levels of control over chemical transformations. Additionally, there is growing interest in developing stimuli-responsive catalysts that can switch between different geometric isomers in response to external stimuli, enabling dynamic control of catalytic activity and selectivity.
The study of geometric isomers in catalysis can be traced back to the mid-20th century when scientists began to recognize the importance of molecular structure in determining catalytic activity. Early work focused on simple coordination compounds, such as square planar complexes of platinum and palladium, which exhibit cis-trans isomerism. These studies laid the foundation for understanding how subtle changes in molecular geometry can significantly impact catalytic performance.
As the field progressed, researchers expanded their investigations to include more complex systems, such as organometallic catalysts used in industrial processes. The discovery of Ziegler-Natta catalysts in the 1950s marked a significant milestone, demonstrating the profound influence of geometric isomerism on polymerization reactions. This breakthrough led to the development of stereospecific catalysts, capable of producing polymers with controlled tacticity and enhanced properties.
In recent decades, the focus has shifted towards understanding the stereochemical effects of geometric isomers in asymmetric catalysis. This area has gained tremendous importance due to its potential applications in the pharmaceutical and fine chemical industries, where the production of enantiomerically pure compounds is crucial. Researchers have explored various chiral ligands and metal complexes, investigating how the spatial arrangement of atoms in these catalysts can induce stereoselectivity in organic transformations.
The advent of advanced spectroscopic techniques and computational methods has greatly facilitated the study of geometric isomers in catalysis. These tools have enabled scientists to elucidate reaction mechanisms, predict catalytic activity, and design novel catalysts with improved performance. X-ray crystallography, NMR spectroscopy, and density functional theory (DFT) calculations have become indispensable in characterizing the structure and behavior of geometric isomers in catalytic systems.
The primary objective of current research in this field is to develop a comprehensive understanding of how the stereochemical properties of geometric isomers influence catalytic processes at the molecular level. This includes investigating the effects of isomerism on catalyst activation, substrate binding, transition state stabilization, and product release. By gaining insights into these fundamental aspects, researchers aim to establish structure-activity relationships that can guide the rational design of more efficient and selective catalysts.
Another important goal is to explore the potential of geometric isomers in emerging areas of catalysis, such as photocatalysis, electrocatalysis, and biocatalysis. These fields offer new opportunities for exploiting the unique properties of geometric isomers to achieve unprecedented levels of control over chemical transformations. Additionally, there is growing interest in developing stimuli-responsive catalysts that can switch between different geometric isomers in response to external stimuli, enabling dynamic control of catalytic activity and selectivity.
Industrial Demand for Stereospecific Catalysts
The demand for stereospecific catalysts in industrial processes has seen a significant surge in recent years, driven by the growing need for precise control over chemical reactions and the production of high-value compounds. This trend is particularly evident in the pharmaceutical, agrochemical, and fine chemical industries, where the stereochemistry of molecules plays a crucial role in their efficacy and safety profiles.
In the pharmaceutical sector, the importance of stereochemistry cannot be overstated. Many drugs are chiral molecules, and their biological activity often depends on the specific three-dimensional arrangement of atoms. The thalidomide tragedy of the 1960s serves as a stark reminder of the critical nature of stereochemistry in drug development. Since then, regulatory bodies have mandated stringent controls on the stereochemical purity of pharmaceutical products, driving the demand for stereospecific catalysts that can selectively produce the desired enantiomer.
The agrochemical industry has also recognized the significance of stereochemistry in developing more effective and environmentally friendly pesticides and herbicides. Stereospecific catalysts enable the production of compounds with enhanced target specificity and reduced environmental impact. This aligns with the global push for sustainable agricultural practices and stricter regulations on chemical usage in farming.
In the realm of fine chemicals and specialty materials, stereospecific catalysis has opened new avenues for innovation. The production of advanced polymers, liquid crystals, and other functional materials often requires precise control over molecular architecture. Stereospecific catalysts provide the tools necessary to achieve this level of control, enabling the development of materials with tailored properties for applications in electronics, optics, and advanced manufacturing.
The flavor and fragrance industry has also benefited from advancements in stereospecific catalysis. Many aroma compounds exhibit different olfactory properties depending on their stereochemistry. The ability to selectively produce specific stereoisomers has allowed for the creation of more nuanced and complex scent profiles, driving innovation in perfumery and food additives.
As industries continue to demand higher levels of precision and efficiency in chemical processes, the market for stereospecific catalysts is expected to grow. This demand is further fueled by the push towards green chemistry and sustainable manufacturing practices. Stereospecific catalysts often offer improved atom economy and reduced waste generation compared to traditional synthetic methods, aligning with the principles of sustainable development.
In the pharmaceutical sector, the importance of stereochemistry cannot be overstated. Many drugs are chiral molecules, and their biological activity often depends on the specific three-dimensional arrangement of atoms. The thalidomide tragedy of the 1960s serves as a stark reminder of the critical nature of stereochemistry in drug development. Since then, regulatory bodies have mandated stringent controls on the stereochemical purity of pharmaceutical products, driving the demand for stereospecific catalysts that can selectively produce the desired enantiomer.
The agrochemical industry has also recognized the significance of stereochemistry in developing more effective and environmentally friendly pesticides and herbicides. Stereospecific catalysts enable the production of compounds with enhanced target specificity and reduced environmental impact. This aligns with the global push for sustainable agricultural practices and stricter regulations on chemical usage in farming.
In the realm of fine chemicals and specialty materials, stereospecific catalysis has opened new avenues for innovation. The production of advanced polymers, liquid crystals, and other functional materials often requires precise control over molecular architecture. Stereospecific catalysts provide the tools necessary to achieve this level of control, enabling the development of materials with tailored properties for applications in electronics, optics, and advanced manufacturing.
The flavor and fragrance industry has also benefited from advancements in stereospecific catalysis. Many aroma compounds exhibit different olfactory properties depending on their stereochemistry. The ability to selectively produce specific stereoisomers has allowed for the creation of more nuanced and complex scent profiles, driving innovation in perfumery and food additives.
As industries continue to demand higher levels of precision and efficiency in chemical processes, the market for stereospecific catalysts is expected to grow. This demand is further fueled by the push towards green chemistry and sustainable manufacturing practices. Stereospecific catalysts often offer improved atom economy and reduced waste generation compared to traditional synthetic methods, aligning with the principles of sustainable development.
Current Challenges in Stereochemical Control
The control of stereochemistry in catalytic processes involving geometric isomers presents several significant challenges that researchers and industry professionals are actively working to overcome. One of the primary difficulties lies in the selective formation of specific stereoisomers during catalytic reactions. The presence of geometric isomers, which differ in the spatial arrangement of atoms around a double bond or ring structure, can lead to the production of multiple stereoisomers, often with varying properties and biological activities.
A major challenge in this field is the development of catalysts that can effectively discriminate between different geometric isomers and promote the formation of the desired stereoisomer with high selectivity. This requires a deep understanding of the molecular interactions between the catalyst, substrate, and reaction environment. Factors such as steric hindrance, electronic effects, and substrate orientation play crucial roles in determining the stereochemical outcome of the reaction.
Another significant hurdle is the control of reaction kinetics and thermodynamics to favor the formation of the desired geometric isomer. In many cases, the thermodynamically more stable isomer may not be the desired product, necessitating careful manipulation of reaction conditions to overcome energetic barriers and drive the reaction towards the kinetically favored product. This often involves precise control of temperature, pressure, and reaction time, as well as the use of specialized catalysts or additives.
The complexity of reaction mechanisms involving geometric isomers also poses a challenge in stereochemical control. Many catalytic processes involve multiple steps and intermediates, each of which can influence the final stereochemical outcome. Elucidating these mechanisms and identifying key intermediates is crucial for developing effective strategies to control stereochemistry. Advanced spectroscopic and computational techniques are often required to gain insights into these complex reaction pathways.
Furthermore, the scalability of stereoselective catalytic processes remains a significant challenge, particularly in industrial applications. Maintaining high levels of stereoselectivity while increasing reaction scale can be problematic due to changes in heat and mass transfer properties. This often necessitates the redesign of catalysts and reaction conditions to ensure consistent performance at larger scales.
Environmental considerations also present challenges in stereochemical control. There is a growing emphasis on developing more sustainable catalytic processes that minimize waste and energy consumption. This has led to increased research into recyclable catalysts, greener solvents, and more atom-efficient reactions. However, balancing these environmental concerns with the need for high stereoselectivity can be challenging and often requires innovative approaches to catalyst design and process optimization.
A major challenge in this field is the development of catalysts that can effectively discriminate between different geometric isomers and promote the formation of the desired stereoisomer with high selectivity. This requires a deep understanding of the molecular interactions between the catalyst, substrate, and reaction environment. Factors such as steric hindrance, electronic effects, and substrate orientation play crucial roles in determining the stereochemical outcome of the reaction.
Another significant hurdle is the control of reaction kinetics and thermodynamics to favor the formation of the desired geometric isomer. In many cases, the thermodynamically more stable isomer may not be the desired product, necessitating careful manipulation of reaction conditions to overcome energetic barriers and drive the reaction towards the kinetically favored product. This often involves precise control of temperature, pressure, and reaction time, as well as the use of specialized catalysts or additives.
The complexity of reaction mechanisms involving geometric isomers also poses a challenge in stereochemical control. Many catalytic processes involve multiple steps and intermediates, each of which can influence the final stereochemical outcome. Elucidating these mechanisms and identifying key intermediates is crucial for developing effective strategies to control stereochemistry. Advanced spectroscopic and computational techniques are often required to gain insights into these complex reaction pathways.
Furthermore, the scalability of stereoselective catalytic processes remains a significant challenge, particularly in industrial applications. Maintaining high levels of stereoselectivity while increasing reaction scale can be problematic due to changes in heat and mass transfer properties. This often necessitates the redesign of catalysts and reaction conditions to ensure consistent performance at larger scales.
Environmental considerations also present challenges in stereochemical control. There is a growing emphasis on developing more sustainable catalytic processes that minimize waste and energy consumption. This has led to increased research into recyclable catalysts, greener solvents, and more atom-efficient reactions. However, balancing these environmental concerns with the need for high stereoselectivity can be challenging and often requires innovative approaches to catalyst design and process optimization.
State-of-the-Art Stereocontrol Strategies
01 Synthesis and separation of geometric isomers
Geometric isomers are compounds with the same molecular formula but different spatial arrangements of atoms. The synthesis and separation of these isomers are crucial in understanding their stereochemical effects. Various techniques, such as selective crystallization, chromatography, and asymmetric synthesis, are employed to obtain pure geometric isomers for further study and application.- Synthesis and separation of geometric isomers: Methods for synthesizing and separating geometric isomers are crucial in understanding their stereochemical effects. These techniques involve specialized reaction conditions and purification processes to isolate specific isomers. The separation of geometric isomers often requires advanced chromatographic techniques or selective crystallization methods.
- Stereochemical effects on biological activity: Geometric isomers can exhibit different biological activities due to their spatial arrangements. This is particularly important in pharmaceutical research, where the stereochemistry of a compound can significantly affect its efficacy, toxicity, and metabolism. Understanding these effects is crucial for drug development and optimization.
- Computational modeling of geometric isomers: Advanced computational techniques are employed to model and predict the properties and behaviors of geometric isomers. These methods include molecular dynamics simulations, quantum mechanical calculations, and machine learning approaches. Such models help in understanding the relationship between molecular structure and stereochemical effects.
- Industrial applications of geometric isomers: Geometric isomers find applications in various industries, including polymer science, materials engineering, and catalysis. The stereochemical effects of these isomers can be exploited to create materials with specific properties or to enhance the efficiency of chemical processes. This includes the development of novel catalysts, advanced materials, and specialized chemical products.
- Analytical methods for characterizing geometric isomers: Sophisticated analytical techniques are essential for characterizing and quantifying geometric isomers. These methods include spectroscopic techniques such as NMR and IR spectroscopy, as well as advanced chromatographic methods. The development of these analytical tools is crucial for studying the stereochemical effects of geometric isomers in complex systems.
02 Stereochemical effects on biological activity
The spatial arrangement of atoms in geometric isomers can significantly influence their biological activity. Different isomers may interact differently with biological targets, such as enzymes or receptors, leading to varied pharmacological effects. Understanding these stereochemical effects is crucial in drug design and development, as well as in the study of natural products and their biological functions.Expand Specific Solutions03 Analytical methods for characterizing geometric isomers
Various analytical techniques are used to characterize and distinguish between geometric isomers. These methods include spectroscopic techniques such as NMR, IR, and UV-Vis spectroscopy, as well as X-ray crystallography and circular dichroism. Advanced chromatographic techniques, like chiral HPLC, are also employed to separate and analyze geometric isomers, providing insights into their stereochemical properties.Expand Specific Solutions04 Computational modeling of geometric isomers
Computational methods play a crucial role in studying the stereochemical effects of geometric isomers. Molecular modeling and quantum chemical calculations are used to predict the stability, reactivity, and properties of different isomers. These computational approaches help in understanding the relationship between molecular structure and stereochemical effects, guiding experimental design and interpretation of results.Expand Specific Solutions05 Industrial applications of geometric isomers
The stereochemical effects of geometric isomers have significant implications in various industrial applications. In the pharmaceutical industry, understanding these effects is crucial for drug efficacy and safety. In materials science, geometric isomers can influence the properties of polymers and other materials. The food and fragrance industries also utilize the distinct properties of geometric isomers in product development and quality control.Expand Specific Solutions
Key Players in Stereospecific Catalyst Research
The field of stereochemical effects in catalytic processes is currently in a mature development stage, with significant market potential due to its applications in pharmaceutical and chemical industries. The global market size for catalysts is projected to reach $34.3 billion by 2024, with a growing emphasis on stereospecific reactions. Technological maturity varies among key players, with pharmaceutical giants like Pfizer Inc. and Bristol Myers Squibb Co. leading in drug development applications. Research institutions such as The Rockefeller University and Dalian Institute of Chemical Physics contribute to fundamental advancements. Companies like China Petroleum & Chemical Corp. and PetroChina Co., Ltd. focus on industrial-scale catalytic processes, while specialized firms like Revolution Medicines, Inc. and Inspirna, Inc. are developing novel approaches to exploit stereochemical effects in drug discovery and development.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced catalytic processes that leverage stereochemical effects of geometric isomers. Their approach involves using chiral metal-organic frameworks (MOFs) as heterogeneous catalysts for asymmetric reactions[1]. These MOFs are designed with specific pore geometries to enhance stereoselectivity in catalytic transformations. Sinopec has also implemented continuous-flow reactors with immobilized chiral catalysts, allowing for efficient production of optically pure compounds[2]. Their research extends to the use of supercritical CO2 as a reaction medium, which has shown to improve stereoselectivity in certain catalytic processes due to its unique solvation properties[3].
Strengths: Large-scale implementation capability, integration with existing petrochemical processes. Weaknesses: Potential high costs for specialized catalyst development, challenges in maintaining catalyst stability under industrial conditions.
Dow Global Technologies LLC
Technical Solution: Dow Global Technologies has developed innovative approaches to exploit stereochemical effects in catalytic processes, particularly in polymer production. They have pioneered the use of single-site metallocene catalysts with specific geometric configurations to control polymer tacticity and molecular weight distribution[7]. Their research extends to the development of stereospecific catalysts for the production of specialty chemicals, including the use of chiral ligands in transition metal complexes for asymmetric hydroformylation reactions[8]. Dow has also implemented continuous manufacturing techniques that leverage the stereochemical control offered by their catalysts, enabling the production of polymers with tailored properties and reduced environmental impact[9].
Strengths: Broad application across multiple industries, strong commercialization capabilities. Weaknesses: Potential intellectual property constraints, high R&D costs for developing new catalyst systems.
Breakthrough Innovations in Geometric Isomer Catalysis
Compounds and uses thereof
PatentPendingUS20230145003A1
Innovation
- Development of specific compounds that modulate the BAF complex by inhibiting BRG1 and/or BRM activity, which can be used alone or in combination with other pharmaceutically active agents to treat disorders like cancer.
Multi-component catalyst systems for the production of reactor blends of polypropylene
PatentInactiveUS20120322960A1
Innovation
- A multicomponent catalyst system comprising an isotactic directing metallocene catalyst and a syndiotactic directing metallocene catalyst is introduced into a reaction zone with an olefin monomer to form a polyolefin blend of metallocene isotactic polypropylene and syndiotactic polypropylene without the need for separate melt blending.
Green Chemistry Implications of Stereospecific Catalysis
The implications of stereospecific catalysis for green chemistry are profound and far-reaching. This approach aligns closely with the principles of green chemistry by enhancing reaction efficiency, reducing waste, and minimizing energy consumption. Stereospecific catalysts enable the selective production of desired stereoisomers, significantly reducing the formation of unwanted by-products and simplifying downstream separation processes.
One of the key benefits of stereospecific catalysis in green chemistry is the improvement in atom economy. By selectively producing the desired stereoisomer, these catalysts maximize the conversion of reactants into valuable products, minimizing the generation of waste materials. This not only reduces the environmental impact of chemical processes but also improves overall resource utilization.
Stereospecific catalysis also contributes to energy efficiency in chemical processes. By directing reactions towards specific stereochemical outcomes, these catalysts often allow for milder reaction conditions, including lower temperatures and pressures. This reduction in energy requirements aligns with the green chemistry goal of designing more sustainable and environmentally friendly processes.
Furthermore, the use of stereospecific catalysts can lead to the development of more efficient and streamlined synthetic routes. By enabling the direct synthesis of desired stereoisomers, complex multi-step processes can often be simplified or eliminated. This not only reduces the overall environmental footprint of chemical production but also enhances process economics.
In the pharmaceutical industry, stereospecific catalysis plays a crucial role in green chemistry applications. The ability to selectively produce single enantiomers of drug molecules can significantly reduce the use of raw materials, minimize waste generation, and improve the safety and efficacy of medications. This approach aligns with the principles of green chemistry by enhancing both environmental sustainability and human health outcomes.
The development of recyclable and reusable stereospecific catalysts further enhances their green chemistry credentials. Heterogeneous catalysts and immobilized homogeneous catalysts that maintain their stereospecificity over multiple reaction cycles contribute to reduced waste generation and improved process sustainability.
As research in this field progresses, the integration of stereospecific catalysis with other green chemistry technologies, such as continuous flow processes and biocatalysis, holds promise for even greater environmental benefits. These synergistic approaches have the potential to revolutionize chemical manufacturing, making it more sustainable and environmentally responsible.
One of the key benefits of stereospecific catalysis in green chemistry is the improvement in atom economy. By selectively producing the desired stereoisomer, these catalysts maximize the conversion of reactants into valuable products, minimizing the generation of waste materials. This not only reduces the environmental impact of chemical processes but also improves overall resource utilization.
Stereospecific catalysis also contributes to energy efficiency in chemical processes. By directing reactions towards specific stereochemical outcomes, these catalysts often allow for milder reaction conditions, including lower temperatures and pressures. This reduction in energy requirements aligns with the green chemistry goal of designing more sustainable and environmentally friendly processes.
Furthermore, the use of stereospecific catalysts can lead to the development of more efficient and streamlined synthetic routes. By enabling the direct synthesis of desired stereoisomers, complex multi-step processes can often be simplified or eliminated. This not only reduces the overall environmental footprint of chemical production but also enhances process economics.
In the pharmaceutical industry, stereospecific catalysis plays a crucial role in green chemistry applications. The ability to selectively produce single enantiomers of drug molecules can significantly reduce the use of raw materials, minimize waste generation, and improve the safety and efficacy of medications. This approach aligns with the principles of green chemistry by enhancing both environmental sustainability and human health outcomes.
The development of recyclable and reusable stereospecific catalysts further enhances their green chemistry credentials. Heterogeneous catalysts and immobilized homogeneous catalysts that maintain their stereospecificity over multiple reaction cycles contribute to reduced waste generation and improved process sustainability.
As research in this field progresses, the integration of stereospecific catalysis with other green chemistry technologies, such as continuous flow processes and biocatalysis, holds promise for even greater environmental benefits. These synergistic approaches have the potential to revolutionize chemical manufacturing, making it more sustainable and environmentally responsible.
Computational Modeling for Stereochemical Prediction
Computational modeling has emerged as a powerful tool for predicting stereochemical effects in catalytic processes involving geometric isomers. These models leverage advanced algorithms and quantum mechanical calculations to simulate the behavior of molecules and catalysts at the atomic level. By incorporating factors such as molecular geometry, electronic structure, and reaction energetics, computational models can provide valuable insights into the stereochemical outcomes of catalytic reactions.
One of the primary approaches in computational modeling for stereochemical prediction is density functional theory (DFT). DFT calculations allow researchers to optimize molecular structures, determine transition state geometries, and calculate reaction energies with high accuracy. This method has proven particularly effective in predicting the stereoselectivity of catalytic reactions involving geometric isomers, as it can account for subtle differences in molecular interactions that influence product formation.
Machine learning techniques have also been integrated into computational modeling frameworks to enhance predictive capabilities. By training on large datasets of known stereochemical outcomes, machine learning algorithms can identify complex patterns and relationships that may not be immediately apparent through traditional computational methods. This approach has shown promise in predicting stereoselectivity for a wide range of catalytic systems, including those involving geometric isomers.
Molecular dynamics simulations provide another valuable tool for understanding stereochemical effects in catalytic processes. These simulations allow researchers to observe the time-dependent behavior of molecules and catalysts, offering insights into the dynamic aspects of stereochemical control. By incorporating explicit solvent molecules and considering thermal fluctuations, molecular dynamics simulations can capture important environmental factors that influence stereochemical outcomes.
Recent advancements in computational power and algorithm efficiency have enabled the development of multiscale modeling approaches. These methods combine different levels of theory, from quantum mechanics to coarse-grained models, to provide a comprehensive understanding of stereochemical effects across various time and length scales. This holistic approach is particularly valuable for studying complex catalytic systems where stereochemical control is influenced by factors operating at different hierarchical levels.
As computational modeling techniques continue to evolve, their integration with experimental methods is becoming increasingly important. High-throughput virtual screening of catalyst candidates, combined with machine learning-driven optimization, is accelerating the discovery of novel catalytic systems with improved stereochemical control. This synergy between computation and experiment is paving the way for more efficient and targeted development of stereoselective catalytic processes involving geometric isomers.
One of the primary approaches in computational modeling for stereochemical prediction is density functional theory (DFT). DFT calculations allow researchers to optimize molecular structures, determine transition state geometries, and calculate reaction energies with high accuracy. This method has proven particularly effective in predicting the stereoselectivity of catalytic reactions involving geometric isomers, as it can account for subtle differences in molecular interactions that influence product formation.
Machine learning techniques have also been integrated into computational modeling frameworks to enhance predictive capabilities. By training on large datasets of known stereochemical outcomes, machine learning algorithms can identify complex patterns and relationships that may not be immediately apparent through traditional computational methods. This approach has shown promise in predicting stereoselectivity for a wide range of catalytic systems, including those involving geometric isomers.
Molecular dynamics simulations provide another valuable tool for understanding stereochemical effects in catalytic processes. These simulations allow researchers to observe the time-dependent behavior of molecules and catalysts, offering insights into the dynamic aspects of stereochemical control. By incorporating explicit solvent molecules and considering thermal fluctuations, molecular dynamics simulations can capture important environmental factors that influence stereochemical outcomes.
Recent advancements in computational power and algorithm efficiency have enabled the development of multiscale modeling approaches. These methods combine different levels of theory, from quantum mechanics to coarse-grained models, to provide a comprehensive understanding of stereochemical effects across various time and length scales. This holistic approach is particularly valuable for studying complex catalytic systems where stereochemical control is influenced by factors operating at different hierarchical levels.
As computational modeling techniques continue to evolve, their integration with experimental methods is becoming increasingly important. High-throughput virtual screening of catalyst candidates, combined with machine learning-driven optimization, is accelerating the discovery of novel catalytic systems with improved stereochemical control. This synergy between computation and experiment is paving the way for more efficient and targeted development of stereoselective catalytic processes involving geometric isomers.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!