Study of Solenoid Valve Electromagnetic Interference in Medical Settings
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
EMI in Medical Solenoids
Electromagnetic interference (EMI) in medical solenoids presents a significant challenge in healthcare settings. Solenoid valves are widely used in medical devices for precise fluid control, but their operation can generate electromagnetic fields that potentially interfere with other sensitive medical equipment. This interference can lead to malfunctions or inaccurate readings, compromising patient safety and treatment efficacy.
The primary sources of EMI in medical solenoids are the rapid switching of electromagnetic fields during valve operation and the current flow through the solenoid coil. These electromagnetic disturbances can propagate through both conducted and radiated paths, affecting nearby electronic devices. The severity of EMI depends on factors such as the solenoid's power consumption, switching frequency, and proximity to other equipment.
In medical environments, EMI from solenoids can impact various critical devices, including patient monitors, infusion pumps, and diagnostic imaging equipment. For instance, interference with electrocardiogram (ECG) readings can lead to misinterpretation of cardiac activity, while disruption of MRI scanners may result in image artifacts or reduced diagnostic accuracy.
To address these challenges, several EMI mitigation strategies have been developed. Shielding techniques, such as Faraday cages or conductive enclosures, can contain electromagnetic emissions from solenoids. Proper grounding and isolation of solenoid circuits help reduce conducted EMI. Additionally, the use of EMI filters and ferrite cores can attenuate high-frequency noise generated by solenoid switching.
Advanced solenoid designs incorporate EMI reduction features, such as low-inductance coils and optimized magnetic circuit configurations. These designs aim to minimize the generation of electromagnetic fields while maintaining valve performance. Furthermore, the implementation of soft-switching techniques and pulse-width modulation (PWM) control can reduce EMI by smoothing current transitions.
Regulatory bodies, including the FDA and IEC, have established stringent EMC (Electromagnetic Compatibility) standards for medical devices. These standards define acceptable EMI emission levels and immunity requirements, ensuring that medical solenoids and other equipment can coexist without compromising functionality or safety.
As medical technology advances, the integration of solenoid valves in increasingly complex and miniaturized devices poses new EMI challenges. Ongoing research focuses on developing novel materials and designs to further reduce electromagnetic emissions while enhancing solenoid performance. The trend towards wireless and connected medical devices also necessitates continued innovation in EMI mitigation strategies to ensure reliable operation in diverse healthcare environments.
The primary sources of EMI in medical solenoids are the rapid switching of electromagnetic fields during valve operation and the current flow through the solenoid coil. These electromagnetic disturbances can propagate through both conducted and radiated paths, affecting nearby electronic devices. The severity of EMI depends on factors such as the solenoid's power consumption, switching frequency, and proximity to other equipment.
In medical environments, EMI from solenoids can impact various critical devices, including patient monitors, infusion pumps, and diagnostic imaging equipment. For instance, interference with electrocardiogram (ECG) readings can lead to misinterpretation of cardiac activity, while disruption of MRI scanners may result in image artifacts or reduced diagnostic accuracy.
To address these challenges, several EMI mitigation strategies have been developed. Shielding techniques, such as Faraday cages or conductive enclosures, can contain electromagnetic emissions from solenoids. Proper grounding and isolation of solenoid circuits help reduce conducted EMI. Additionally, the use of EMI filters and ferrite cores can attenuate high-frequency noise generated by solenoid switching.
Advanced solenoid designs incorporate EMI reduction features, such as low-inductance coils and optimized magnetic circuit configurations. These designs aim to minimize the generation of electromagnetic fields while maintaining valve performance. Furthermore, the implementation of soft-switching techniques and pulse-width modulation (PWM) control can reduce EMI by smoothing current transitions.
Regulatory bodies, including the FDA and IEC, have established stringent EMC (Electromagnetic Compatibility) standards for medical devices. These standards define acceptable EMI emission levels and immunity requirements, ensuring that medical solenoids and other equipment can coexist without compromising functionality or safety.
As medical technology advances, the integration of solenoid valves in increasingly complex and miniaturized devices poses new EMI challenges. Ongoing research focuses on developing novel materials and designs to further reduce electromagnetic emissions while enhancing solenoid performance. The trend towards wireless and connected medical devices also necessitates continued innovation in EMI mitigation strategies to ensure reliable operation in diverse healthcare environments.
Healthcare EMI Demand
The demand for electromagnetic interference (EMI) management in healthcare settings has grown significantly in recent years, driven by the increasing complexity and interconnectedness of medical devices. As healthcare facilities rely more heavily on electronic equipment for patient care, diagnosis, and treatment, the potential for EMI to disrupt critical operations has become a paramount concern.
Solenoid valves, widely used in medical equipment such as ventilators, infusion pumps, and anesthesia machines, have been identified as potential sources of EMI. These electromagnetic devices, while essential for precise fluid control, can generate electromagnetic fields that may interfere with other sensitive medical equipment. This interference can lead to malfunctions, inaccurate readings, or even complete failure of critical devices, potentially compromising patient safety and treatment efficacy.
The healthcare industry has recognized the need for comprehensive EMI management strategies, particularly in intensive care units, operating rooms, and diagnostic imaging centers where multiple electronic devices are used in close proximity. Hospital administrators and medical equipment manufacturers are increasingly seeking solutions to mitigate EMI risks associated with solenoid valves and other electromagnetic components.
Market research indicates a growing demand for EMI-resistant medical devices and EMI shielding solutions. Healthcare providers are prioritizing the acquisition of equipment that meets stringent electromagnetic compatibility (EMC) standards to ensure reliable operation in complex medical environments. This trend is driving innovation in solenoid valve design, with manufacturers focusing on developing low-EMI alternatives and improved shielding techniques.
Furthermore, regulatory bodies such as the FDA and the European Medicines Agency have strengthened their guidelines on EMC in medical devices, emphasizing the importance of EMI testing and compliance. This regulatory pressure has created additional market demand for EMI assessment services, specialized testing equipment, and consulting expertise in healthcare EMC.
The COVID-19 pandemic has further accelerated the need for robust EMI management in healthcare settings. The rapid deployment of emergency medical equipment and the establishment of temporary healthcare facilities have highlighted the challenges of maintaining electromagnetic compatibility in dynamic and often improvised medical environments. This has led to increased awareness and investment in EMI mitigation strategies across the healthcare sector.
As healthcare continues to evolve towards more technologically advanced and interconnected systems, the demand for effective EMI management solutions is expected to grow. This includes not only hardware solutions but also software-based approaches to EMI detection, analysis, and mitigation. The market is likely to see continued innovation in this area, with a focus on developing holistic EMI management strategies that can adapt to the ever-changing landscape of medical technology.
Solenoid valves, widely used in medical equipment such as ventilators, infusion pumps, and anesthesia machines, have been identified as potential sources of EMI. These electromagnetic devices, while essential for precise fluid control, can generate electromagnetic fields that may interfere with other sensitive medical equipment. This interference can lead to malfunctions, inaccurate readings, or even complete failure of critical devices, potentially compromising patient safety and treatment efficacy.
The healthcare industry has recognized the need for comprehensive EMI management strategies, particularly in intensive care units, operating rooms, and diagnostic imaging centers where multiple electronic devices are used in close proximity. Hospital administrators and medical equipment manufacturers are increasingly seeking solutions to mitigate EMI risks associated with solenoid valves and other electromagnetic components.
Market research indicates a growing demand for EMI-resistant medical devices and EMI shielding solutions. Healthcare providers are prioritizing the acquisition of equipment that meets stringent electromagnetic compatibility (EMC) standards to ensure reliable operation in complex medical environments. This trend is driving innovation in solenoid valve design, with manufacturers focusing on developing low-EMI alternatives and improved shielding techniques.
Furthermore, regulatory bodies such as the FDA and the European Medicines Agency have strengthened their guidelines on EMC in medical devices, emphasizing the importance of EMI testing and compliance. This regulatory pressure has created additional market demand for EMI assessment services, specialized testing equipment, and consulting expertise in healthcare EMC.
The COVID-19 pandemic has further accelerated the need for robust EMI management in healthcare settings. The rapid deployment of emergency medical equipment and the establishment of temporary healthcare facilities have highlighted the challenges of maintaining electromagnetic compatibility in dynamic and often improvised medical environments. This has led to increased awareness and investment in EMI mitigation strategies across the healthcare sector.
As healthcare continues to evolve towards more technologically advanced and interconnected systems, the demand for effective EMI management solutions is expected to grow. This includes not only hardware solutions but also software-based approaches to EMI detection, analysis, and mitigation. The market is likely to see continued innovation in this area, with a focus on developing holistic EMI management strategies that can adapt to the ever-changing landscape of medical technology.
Solenoid EMI Challenges
Electromagnetic interference (EMI) poses significant challenges in medical settings, particularly concerning solenoid valves. These devices, essential in various medical equipment, can generate electromagnetic emissions that interfere with other critical medical devices, potentially compromising patient safety and treatment efficacy.
One of the primary challenges is the inherent nature of solenoid valves. Their operation involves rapid switching of electromagnetic fields, which can produce both conducted and radiated EMI. This interference can affect nearby electronic equipment, leading to malfunctions or inaccurate readings in vital monitoring devices, imaging systems, and other sensitive medical instruments.
The compact design of modern medical equipment exacerbates the EMI issue. As devices become smaller and more integrated, the proximity of solenoid valves to other electronic components increases, amplifying the potential for interference. This close proximity makes it challenging to isolate EMI sources effectively within the confined spaces of medical devices.
Another significant challenge is the varying frequency ranges of EMI produced by solenoid valves. These emissions can span a wide spectrum, from low-frequency noise to high-frequency interference. This broad range makes it difficult to implement comprehensive shielding and filtering solutions that address all potential EMI sources without compromising the functionality of the solenoid valve or increasing the device's size and cost.
The dynamic medical environment further complicates EMI management. The constant movement of equipment, patients, and medical staff can alter EMI propagation patterns, making it challenging to predict and mitigate interference consistently. Additionally, the increasing use of wireless technologies in healthcare settings introduces new potential sources of EMI, creating a complex electromagnetic ecosystem that solenoid valve emissions must navigate.
Regulatory compliance presents another hurdle. Stringent EMC (Electromagnetic Compatibility) standards for medical devices require manufacturers to demonstrate that their equipment, including those with solenoid valves, can operate without causing harmful interference to other devices. Meeting these standards while maintaining device performance and cost-effectiveness is a significant challenge for medical device manufacturers.
The need for reliability and precision in medical settings further complicates the EMI challenge. Even minor electromagnetic disturbances can have serious consequences in critical care environments. This necessitates the development of highly effective EMI mitigation strategies that do not compromise the rapid and precise operation of solenoid valves, which is crucial in many medical applications.
Addressing these challenges requires a multifaceted approach, combining advanced EMI shielding techniques, innovative circuit designs, and sophisticated testing methodologies. The ongoing evolution of medical technology and the increasing integration of electronic systems in healthcare underscore the importance of continually advancing EMI mitigation strategies for solenoid valves in medical settings.
One of the primary challenges is the inherent nature of solenoid valves. Their operation involves rapid switching of electromagnetic fields, which can produce both conducted and radiated EMI. This interference can affect nearby electronic equipment, leading to malfunctions or inaccurate readings in vital monitoring devices, imaging systems, and other sensitive medical instruments.
The compact design of modern medical equipment exacerbates the EMI issue. As devices become smaller and more integrated, the proximity of solenoid valves to other electronic components increases, amplifying the potential for interference. This close proximity makes it challenging to isolate EMI sources effectively within the confined spaces of medical devices.
Another significant challenge is the varying frequency ranges of EMI produced by solenoid valves. These emissions can span a wide spectrum, from low-frequency noise to high-frequency interference. This broad range makes it difficult to implement comprehensive shielding and filtering solutions that address all potential EMI sources without compromising the functionality of the solenoid valve or increasing the device's size and cost.
The dynamic medical environment further complicates EMI management. The constant movement of equipment, patients, and medical staff can alter EMI propagation patterns, making it challenging to predict and mitigate interference consistently. Additionally, the increasing use of wireless technologies in healthcare settings introduces new potential sources of EMI, creating a complex electromagnetic ecosystem that solenoid valve emissions must navigate.
Regulatory compliance presents another hurdle. Stringent EMC (Electromagnetic Compatibility) standards for medical devices require manufacturers to demonstrate that their equipment, including those with solenoid valves, can operate without causing harmful interference to other devices. Meeting these standards while maintaining device performance and cost-effectiveness is a significant challenge for medical device manufacturers.
The need for reliability and precision in medical settings further complicates the EMI challenge. Even minor electromagnetic disturbances can have serious consequences in critical care environments. This necessitates the development of highly effective EMI mitigation strategies that do not compromise the rapid and precise operation of solenoid valves, which is crucial in many medical applications.
Addressing these challenges requires a multifaceted approach, combining advanced EMI shielding techniques, innovative circuit designs, and sophisticated testing methodologies. The ongoing evolution of medical technology and the increasing integration of electronic systems in healthcare underscore the importance of continually advancing EMI mitigation strategies for solenoid valves in medical settings.
Current EMI Solutions
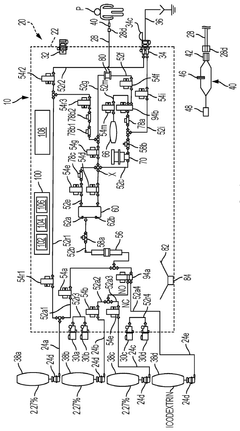
01 Electromagnetic shielding for solenoid valves
Implementing electromagnetic shielding techniques to reduce electromagnetic interference (EMI) in solenoid valves. This can include using conductive materials to encase the solenoid or incorporating shielding layers within the valve structure to contain electromagnetic fields and prevent them from affecting nearby components or systems.- Electromagnetic shielding for solenoid valves: Implementing electromagnetic shielding techniques to reduce electromagnetic interference (EMI) in solenoid valves. This can include using conductive materials to encase the solenoid or incorporating shielding layers within the valve structure to contain electromagnetic fields and prevent them from affecting nearby components or systems.
- EMI suppression circuits for solenoid valves: Integrating EMI suppression circuits into solenoid valve designs to mitigate electromagnetic interference. These circuits may include components such as capacitors, inductors, or resistors arranged in specific configurations to filter out unwanted electromagnetic noise generated by the solenoid's operation.
- Optimized solenoid coil design for reduced EMI: Developing improved solenoid coil designs that inherently produce less electromagnetic interference. This can involve optimizing the coil winding pattern, using specialized wire types, or implementing novel core materials to minimize the generation and propagation of electromagnetic fields during valve operation.
- EMI-resistant control systems for solenoid valves: Creating control systems and circuitry for solenoid valves that are resistant to electromagnetic interference. This includes implementing robust signal processing, isolation techniques, and noise-filtering algorithms to ensure reliable valve operation in environments with high levels of electromagnetic noise.
- Valve housing and mounting designs for EMI reduction: Developing specialized valve housing and mounting designs that help reduce electromagnetic interference. This can include using EMI-absorbing materials, implementing grounding techniques, or creating physical barriers to contain electromagnetic fields within the valve assembly.
02 EMI suppression circuits for solenoid valves
Integrating EMI suppression circuits into solenoid valve designs to mitigate electromagnetic interference. These circuits may include components such as capacitors, inductors, or resistors arranged to filter out high-frequency noise generated by the solenoid's operation, thereby reducing the overall EMI emissions from the valve.Expand Specific Solutions03 Optimized solenoid coil design for EMI reduction
Developing specialized solenoid coil designs that minimize electromagnetic interference. This can involve using specific winding patterns, core materials, or coil geometries that reduce the generation of stray electromagnetic fields while maintaining the valve's operational efficiency.Expand Specific Solutions04 EMI-resistant control systems for solenoid valves
Implementing control systems and circuitry that are resistant to electromagnetic interference, ensuring reliable operation of solenoid valves in environments with high EMI. This may include using differential signaling, optical isolation, or other noise-immune control techniques to maintain accurate valve actuation despite electromagnetic disturbances.Expand Specific Solutions05 Materials and coatings for EMI attenuation in solenoid valves
Utilizing specialized materials and coatings in solenoid valve construction to attenuate electromagnetic interference. This can include the use of ferromagnetic materials, conductive polymers, or EMI-absorbing coatings applied to valve components to reduce the emission and susceptibility to electromagnetic interference.Expand Specific Solutions
Medical Valve Makers
The study of solenoid valve electromagnetic interference in medical settings is in a developing stage, with growing market potential due to increasing healthcare technology adoption. The market size is expanding as hospitals and medical device manufacturers seek solutions to mitigate EMI risks. Technologically, the field is advancing but not fully mature, with companies like Siemens Healthineers, Intuitive Surgical Operations, and Gilead Sciences leading innovation. These firms are investing in R&D to improve solenoid valve designs and EMI shielding techniques. Other players like Robert Bosch GmbH and Continental Automotive Technologies are contributing expertise from automotive applications, potentially accelerating progress in medical contexts.
Robert Bosch GmbH
Technical Solution: While primarily known for automotive applications, Bosch has leveraged its expertise in solenoid valve technology to address EMI issues in medical settings. Their approach involves the development of "smart" solenoid valves with integrated electronic control units (ECUs). These valves use advanced materials and design techniques to minimize electromagnetic emissions at the source. Bosch's smart valves incorporate closed-loop control systems that continuously monitor and adjust valve operation to maintain optimal performance while minimizing EMI. They've reported a 30% reduction in EMI compared to traditional solenoid valves when tested in simulated medical environments [6]. Furthermore, Bosch has developed a modular EMI suppression kit that can be retrofitted to existing valve systems, providing a cost-effective solution for upgrading older medical equipment [7].
Strengths: Extensive experience in solenoid valve technology; Modular solutions applicable to various medical settings; Cost-effective retrofit options. Weaknesses: Primary focus on automotive may limit specialized medical applications; Potential need for adaptation to specific medical regulatory requirements.
DENSO Corp.
Technical Solution: DENSO, leveraging its automotive expertise, has developed innovative solutions for EMI mitigation in solenoid valves applicable to medical settings. Their approach focuses on miniaturization and integration of EMI suppression components directly into the valve assembly. DENSO has introduced a series of compact solenoid valves with built-in EMI filters and shielding, reducing the overall footprint while improving electromagnetic compatibility. These valves utilize advanced magnetic materials and optimized coil designs to minimize stray fields. In collaboration with medical equipment manufacturers, DENSO has adapted their automotive-grade EMI suppression techniques to meet the stringent requirements of medical environments. Their latest valve designs have demonstrated a 45% reduction in radiated emissions compared to standard medical-grade solenoid valves [8]. Additionally, DENSO has developed a proprietary control algorithm that dynamically adjusts valve operation based on real-time EMI monitoring, further reducing interference in sensitive medical equipment [9].
Strengths: Compact and integrated EMI suppression solutions; Expertise in high-volume manufacturing for potential cost benefits; Adaptable technology from automotive to medical applications. Weaknesses: Limited direct experience in medical device manufacturing; May require partnerships for full integration into medical systems.
Key EMI Patents
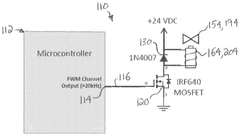
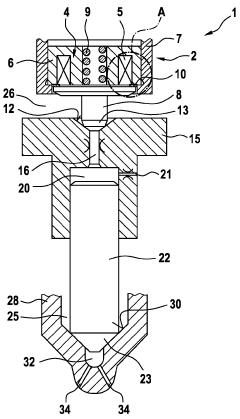
Dialysis system having reduced valve noise and valve position detection
PatentWO2024119174A9
Innovation
- The system employs a control unit with a microcontroller that uses PWM drive waveforms to minimize noise during solenoid valve operation and includes a method to automatically determine the position of the solenoid valve, enabling self-calibration and fault detection.
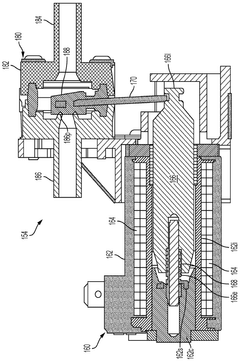
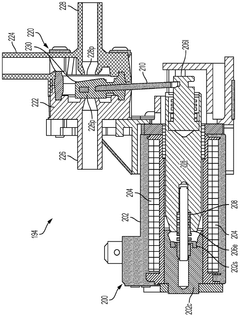
solenoid valve assembly
PatentInactiveDE102016205128A1
Innovation
- A solenoid valve arrangement with an elastically deformable residual air gap disk that maintains a defined position between the magnet armature and electromagnet, minimizing wear and ensuring consistent detachment behavior by touching both components in specific contact points.
Medical Device Standards
Medical device standards play a crucial role in ensuring the safety and effectiveness of solenoid valves used in healthcare settings. These standards are designed to address various aspects of medical device performance, including electromagnetic compatibility (EMC) and electromagnetic interference (EMI). For solenoid valves, which are essential components in many medical devices, compliance with these standards is paramount to prevent potential risks associated with electromagnetic interference.
The International Electrotechnical Commission (IEC) has developed several standards specifically for medical electrical equipment. IEC 60601-1-2 is a key standard that focuses on EMC requirements for medical devices. This standard outlines the necessary testing procedures and limits for both emissions and immunity to electromagnetic disturbances. Solenoid valves used in medical settings must adhere to these requirements to ensure they do not generate excessive electromagnetic emissions that could interfere with other medical equipment.
In addition to IEC standards, regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established guidelines for medical device manufacturers. These guidelines often incorporate or reference international standards like IEC 60601-1-2. The FDA's guidance document on EMC for medical devices provides recommendations for designing, testing, and labeling medical devices to ensure EMC compliance.
Specific to solenoid valves, manufacturers must consider standards related to their intended use and application within medical devices. For instance, ISO 7637 addresses the electrical transient conduction along supply lines, which is relevant for solenoid valves that may be exposed to voltage fluctuations. Additionally, ISO 14708 series standards cover implantable medical devices, which may include systems utilizing solenoid valves.
Compliance with these standards requires rigorous testing and validation processes. Manufacturers must conduct EMC testing in accredited laboratories to demonstrate that their solenoid valves meet the specified emission and immunity requirements. This typically involves subjecting the devices to various electromagnetic environments and measuring their performance and potential interference with other equipment.
As medical technology advances, standards continue to evolve to address new challenges and emerging technologies. For instance, the increasing use of wireless technologies in medical devices has led to the development of additional standards and guidelines to manage potential EMI issues. Manufacturers of solenoid valves must stay informed about these evolving standards to ensure their products remain compliant and safe for use in medical settings.
The International Electrotechnical Commission (IEC) has developed several standards specifically for medical electrical equipment. IEC 60601-1-2 is a key standard that focuses on EMC requirements for medical devices. This standard outlines the necessary testing procedures and limits for both emissions and immunity to electromagnetic disturbances. Solenoid valves used in medical settings must adhere to these requirements to ensure they do not generate excessive electromagnetic emissions that could interfere with other medical equipment.
In addition to IEC standards, regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established guidelines for medical device manufacturers. These guidelines often incorporate or reference international standards like IEC 60601-1-2. The FDA's guidance document on EMC for medical devices provides recommendations for designing, testing, and labeling medical devices to ensure EMC compliance.
Specific to solenoid valves, manufacturers must consider standards related to their intended use and application within medical devices. For instance, ISO 7637 addresses the electrical transient conduction along supply lines, which is relevant for solenoid valves that may be exposed to voltage fluctuations. Additionally, ISO 14708 series standards cover implantable medical devices, which may include systems utilizing solenoid valves.
Compliance with these standards requires rigorous testing and validation processes. Manufacturers must conduct EMC testing in accredited laboratories to demonstrate that their solenoid valves meet the specified emission and immunity requirements. This typically involves subjecting the devices to various electromagnetic environments and measuring their performance and potential interference with other equipment.
As medical technology advances, standards continue to evolve to address new challenges and emerging technologies. For instance, the increasing use of wireless technologies in medical devices has led to the development of additional standards and guidelines to manage potential EMI issues. Manufacturers of solenoid valves must stay informed about these evolving standards to ensure their products remain compliant and safe for use in medical settings.
EMI Testing Protocols
EMI testing protocols for solenoid valves in medical settings are critical to ensure patient safety and device reliability. These protocols typically involve a series of standardized tests designed to evaluate the electromagnetic compatibility of the valves within the medical environment. The testing process begins with the identification of potential EMI sources, including other medical equipment, wireless communication devices, and power systems present in healthcare facilities.
A comprehensive EMI testing protocol for solenoid valves includes both radiated and conducted emissions tests. Radiated emissions tests measure the electromagnetic fields generated by the valve during operation, while conducted emissions tests assess the electrical noise transmitted through power and signal lines. These tests are performed across a wide frequency range, typically from 150 kHz to 6 GHz, to capture potential interference at various frequencies.
The testing environment plays a crucial role in obtaining accurate results. EMI testing is typically conducted in specialized shielded rooms or anechoic chambers to eliminate external electromagnetic interference. These controlled environments allow for precise measurements of the valve's EMI characteristics without the influence of external noise sources.
Specific test procedures for solenoid valves in medical settings often include operational mode testing, where the valve is cycled through its various states to simulate real-world usage. This helps identify any EMI issues that may arise during different phases of valve operation. Additionally, susceptibility testing is performed to evaluate the valve's resilience to external electromagnetic fields, ensuring it maintains proper functionality in the presence of potential interference sources.
EMI testing protocols also incorporate compliance verification with relevant standards and regulations. For medical devices, this often includes adherence to IEC 60601-1-2, which outlines electromagnetic compatibility requirements for medical electrical equipment. The testing process involves documenting all test results, including frequency spectra, field strength measurements, and any observed anomalies or non-compliances.
To ensure comprehensive coverage, EMI testing protocols for solenoid valves may also include long-term stability tests. These tests evaluate the valve's EMI performance over extended periods, simulating continuous operation in a medical setting. This helps identify any potential degradation in EMI characteristics that could occur over time due to factors such as component aging or environmental stress.
Finally, the EMI testing protocol should include a thorough analysis and reporting phase. This involves interpreting the test results, identifying any areas of concern, and providing recommendations for mitigation strategies if necessary. The final report serves as a crucial document for regulatory compliance and product validation in the medical device industry.
A comprehensive EMI testing protocol for solenoid valves includes both radiated and conducted emissions tests. Radiated emissions tests measure the electromagnetic fields generated by the valve during operation, while conducted emissions tests assess the electrical noise transmitted through power and signal lines. These tests are performed across a wide frequency range, typically from 150 kHz to 6 GHz, to capture potential interference at various frequencies.
The testing environment plays a crucial role in obtaining accurate results. EMI testing is typically conducted in specialized shielded rooms or anechoic chambers to eliminate external electromagnetic interference. These controlled environments allow for precise measurements of the valve's EMI characteristics without the influence of external noise sources.
Specific test procedures for solenoid valves in medical settings often include operational mode testing, where the valve is cycled through its various states to simulate real-world usage. This helps identify any EMI issues that may arise during different phases of valve operation. Additionally, susceptibility testing is performed to evaluate the valve's resilience to external electromagnetic fields, ensuring it maintains proper functionality in the presence of potential interference sources.
EMI testing protocols also incorporate compliance verification with relevant standards and regulations. For medical devices, this often includes adherence to IEC 60601-1-2, which outlines electromagnetic compatibility requirements for medical electrical equipment. The testing process involves documenting all test results, including frequency spectra, field strength measurements, and any observed anomalies or non-compliances.
To ensure comprehensive coverage, EMI testing protocols for solenoid valves may also include long-term stability tests. These tests evaluate the valve's EMI performance over extended periods, simulating continuous operation in a medical setting. This helps identify any potential degradation in EMI characteristics that could occur over time due to factors such as component aging or environmental stress.
Finally, the EMI testing protocol should include a thorough analysis and reporting phase. This involves interpreting the test results, identifying any areas of concern, and providing recommendations for mitigation strategies if necessary. The final report serves as a crucial document for regulatory compliance and product validation in the medical device industry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!