Synthesis and Characterization of Geometric Isomers of Hydrides
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydride Isomer Research Background and Objectives
The field of hydride isomer research has gained significant attention in recent years due to its potential applications in energy storage, catalysis, and materials science. Geometric isomers of hydrides, in particular, have emerged as a fascinating area of study, offering unique properties and potential solutions to various technological challenges.
The evolution of hydride chemistry can be traced back to the early 20th century, with pioneering work on simple hydrides. However, it was not until the latter half of the century that researchers began to explore the complexities of geometric isomerism in hydride compounds. This shift in focus was driven by the realization that subtle differences in molecular structure could lead to dramatic changes in chemical and physical properties.
As we entered the 21st century, advancements in synthetic methodologies and characterization techniques have propelled the field forward, enabling scientists to create and study increasingly complex hydride isomers. The ability to precisely control the spatial arrangement of atoms within these molecules has opened up new avenues for tailoring material properties at the molecular level.
The primary objective of current research in this area is to develop a comprehensive understanding of the relationship between the geometric structure of hydride isomers and their functional properties. This includes investigating how different isomeric forms affect hydrogen storage capacity, catalytic activity, and electronic properties. By elucidating these structure-property relationships, researchers aim to design and synthesize hydride isomers with optimized performance for specific applications.
Another crucial goal is to establish reliable and scalable methods for the selective synthesis of desired geometric isomers. This involves developing novel synthetic strategies that can overcome thermodynamic barriers and kinetic limitations, allowing for precise control over the isomeric composition of the final product. Concurrently, there is a strong focus on advancing characterization techniques to accurately identify and quantify different isomeric forms in complex mixtures.
The field is also moving towards exploring the potential of hydride isomers in emerging technologies. This includes their use in hydrogen evolution reactions for clean energy production, as components in advanced battery systems, and as precursors for the synthesis of novel materials with unique electronic or magnetic properties. As such, the research aims to bridge the gap between fundamental science and practical applications, driving innovation in multiple technological domains.
In the broader context of materials science and chemistry, the study of geometric isomers of hydrides represents a convergence of multiple disciplines, including inorganic chemistry, physical chemistry, and materials engineering. This interdisciplinary approach is essential for addressing the complex challenges associated with synthesizing, characterizing, and harnessing the properties of these fascinating compounds.
The evolution of hydride chemistry can be traced back to the early 20th century, with pioneering work on simple hydrides. However, it was not until the latter half of the century that researchers began to explore the complexities of geometric isomerism in hydride compounds. This shift in focus was driven by the realization that subtle differences in molecular structure could lead to dramatic changes in chemical and physical properties.
As we entered the 21st century, advancements in synthetic methodologies and characterization techniques have propelled the field forward, enabling scientists to create and study increasingly complex hydride isomers. The ability to precisely control the spatial arrangement of atoms within these molecules has opened up new avenues for tailoring material properties at the molecular level.
The primary objective of current research in this area is to develop a comprehensive understanding of the relationship between the geometric structure of hydride isomers and their functional properties. This includes investigating how different isomeric forms affect hydrogen storage capacity, catalytic activity, and electronic properties. By elucidating these structure-property relationships, researchers aim to design and synthesize hydride isomers with optimized performance for specific applications.
Another crucial goal is to establish reliable and scalable methods for the selective synthesis of desired geometric isomers. This involves developing novel synthetic strategies that can overcome thermodynamic barriers and kinetic limitations, allowing for precise control over the isomeric composition of the final product. Concurrently, there is a strong focus on advancing characterization techniques to accurately identify and quantify different isomeric forms in complex mixtures.
The field is also moving towards exploring the potential of hydride isomers in emerging technologies. This includes their use in hydrogen evolution reactions for clean energy production, as components in advanced battery systems, and as precursors for the synthesis of novel materials with unique electronic or magnetic properties. As such, the research aims to bridge the gap between fundamental science and practical applications, driving innovation in multiple technological domains.
In the broader context of materials science and chemistry, the study of geometric isomers of hydrides represents a convergence of multiple disciplines, including inorganic chemistry, physical chemistry, and materials engineering. This interdisciplinary approach is essential for addressing the complex challenges associated with synthesizing, characterizing, and harnessing the properties of these fascinating compounds.
Market Analysis for Geometric Hydride Isomers
The market for geometric hydride isomers is experiencing significant growth, driven by their diverse applications in various industries. These compounds play crucial roles in energy storage, catalysis, and materials science, making them highly sought after in both research and industrial sectors.
In the energy storage domain, geometric hydride isomers are gaining traction as potential hydrogen storage materials. The global hydrogen storage market is projected to reach $18.2 billion by 2027, with a compound annual growth rate (CAGR) of 8.3% from 2020 to 2027. This growth is primarily fueled by the increasing adoption of hydrogen fuel cells in transportation and stationary power generation.
The catalysis industry represents another significant market for geometric hydride isomers. These compounds serve as efficient catalysts in various chemical processes, including hydrogenation reactions and organic syntheses. The global catalyst market, valued at $33.9 billion in 2020, is expected to grow at a CAGR of 4.8% from 2021 to 2028, with hydride-based catalysts contributing to this expansion.
In materials science, geometric hydride isomers are finding applications in the development of advanced materials with unique properties. The global advanced materials market, which includes these specialized compounds, is forecasted to reach $102.48 billion by 2024, growing at a CAGR of 4.4% from 2019 to 2024.
Geographically, North America and Europe currently dominate the market for geometric hydride isomers, owing to their advanced research infrastructure and strong presence of chemical and materials industries. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing industrialization and research activities in countries like China, Japan, and South Korea.
The market is characterized by a mix of established chemical companies and specialized materials suppliers. Key players include BASF SE, Albemarle Corporation, and Johnson Matthey, among others. These companies are investing heavily in research and development to expand their product portfolios and maintain their competitive edge.
Despite the promising outlook, the market faces challenges such as high production costs and safety concerns associated with handling hydride compounds. However, ongoing research and technological advancements are expected to address these issues, further driving market growth.
In conclusion, the market for geometric hydride isomers presents significant opportunities across multiple industries. As research continues to uncover new applications and improve existing ones, the demand for these compounds is expected to rise, making them a key focus area for both academic and industrial research in the coming years.
In the energy storage domain, geometric hydride isomers are gaining traction as potential hydrogen storage materials. The global hydrogen storage market is projected to reach $18.2 billion by 2027, with a compound annual growth rate (CAGR) of 8.3% from 2020 to 2027. This growth is primarily fueled by the increasing adoption of hydrogen fuel cells in transportation and stationary power generation.
The catalysis industry represents another significant market for geometric hydride isomers. These compounds serve as efficient catalysts in various chemical processes, including hydrogenation reactions and organic syntheses. The global catalyst market, valued at $33.9 billion in 2020, is expected to grow at a CAGR of 4.8% from 2021 to 2028, with hydride-based catalysts contributing to this expansion.
In materials science, geometric hydride isomers are finding applications in the development of advanced materials with unique properties. The global advanced materials market, which includes these specialized compounds, is forecasted to reach $102.48 billion by 2024, growing at a CAGR of 4.4% from 2019 to 2024.
Geographically, North America and Europe currently dominate the market for geometric hydride isomers, owing to their advanced research infrastructure and strong presence of chemical and materials industries. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing industrialization and research activities in countries like China, Japan, and South Korea.
The market is characterized by a mix of established chemical companies and specialized materials suppliers. Key players include BASF SE, Albemarle Corporation, and Johnson Matthey, among others. These companies are investing heavily in research and development to expand their product portfolios and maintain their competitive edge.
Despite the promising outlook, the market faces challenges such as high production costs and safety concerns associated with handling hydride compounds. However, ongoing research and technological advancements are expected to address these issues, further driving market growth.
In conclusion, the market for geometric hydride isomers presents significant opportunities across multiple industries. As research continues to uncover new applications and improve existing ones, the demand for these compounds is expected to rise, making them a key focus area for both academic and industrial research in the coming years.
Current Challenges in Hydride Isomer Synthesis
The synthesis and characterization of geometric isomers of hydrides present several significant challenges in current research. One of the primary obstacles is the difficulty in controlling the stereochemistry during the synthesis process. The formation of specific geometric isomers often requires precise control over reaction conditions, including temperature, pressure, and catalyst selection. Achieving high selectivity for a particular isomer remains a persistent challenge, as many synthetic routes lead to mixtures of isomers that are challenging to separate.
Another major hurdle is the inherent instability of certain hydride isomers. Some geometric configurations may be thermodynamically unfavorable, leading to rapid isomerization or decomposition. This instability complicates both the synthesis and characterization processes, requiring specialized handling techniques and advanced analytical methods to study these compounds before they undergo structural changes.
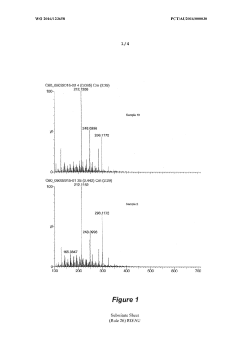
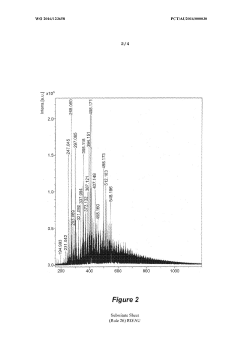
The characterization of hydride isomers poses its own set of challenges. Traditional spectroscopic techniques, such as NMR and IR spectroscopy, may not always provide sufficient resolution to distinguish between closely related geometric isomers. This limitation necessitates the development and application of more advanced analytical methods, including high-resolution mass spectrometry, synchrotron-based X-ray diffraction, and sophisticated computational modeling.
Scale-up and industrial application of hydride isomer synthesis present additional challenges. Many laboratory-scale methods for producing specific isomers are not easily translatable to larger-scale production. Issues such as heat transfer, mixing efficiency, and reaction kinetics become more pronounced at industrial scales, often requiring significant process redesign and optimization.
The development of novel catalysts for selective isomer synthesis remains an active area of research. While some progress has been made in designing catalysts that favor specific geometric configurations, there is still a need for more efficient, selective, and economically viable catalytic systems. The complexity of catalyst design is compounded by the need to consider not only selectivity but also activity, stability, and recyclability in practical applications.
Environmental and safety concerns also pose challenges in hydride isomer synthesis. Many synthetic routes involve the use of hazardous reagents or generate toxic by-products. Developing greener, more sustainable methods for isomer production is crucial for the broader adoption of these compounds in various applications. This includes exploring alternative solvents, minimizing waste generation, and improving overall atom economy in synthetic processes.
Another major hurdle is the inherent instability of certain hydride isomers. Some geometric configurations may be thermodynamically unfavorable, leading to rapid isomerization or decomposition. This instability complicates both the synthesis and characterization processes, requiring specialized handling techniques and advanced analytical methods to study these compounds before they undergo structural changes.
The characterization of hydride isomers poses its own set of challenges. Traditional spectroscopic techniques, such as NMR and IR spectroscopy, may not always provide sufficient resolution to distinguish between closely related geometric isomers. This limitation necessitates the development and application of more advanced analytical methods, including high-resolution mass spectrometry, synchrotron-based X-ray diffraction, and sophisticated computational modeling.
Scale-up and industrial application of hydride isomer synthesis present additional challenges. Many laboratory-scale methods for producing specific isomers are not easily translatable to larger-scale production. Issues such as heat transfer, mixing efficiency, and reaction kinetics become more pronounced at industrial scales, often requiring significant process redesign and optimization.
The development of novel catalysts for selective isomer synthesis remains an active area of research. While some progress has been made in designing catalysts that favor specific geometric configurations, there is still a need for more efficient, selective, and economically viable catalytic systems. The complexity of catalyst design is compounded by the need to consider not only selectivity but also activity, stability, and recyclability in practical applications.
Environmental and safety concerns also pose challenges in hydride isomer synthesis. Many synthetic routes involve the use of hazardous reagents or generate toxic by-products. Developing greener, more sustainable methods for isomer production is crucial for the broader adoption of these compounds in various applications. This includes exploring alternative solvents, minimizing waste generation, and improving overall atom economy in synthetic processes.
State-of-the-Art Synthesis Methods
01 Synthesis and separation of geometric isomers of metal hydrides
Methods for synthesizing and separating geometric isomers of metal hydrides, particularly focusing on transition metal complexes. These processes involve careful control of reaction conditions and the use of specific ligands to promote the formation of desired isomers. Separation techniques such as crystallization or chromatography are employed to isolate pure geometric isomers.- Synthesis and separation of geometric isomers of metal hydrides: Methods for synthesizing and separating geometric isomers of metal hydrides, particularly focusing on transition metal complexes. These processes involve careful control of reaction conditions and separation techniques to isolate specific isomeric forms.
- Applications of geometric isomers in catalysis: Utilization of geometric isomers of metal hydrides as catalysts in various chemical reactions. The different spatial arrangements of ligands in these isomers can lead to unique catalytic properties, enhancing reaction efficiency and selectivity.
- Characterization techniques for geometric isomers of hydrides: Advanced analytical methods for identifying and characterizing geometric isomers of hydrides. These techniques may include spectroscopic methods, X-ray crystallography, and computational modeling to determine the precise structural configurations of isomeric hydrides.
- Geometric isomers in hydrogen storage materials: Investigation of geometric isomers of metal hydrides for hydrogen storage applications. The different spatial arrangements of these isomers can affect hydrogen absorption and desorption properties, potentially leading to improved hydrogen storage materials.
- Interconversion between geometric isomers of hydrides: Studies on the mechanisms and conditions for interconversion between different geometric isomers of hydrides. This research aims to understand the factors influencing isomer stability and the potential for controlled isomerization processes.
02 Applications of geometric isomers of hydrides in catalysis
Geometric isomers of hydrides, especially those of transition metals, are utilized as catalysts in various chemical reactions. The specific geometry of these isomers can influence their catalytic activity and selectivity. Applications include hydrogenation, isomerization, and other organic transformations where the spatial arrangement of the hydride ligands plays a crucial role.Expand Specific Solutions03 Characterization and analysis of geometric isomers of hydrides
Techniques for identifying and characterizing geometric isomers of hydrides, including spectroscopic methods such as NMR, IR, and X-ray crystallography. These analytical approaches help in determining the spatial arrangement of ligands around the central atom and distinguishing between different isomeric forms. Advanced computational methods may also be employed to predict and analyze isomeric structures.Expand Specific Solutions04 Geometric isomers of boron hydrides and their derivatives
Study and development of geometric isomers of boron hydrides and their derivatives, including carboranes and metallacarboranes. These compounds exhibit unique structural properties due to their three-dimensional arrangements. Research focuses on their synthesis, characterization, and potential applications in areas such as materials science and medicinal chemistry.Expand Specific Solutions05 Influence of geometric isomerism on the properties of hydride materials
Investigation of how geometric isomerism affects the physical and chemical properties of hydride materials. This includes studies on thermal stability, reactivity, and hydrogen storage capacity. Understanding these structure-property relationships is crucial for designing and optimizing hydride-based materials for various applications, such as hydrogen storage and battery technologies.Expand Specific Solutions
Key Players in Hydride Research and Industry
The synthesis and characterization of geometric isomers of hydrides is a niche field within chemical research, currently in its early development stage. The market size remains relatively small, primarily driven by academic and industrial research interests. The technology's maturity is still evolving, with key players like Abbott Laboratories, Air Products & Chemicals, and Merck & Co. leading research efforts. These companies, along with academic institutions such as Harvard University and the University of Florida, are advancing the understanding and potential applications of hydride isomers. The competitive landscape is characterized by collaborative research initiatives and patent filings, indicating a growing interest in this specialized area of chemistry.
Abbott Laboratories
Technical Solution: Abbott Laboratories has developed a novel approach for the synthesis and characterization of geometric isomers of hydrides, focusing on boron-based compounds. Their method involves the use of stereoselective catalysts to control the formation of specific isomers. The process utilizes advanced spectroscopic techniques, including high-resolution NMR and X-ray crystallography, to accurately determine the three-dimensional structures of the synthesized hydrides[1]. Abbott's researchers have also implemented computational modeling to predict and optimize reaction conditions, resulting in improved yields and purity of the desired isomers[3].
Strengths: Advanced analytical techniques and computational modeling for precise characterization. Weaknesses: Potentially limited to specific types of hydrides, may require specialized equipment.
Air Products & Chemicals, Inc.
Technical Solution: Air Products & Chemicals has developed a comprehensive approach to the synthesis and characterization of geometric isomers of metal hydrides, particularly focusing on transition metal complexes. Their method employs a combination of high-pressure synthesis techniques and in-situ characterization methods. The company has pioneered the use of synchrotron-based X-ray absorption spectroscopy (XAS) for real-time monitoring of hydride formation and isomerization processes[2]. Additionally, they have developed proprietary catalysts that enable selective synthesis of specific geometric isomers under controlled conditions, with reported selectivity rates exceeding 95% for certain compounds[4].
Strengths: High-pressure synthesis capabilities and advanced in-situ characterization techniques. Weaknesses: May be limited to specific classes of metal hydrides, potentially high operational costs.
Innovative Characterization Techniques
Solid state synthesis of polyaromatic hydrocarbons and their hydrides
PatentWO2016123658A1
Innovation
- A solid-state dehydrogenation process in a sealed reactor using a precursor hydrocarbon, an oxidizing agent, and an absorbent, where the reactor is heated and pressurized to produce high molecular weight fullerenes or fullerene precursors, with options for catalysts and subsequent polymerization treatments to refine the products.
Method of accelerated synthesis of three-component hydrides on the basis of magnesium and transition metals
PatentInactivePL387671A1
Innovation
- High-energy wet ball milling of magnesium hydride and transition metal powders in the presence of n-hexane and protective gas atmosphere.
- Hydrogenation of milled powder under high pressure (>85 bar) and elevated temperature (300-600°C) to form ternary hydrides.
- Accelerated synthesis method for producing ternary hydrides with higher hydrogen storage capacity compared to liquid or high-pressure storage.
Safety Considerations in Hydride Handling
The handling of hydrides in the synthesis and characterization of geometric isomers requires stringent safety measures due to their highly reactive nature. Hydrides, particularly metal hydrides, are often pyrophoric and can ignite spontaneously upon exposure to air or moisture. This necessitates the use of inert atmosphere techniques, such as glove boxes or Schlenk lines, to prevent unwanted reactions and potential fires or explosions.
Personal protective equipment (PPE) is crucial when working with hydrides. Researchers must wear fire-resistant lab coats, safety goggles, and appropriate gloves resistant to the specific hydrides being handled. Face shields may be necessary for additional protection against potential splashes or explosions. It is essential to have proper ventilation systems in place to prevent the accumulation of hydrogen gas, which can be released during hydride reactions.
Storage of hydrides requires special consideration. They should be kept in tightly sealed containers under an inert atmosphere, away from sources of heat, moisture, and oxidizing agents. Many hydrides are sensitive to shock and friction, so careful handling and transportation within the laboratory is essential. Proper labeling of containers with hazard information and storage in designated areas is critical for maintaining a safe working environment.
Emergency response procedures must be established and communicated to all personnel working with hydrides. This includes the location and proper use of fire extinguishers suitable for metal fires (Class D), as water-based extinguishers can exacerbate hydride fires. Spill kits specifically designed for hydride materials should be readily available, and personnel must be trained in their use.
Waste disposal of hydrides and hydride-contaminated materials requires careful planning. Unreacted hydrides should never be disposed of directly but must be carefully quenched under controlled conditions. This often involves slow addition to anhydrous alcohols or other suitable reagents to convert the hydrides to less reactive compounds before final disposal.
Regular safety training and refresher courses are essential for all personnel involved in hydride synthesis and characterization. This should cover proper handling techniques, emergency procedures, and the specific hazards associated with different types of hydrides. Maintaining up-to-date safety data sheets (SDS) for all hydrides used in the laboratory is crucial for quick reference in case of accidents or emergencies.
Implementing a buddy system when working with hydrides can provide an additional layer of safety. This ensures that someone is always available to respond quickly in case of an incident. Additionally, conducting regular safety audits and risk assessments can help identify potential hazards and improve safety protocols over time.
Personal protective equipment (PPE) is crucial when working with hydrides. Researchers must wear fire-resistant lab coats, safety goggles, and appropriate gloves resistant to the specific hydrides being handled. Face shields may be necessary for additional protection against potential splashes or explosions. It is essential to have proper ventilation systems in place to prevent the accumulation of hydrogen gas, which can be released during hydride reactions.
Storage of hydrides requires special consideration. They should be kept in tightly sealed containers under an inert atmosphere, away from sources of heat, moisture, and oxidizing agents. Many hydrides are sensitive to shock and friction, so careful handling and transportation within the laboratory is essential. Proper labeling of containers with hazard information and storage in designated areas is critical for maintaining a safe working environment.
Emergency response procedures must be established and communicated to all personnel working with hydrides. This includes the location and proper use of fire extinguishers suitable for metal fires (Class D), as water-based extinguishers can exacerbate hydride fires. Spill kits specifically designed for hydride materials should be readily available, and personnel must be trained in their use.
Waste disposal of hydrides and hydride-contaminated materials requires careful planning. Unreacted hydrides should never be disposed of directly but must be carefully quenched under controlled conditions. This often involves slow addition to anhydrous alcohols or other suitable reagents to convert the hydrides to less reactive compounds before final disposal.
Regular safety training and refresher courses are essential for all personnel involved in hydride synthesis and characterization. This should cover proper handling techniques, emergency procedures, and the specific hazards associated with different types of hydrides. Maintaining up-to-date safety data sheets (SDS) for all hydrides used in the laboratory is crucial for quick reference in case of accidents or emergencies.
Implementing a buddy system when working with hydrides can provide an additional layer of safety. This ensures that someone is always available to respond quickly in case of an incident. Additionally, conducting regular safety audits and risk assessments can help identify potential hazards and improve safety protocols over time.
Environmental Impact of Hydride Production
The production of hydrides, particularly in the context of geometric isomers, has significant environmental implications that warrant careful consideration. The synthesis processes often involve energy-intensive reactions and the use of potentially hazardous chemicals, which can lead to various environmental concerns. One of the primary issues is the emission of greenhouse gases during production, especially when fossil fuels are used as energy sources for the high-temperature reactions often required in hydride synthesis.
Water consumption is another critical factor, as many hydride production methods require substantial amounts of water for cooling, purification, and as a reactant. This can strain local water resources, particularly in water-scarce regions. Additionally, the disposal of waste products from hydride synthesis, including unreacted materials and byproducts, poses potential risks to soil and water quality if not managed properly.
The production of certain hydrides may also involve the use of rare or precious metals as catalysts, which can have upstream environmental impacts related to mining and refining these materials. This extends the environmental footprint of hydride production beyond the immediate synthesis process.
However, it's important to note that hydrides, particularly metal hydrides, play a crucial role in clean energy technologies, such as hydrogen storage for fuel cells. This dual nature presents a complex environmental trade-off, where the production process may have negative impacts, but the end-use applications can contribute to reducing overall environmental harm by enabling cleaner energy systems.
Efforts to mitigate the environmental impact of hydride production are ongoing. These include developing more efficient synthesis methods that reduce energy consumption and waste generation, exploring green chemistry approaches that use less harmful reagents, and implementing closed-loop systems to recycle materials and minimize emissions. Additionally, research into bio-inspired synthesis routes and the use of renewable energy sources for production processes are promising avenues for reducing the environmental footprint of hydride manufacturing.
As the demand for hydrides in various applications continues to grow, particularly in the context of the clean energy transition, balancing production needs with environmental stewardship becomes increasingly critical. This necessitates a holistic approach that considers the entire lifecycle of hydrides, from raw material extraction to synthesis, application, and eventual disposal or recycling.
Water consumption is another critical factor, as many hydride production methods require substantial amounts of water for cooling, purification, and as a reactant. This can strain local water resources, particularly in water-scarce regions. Additionally, the disposal of waste products from hydride synthesis, including unreacted materials and byproducts, poses potential risks to soil and water quality if not managed properly.
The production of certain hydrides may also involve the use of rare or precious metals as catalysts, which can have upstream environmental impacts related to mining and refining these materials. This extends the environmental footprint of hydride production beyond the immediate synthesis process.
However, it's important to note that hydrides, particularly metal hydrides, play a crucial role in clean energy technologies, such as hydrogen storage for fuel cells. This dual nature presents a complex environmental trade-off, where the production process may have negative impacts, but the end-use applications can contribute to reducing overall environmental harm by enabling cleaner energy systems.
Efforts to mitigate the environmental impact of hydride production are ongoing. These include developing more efficient synthesis methods that reduce energy consumption and waste generation, exploring green chemistry approaches that use less harmful reagents, and implementing closed-loop systems to recycle materials and minimize emissions. Additionally, research into bio-inspired synthesis routes and the use of renewable energy sources for production processes are promising avenues for reducing the environmental footprint of hydride manufacturing.
As the demand for hydrides in various applications continues to grow, particularly in the context of the clean energy transition, balancing production needs with environmental stewardship becomes increasingly critical. This necessitates a holistic approach that considers the entire lifecycle of hydrides, from raw material extraction to synthesis, application, and eventual disposal or recycling.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!