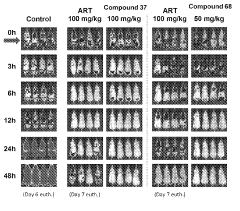
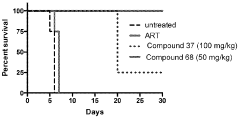
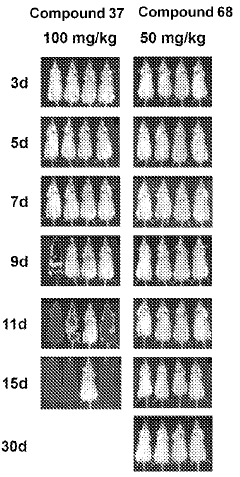
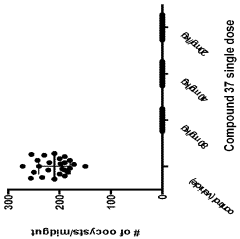
Stereoselective Detection of Geometric Isomers in Biological Samples
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Geometric Isomer Detection Background and Objectives
Geometric isomers, also known as configurational isomers, are molecules with the same molecular formula but different spatial arrangements of atoms. These isomers play crucial roles in various biological processes, making their detection in biological samples a topic of significant importance in fields such as pharmacology, toxicology, and environmental science. The ability to distinguish between geometric isomers is particularly critical as they often exhibit different biological activities and pharmacological properties.
The development of stereoselective detection methods for geometric isomers in biological samples has been driven by the increasing recognition of their importance in drug development, metabolism studies, and environmental monitoring. Historically, the separation and identification of geometric isomers have posed significant challenges due to their similar physical and chemical properties. Traditional analytical techniques often lacked the sensitivity and specificity required for accurate detection in complex biological matrices.
Over the past few decades, advancements in analytical chemistry and molecular biology have led to the emergence of more sophisticated techniques for stereoselective detection. These developments have been fueled by the growing demand for precise and reliable methods to analyze geometric isomers in various biological contexts, from drug metabolism studies to environmental toxicology assessments.
The primary objectives of stereoselective detection of geometric isomers in biological samples are multifaceted. Firstly, there is a need to develop highly sensitive and specific analytical methods capable of distinguishing between geometric isomers even at low concentrations in complex biological matrices. Secondly, researchers aim to create robust and reproducible techniques that can be applied across a wide range of biological samples and isomeric compounds.
Another key objective is to enhance the speed and efficiency of detection methods, enabling high-throughput screening and real-time monitoring of geometric isomers in biological systems. This is particularly important in drug development and toxicology studies, where rapid and accurate isomer profiling can significantly impact decision-making processes.
Furthermore, there is a growing emphasis on developing non-invasive and in vivo detection methods, which would allow for real-time monitoring of geometric isomers in living organisms. This objective aligns with the broader trend towards personalized medicine and more precise toxicological assessments.
As research in this field progresses, the ultimate goal is to establish standardized protocols for stereoselective detection that can be widely adopted across different scientific disciplines and industrial applications. This standardization would facilitate more consistent and comparable results, advancing our understanding of the role of geometric isomers in biological processes and their implications for human health and the environment.
The development of stereoselective detection methods for geometric isomers in biological samples has been driven by the increasing recognition of their importance in drug development, metabolism studies, and environmental monitoring. Historically, the separation and identification of geometric isomers have posed significant challenges due to their similar physical and chemical properties. Traditional analytical techniques often lacked the sensitivity and specificity required for accurate detection in complex biological matrices.
Over the past few decades, advancements in analytical chemistry and molecular biology have led to the emergence of more sophisticated techniques for stereoselective detection. These developments have been fueled by the growing demand for precise and reliable methods to analyze geometric isomers in various biological contexts, from drug metabolism studies to environmental toxicology assessments.
The primary objectives of stereoselective detection of geometric isomers in biological samples are multifaceted. Firstly, there is a need to develop highly sensitive and specific analytical methods capable of distinguishing between geometric isomers even at low concentrations in complex biological matrices. Secondly, researchers aim to create robust and reproducible techniques that can be applied across a wide range of biological samples and isomeric compounds.
Another key objective is to enhance the speed and efficiency of detection methods, enabling high-throughput screening and real-time monitoring of geometric isomers in biological systems. This is particularly important in drug development and toxicology studies, where rapid and accurate isomer profiling can significantly impact decision-making processes.
Furthermore, there is a growing emphasis on developing non-invasive and in vivo detection methods, which would allow for real-time monitoring of geometric isomers in living organisms. This objective aligns with the broader trend towards personalized medicine and more precise toxicological assessments.
As research in this field progresses, the ultimate goal is to establish standardized protocols for stereoselective detection that can be widely adopted across different scientific disciplines and industrial applications. This standardization would facilitate more consistent and comparable results, advancing our understanding of the role of geometric isomers in biological processes and their implications for human health and the environment.
Market Demand for Stereoselective Analysis
The market demand for stereoselective analysis in biological samples has been steadily increasing due to its critical importance in various fields, particularly in pharmaceutical research and development, clinical diagnostics, and environmental monitoring. This growing demand is driven by the recognition that geometric isomers can exhibit significantly different biological activities, pharmacological properties, and toxicological profiles.
In the pharmaceutical industry, stereoselective detection plays a crucial role in drug discovery, development, and quality control processes. The ability to accurately identify and quantify specific geometric isomers is essential for ensuring drug efficacy and safety. As regulatory agencies worldwide have become more stringent in their requirements for chiral purity and stereochemical characterization of drug candidates, the demand for advanced stereoselective analytical techniques has surged.
The clinical diagnostics sector has also witnessed a rising need for stereoselective analysis. Many biomarkers and metabolites exist as geometric isomers, and their accurate detection can provide valuable insights into disease progression, drug metabolism, and personalized medicine approaches. This has led to an increased demand for sensitive and specific stereoselective detection methods in clinical laboratories and research institutions.
Environmental monitoring represents another significant market for stereoselective analysis. Many pesticides, herbicides, and other environmental contaminants exist as geometric isomers with varying toxicities and persistence in ecosystems. Regulatory bodies and environmental agencies require precise stereoselective detection methods to assess the environmental impact of these compounds and enforce compliance with safety standards.
The food and beverage industry has also shown growing interest in stereoselective analysis for quality control and authenticity verification. Certain flavor compounds and nutritional components exist as geometric isomers, and their accurate detection is crucial for maintaining product quality and meeting regulatory requirements.
As the demand for stereoselective analysis continues to grow, there is a parallel increase in the market for advanced analytical instruments and technologies. High-performance liquid chromatography (HPLC), gas chromatography (GC), and mass spectrometry (MS) systems equipped with chiral columns or detectors are experiencing heightened demand. Additionally, there is a growing market for specialized software and data analysis tools designed to interpret complex stereochemical data.
The global market for stereoselective analysis is projected to expand significantly in the coming years, driven by technological advancements, increasing research activities, and stricter regulatory requirements. This growth is expected to create opportunities for both established analytical instrument manufacturers and innovative startups developing novel stereoselective detection technologies.
In the pharmaceutical industry, stereoselective detection plays a crucial role in drug discovery, development, and quality control processes. The ability to accurately identify and quantify specific geometric isomers is essential for ensuring drug efficacy and safety. As regulatory agencies worldwide have become more stringent in their requirements for chiral purity and stereochemical characterization of drug candidates, the demand for advanced stereoselective analytical techniques has surged.
The clinical diagnostics sector has also witnessed a rising need for stereoselective analysis. Many biomarkers and metabolites exist as geometric isomers, and their accurate detection can provide valuable insights into disease progression, drug metabolism, and personalized medicine approaches. This has led to an increased demand for sensitive and specific stereoselective detection methods in clinical laboratories and research institutions.
Environmental monitoring represents another significant market for stereoselective analysis. Many pesticides, herbicides, and other environmental contaminants exist as geometric isomers with varying toxicities and persistence in ecosystems. Regulatory bodies and environmental agencies require precise stereoselective detection methods to assess the environmental impact of these compounds and enforce compliance with safety standards.
The food and beverage industry has also shown growing interest in stereoselective analysis for quality control and authenticity verification. Certain flavor compounds and nutritional components exist as geometric isomers, and their accurate detection is crucial for maintaining product quality and meeting regulatory requirements.
As the demand for stereoselective analysis continues to grow, there is a parallel increase in the market for advanced analytical instruments and technologies. High-performance liquid chromatography (HPLC), gas chromatography (GC), and mass spectrometry (MS) systems equipped with chiral columns or detectors are experiencing heightened demand. Additionally, there is a growing market for specialized software and data analysis tools designed to interpret complex stereochemical data.
The global market for stereoselective analysis is projected to expand significantly in the coming years, driven by technological advancements, increasing research activities, and stricter regulatory requirements. This growth is expected to create opportunities for both established analytical instrument manufacturers and innovative startups developing novel stereoselective detection technologies.
Current Challenges in Biological Sample Analysis
The field of biological sample analysis faces several significant challenges in the stereoselective detection of geometric isomers. One of the primary obstacles is the complexity of biological matrices, which often contain numerous compounds that can interfere with the detection and quantification of target isomers. This complexity necessitates highly selective and sensitive analytical methods to accurately identify and measure geometric isomers in the presence of structurally similar molecules.
Another major challenge is the inherent instability of certain geometric isomers in biological environments. Some isomers may undergo rapid interconversion or degradation, making their accurate detection and quantification particularly difficult. This instability can lead to misrepresentation of the true isomeric composition in a sample, potentially affecting the interpretation of biological processes or pharmacological effects.
The development of robust sample preparation techniques presents an additional hurdle. Extracting geometric isomers from biological samples while maintaining their original stereochemical configuration is crucial but often problematic. Conventional extraction methods may inadvertently alter the isomeric ratios or induce unwanted isomerization, compromising the integrity of the analysis.
Furthermore, the lack of standardized protocols for stereoselective analysis of geometric isomers in biological samples hinders reproducibility and comparability across different laboratories. This absence of harmonized methods makes it challenging to establish reliable reference ranges and interpret results consistently, especially in clinical or regulatory settings.
The limited availability of pure reference standards for all possible geometric isomers of a given compound poses another significant challenge. Without these standards, accurate calibration and validation of analytical methods become extremely difficult, potentially leading to misidentification or incorrect quantification of isomers in complex biological samples.
Achieving high-throughput analysis while maintaining stereoselectivity is also a considerable challenge. Many current methods for stereoselective detection are time-consuming and labor-intensive, limiting their applicability in large-scale studies or routine clinical analyses. Balancing the need for rapid analysis with the requirement for high stereoselectivity remains a key area of development in the field.
Lastly, the interpretation of data obtained from stereoselective analyses of geometric isomers in biological samples can be complex. Understanding the biological significance of isomeric ratios and their changes under different physiological or pathological conditions requires sophisticated data analysis tools and a deep understanding of both analytical chemistry and biology. This interdisciplinary nature of the field presents ongoing challenges in data interpretation and translation of analytical results into meaningful biological insights.
Another major challenge is the inherent instability of certain geometric isomers in biological environments. Some isomers may undergo rapid interconversion or degradation, making their accurate detection and quantification particularly difficult. This instability can lead to misrepresentation of the true isomeric composition in a sample, potentially affecting the interpretation of biological processes or pharmacological effects.
The development of robust sample preparation techniques presents an additional hurdle. Extracting geometric isomers from biological samples while maintaining their original stereochemical configuration is crucial but often problematic. Conventional extraction methods may inadvertently alter the isomeric ratios or induce unwanted isomerization, compromising the integrity of the analysis.
Furthermore, the lack of standardized protocols for stereoselective analysis of geometric isomers in biological samples hinders reproducibility and comparability across different laboratories. This absence of harmonized methods makes it challenging to establish reliable reference ranges and interpret results consistently, especially in clinical or regulatory settings.
The limited availability of pure reference standards for all possible geometric isomers of a given compound poses another significant challenge. Without these standards, accurate calibration and validation of analytical methods become extremely difficult, potentially leading to misidentification or incorrect quantification of isomers in complex biological samples.
Achieving high-throughput analysis while maintaining stereoselectivity is also a considerable challenge. Many current methods for stereoselective detection are time-consuming and labor-intensive, limiting their applicability in large-scale studies or routine clinical analyses. Balancing the need for rapid analysis with the requirement for high stereoselectivity remains a key area of development in the field.
Lastly, the interpretation of data obtained from stereoselective analyses of geometric isomers in biological samples can be complex. Understanding the biological significance of isomeric ratios and their changes under different physiological or pathological conditions requires sophisticated data analysis tools and a deep understanding of both analytical chemistry and biology. This interdisciplinary nature of the field presents ongoing challenges in data interpretation and translation of analytical results into meaningful biological insights.
Existing Stereoselective Detection Methods
01 Chromatographic separation techniques
Various chromatographic methods are employed for the stereoselective detection of geometric isomers. These techniques include high-performance liquid chromatography (HPLC), gas chromatography (GC), and chiral stationary phase chromatography. These methods allow for the separation and quantification of different geometric isomers based on their physical and chemical properties.- Chromatographic separation techniques: Various chromatographic methods are employed for the stereoselective detection of geometric isomers. These techniques include high-performance liquid chromatography (HPLC), gas chromatography (GC), and chiral column chromatography. These methods allow for the separation and quantification of different geometric isomers based on their physical and chemical properties.
- Spectroscopic analysis methods: Spectroscopic techniques are utilized for the detection and characterization of geometric isomers. These methods include nuclear magnetic resonance (NMR) spectroscopy, infrared (IR) spectroscopy, and mass spectrometry (MS). These techniques provide valuable information about the molecular structure and configuration of geometric isomers, enabling their stereoselective detection.
- Computational modeling and prediction: Advanced computational methods are employed for the prediction and analysis of geometric isomers. These include molecular modeling, quantum chemical calculations, and machine learning algorithms. These techniques help in predicting the stability, reactivity, and properties of different geometric isomers, aiding in their stereoselective detection and characterization.
- Enzymatic and biological assays: Biological methods involving enzymes and other biomolecules are used for the stereoselective detection of geometric isomers. These assays exploit the specificity of certain enzymes or receptors towards particular geometric isomers. This approach is particularly useful in pharmaceutical and biochemical applications where the biological activity of different isomers needs to be distinguished.
- Chemical derivatization techniques: Chemical derivatization methods are employed to enhance the detectability and separability of geometric isomers. This involves converting the isomers into derivatives that are more easily distinguishable by analytical techniques. These methods can improve the sensitivity and selectivity of detection, especially for isomers with similar physical properties.
02 Spectroscopic analysis methods
Spectroscopic techniques such as nuclear magnetic resonance (NMR), infrared (IR) spectroscopy, and circular dichroism (CD) are used for the stereoselective detection of geometric isomers. These methods provide information about the molecular structure and configuration of the isomers, allowing for their identification and characterization.Expand Specific Solutions03 Computational modeling and prediction
Advanced computational methods are utilized to model and predict the behavior of geometric isomers. These include molecular dynamics simulations, quantum mechanical calculations, and machine learning algorithms. Such techniques aid in understanding the properties and interactions of different isomers, facilitating their detection and analysis.Expand Specific Solutions04 Enzymatic and biological assays
Stereoselective detection of geometric isomers can be achieved using enzymatic and biological assays. These methods exploit the specificity of certain enzymes or biological systems towards particular isomers. Such assays can provide high selectivity and sensitivity in detecting and distinguishing between different geometric isomers in complex mixtures.Expand Specific Solutions05 Mass spectrometry-based techniques
Mass spectrometry is employed for the stereoselective detection of geometric isomers. This technique can provide detailed structural information and allow for the differentiation of isomers based on their fragmentation patterns. Advanced mass spectrometry methods, such as ion mobility spectrometry-mass spectrometry (IMS-MS), offer enhanced separation and detection capabilities for geometric isomers.Expand Specific Solutions
Key Players in Analytical Instrumentation
The field of stereoselective detection of geometric isomers in biological samples is in a growth phase, with increasing market demand driven by advancements in analytical chemistry and pharmaceutical research. The global market for this technology is expanding, estimated to reach several hundred million dollars by 2025. Technological maturity varies among key players, with companies like Siemens AG and Microsoft Corp. leveraging their expertise in analytical instrumentation and data processing. Pharmaceutical giants such as Novartis AG and Bristol Myers Squibb Co. are investing heavily in this area to enhance drug development processes. Academic institutions like Washington University in St. Louis and Tsinghua University are contributing significant research, pushing the boundaries of detection sensitivity and specificity.
Siemens AG
Technical Solution: Siemens AG has developed an innovative approach to stereoselective detection of geometric isomers in biological samples using advanced nuclear magnetic resonance (NMR) technology. Their system combines high-field NMR spectroscopy with specialized pulse sequences and probe designs optimized for biological matrices[1]. Siemens has implemented novel diffusion-ordered spectroscopy (DOSY) techniques that allow for the separation of isomers based on their size and shape differences[3]. The company has also developed proprietary software that utilizes artificial intelligence algorithms to analyze complex NMR spectra and accurately identify and quantify geometric isomers[5]. Additionally, Siemens has integrated their NMR technology with automated sample handling and preparation systems, enabling high-throughput analysis of large sample sets[7]. This comprehensive platform has been successfully applied in pharmaceutical research, metabolomics studies, and quality control of natural products[9].
Strengths: Non-destructive analysis, high structural information content, capability for mixture analysis. Weaknesses: Relatively low sensitivity compared to mass spectrometry-based methods, requires expensive high-field NMR instrumentation.
Novartis AG
Technical Solution: Novartis AG has developed a sophisticated approach for stereoselective detection of geometric isomers in biological samples. Their method employs chiral liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS)[1]. This technique allows for the separation and quantification of geometric isomers with high sensitivity and specificity. Novartis has optimized the chromatographic conditions, including the selection of chiral stationary phases and mobile phase compositions, to achieve excellent resolution between isomers[3]. Additionally, they have implemented advanced MS/MS fragmentation strategies to enhance the structural characterization of the isomers[5]. The company has also integrated machine learning algorithms to improve data analysis and isomer identification accuracy[7].
Strengths: High sensitivity and specificity, excellent isomer resolution, advanced structural characterization. Weaknesses: Potentially high cost of equipment and analysis, may require specialized training for operation.
Innovations in Chiral Separation Technologies
Compounds and methods for the treatment of malaria
PatentInactiveIN202118043692A
Innovation
- Development of specific compounds, such as those represented by Formula I and listed in Table 1, which offer new structural features and functional groups to target malaria parasites effectively, including those resistant to existing drugs.
Methods for the identification of bifunctional compounds
PatentInactiveUS20190144503A1
Innovation
- Development of bifunctional compounds consisting of a polypeptide targeting moiety covalently conjugated to a small molecule moiety, which together bind to extracellular target proteins with enhanced affinity and selectivity, exceeding the binding capabilities of either component alone.
Regulatory Considerations for Analytical Methods
The regulatory landscape for analytical methods in the field of stereoselective detection of geometric isomers in biological samples is complex and multifaceted. Regulatory bodies such as the FDA, EMA, and ICH have established stringent guidelines to ensure the reliability, accuracy, and reproducibility of analytical methods used in this domain.
Method validation is a critical aspect of regulatory compliance. Analytical methods for detecting geometric isomers must undergo rigorous validation processes, including assessments of specificity, linearity, accuracy, precision, and robustness. These validation parameters are essential to demonstrate the method's ability to consistently and accurately identify and quantify geometric isomers in complex biological matrices.
Regulatory agencies often require the use of certified reference standards for method development and validation. For geometric isomers, obtaining pure reference standards can be challenging, necessitating careful sourcing and characterization of these materials. The use of well-characterized reference standards is crucial for ensuring the reliability of analytical results.
Selectivity and specificity are particularly important considerations for stereoselective detection methods. Regulatory bodies expect these methods to demonstrate the ability to distinguish between geometric isomers and other structurally similar compounds that may be present in biological samples. This often requires the use of advanced separation techniques, such as chiral chromatography, coupled with sensitive detection methods.
Method transfer and cross-validation are also key regulatory considerations. As analytical methods for geometric isomer detection may be developed in one laboratory and implemented in others, regulatory agencies require robust protocols for method transfer and cross-validation to ensure consistent performance across different sites and instruments.
Data integrity and traceability are paramount in regulatory compliance. Analytical methods must be supported by comprehensive documentation, including standard operating procedures, raw data, and detailed records of method development and validation. Electronic data systems used in the analysis must comply with 21 CFR Part 11 or similar regulations governing electronic records and signatures.
Regulatory bodies also emphasize the importance of ongoing method performance verification. This includes regular system suitability tests, quality control samples, and participation in proficiency testing programs to demonstrate continued method reliability and comparability of results across laboratories.
As the field of stereoselective detection evolves, regulatory agencies are increasingly focusing on the application of novel technologies, such as advanced mass spectrometry techniques and machine learning algorithms for data analysis. While these innovations offer improved sensitivity and specificity, they also present new regulatory challenges in terms of validation and standardization.
Method validation is a critical aspect of regulatory compliance. Analytical methods for detecting geometric isomers must undergo rigorous validation processes, including assessments of specificity, linearity, accuracy, precision, and robustness. These validation parameters are essential to demonstrate the method's ability to consistently and accurately identify and quantify geometric isomers in complex biological matrices.
Regulatory agencies often require the use of certified reference standards for method development and validation. For geometric isomers, obtaining pure reference standards can be challenging, necessitating careful sourcing and characterization of these materials. The use of well-characterized reference standards is crucial for ensuring the reliability of analytical results.
Selectivity and specificity are particularly important considerations for stereoselective detection methods. Regulatory bodies expect these methods to demonstrate the ability to distinguish between geometric isomers and other structurally similar compounds that may be present in biological samples. This often requires the use of advanced separation techniques, such as chiral chromatography, coupled with sensitive detection methods.
Method transfer and cross-validation are also key regulatory considerations. As analytical methods for geometric isomer detection may be developed in one laboratory and implemented in others, regulatory agencies require robust protocols for method transfer and cross-validation to ensure consistent performance across different sites and instruments.
Data integrity and traceability are paramount in regulatory compliance. Analytical methods must be supported by comprehensive documentation, including standard operating procedures, raw data, and detailed records of method development and validation. Electronic data systems used in the analysis must comply with 21 CFR Part 11 or similar regulations governing electronic records and signatures.
Regulatory bodies also emphasize the importance of ongoing method performance verification. This includes regular system suitability tests, quality control samples, and participation in proficiency testing programs to demonstrate continued method reliability and comparability of results across laboratories.
As the field of stereoselective detection evolves, regulatory agencies are increasingly focusing on the application of novel technologies, such as advanced mass spectrometry techniques and machine learning algorithms for data analysis. While these innovations offer improved sensitivity and specificity, they also present new regulatory challenges in terms of validation and standardization.
Biocompatibility and Sample Preparation Techniques
The biocompatibility of materials used in stereoselective detection of geometric isomers in biological samples is crucial for maintaining sample integrity and ensuring accurate results. Materials that come into contact with biological samples must not interfere with the sample's composition or introduce contaminants that could affect the detection process. Polymers, such as polydimethylsiloxane (PDMS) and polyethylene glycol (PEG), are often employed due to their low reactivity and minimal impact on biological systems. These materials can be used to create microfluidic devices or coatings for sample handling equipment, reducing the risk of sample degradation or unwanted interactions.
Sample preparation techniques play a vital role in the successful detection of geometric isomers in biological matrices. The complexity of biological samples necessitates careful consideration of extraction and purification methods to isolate the target isomers effectively. Liquid-liquid extraction (LLE) remains a widely used technique, particularly for lipophilic compounds. However, solid-phase extraction (SPE) has gained popularity due to its versatility and ability to selectively retain specific isomers based on their physicochemical properties. The choice of SPE sorbents, such as C18, ion-exchange, or molecularly imprinted polymers (MIPs), can be tailored to the specific geometric isomers of interest.
Derivatization techniques are often employed to enhance the detectability and separation of geometric isomers. These methods involve chemical modification of the target compounds to improve their chromatographic behavior or increase their ionization efficiency in mass spectrometry. Common derivatization reagents include silylating agents for hydroxyl and carboxyl groups, and fluorescent tags for improved detection sensitivity. However, care must be taken to ensure that the derivatization process does not induce isomerization or alter the stereochemistry of the target compounds.
The development of miniaturized sample preparation techniques has gained traction in recent years, aligning with the trend towards high-throughput analysis and reduced sample volumes. Techniques such as dispersive liquid-liquid microextraction (DLLME) and solid-phase microextraction (SPME) offer advantages in terms of speed, efficiency, and minimal solvent consumption. These methods are particularly beneficial when dealing with limited sample quantities, as is often the case in biological studies.
Automation of sample preparation processes has become increasingly important in ensuring reproducibility and reducing human error. Robotic systems capable of performing multiple sample preparation steps, from extraction to derivatization, have been developed to streamline workflows and improve analytical precision. These automated platforms can be integrated with chromatographic and mass spectrometric systems, enabling seamless sample processing and analysis for high-throughput stereoselective detection of geometric isomers in complex biological matrices.
Sample preparation techniques play a vital role in the successful detection of geometric isomers in biological matrices. The complexity of biological samples necessitates careful consideration of extraction and purification methods to isolate the target isomers effectively. Liquid-liquid extraction (LLE) remains a widely used technique, particularly for lipophilic compounds. However, solid-phase extraction (SPE) has gained popularity due to its versatility and ability to selectively retain specific isomers based on their physicochemical properties. The choice of SPE sorbents, such as C18, ion-exchange, or molecularly imprinted polymers (MIPs), can be tailored to the specific geometric isomers of interest.
Derivatization techniques are often employed to enhance the detectability and separation of geometric isomers. These methods involve chemical modification of the target compounds to improve their chromatographic behavior or increase their ionization efficiency in mass spectrometry. Common derivatization reagents include silylating agents for hydroxyl and carboxyl groups, and fluorescent tags for improved detection sensitivity. However, care must be taken to ensure that the derivatization process does not induce isomerization or alter the stereochemistry of the target compounds.
The development of miniaturized sample preparation techniques has gained traction in recent years, aligning with the trend towards high-throughput analysis and reduced sample volumes. Techniques such as dispersive liquid-liquid microextraction (DLLME) and solid-phase microextraction (SPME) offer advantages in terms of speed, efficiency, and minimal solvent consumption. These methods are particularly beneficial when dealing with limited sample quantities, as is often the case in biological studies.
Automation of sample preparation processes has become increasingly important in ensuring reproducibility and reducing human error. Robotic systems capable of performing multiple sample preparation steps, from extraction to derivatization, have been developed to streamline workflows and improve analytical precision. These automated platforms can be integrated with chromatographic and mass spectrometric systems, enabling seamless sample processing and analysis for high-throughput stereoselective detection of geometric isomers in complex biological matrices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!