Techniques for Modeling Geometric Isomers in Predictive Toxicology
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Geometric Isomer Modeling Background and Objectives
Geometric isomers, a crucial aspect of molecular structure, have gained significant attention in the field of predictive toxicology. The evolution of this technology can be traced back to the early days of chemical modeling, where researchers first recognized the importance of spatial arrangements in determining a compound's properties and biological activities. As our understanding of molecular structures advanced, so did the techniques for modeling geometric isomers.
The primary objective of modeling geometric isomers in predictive toxicology is to accurately represent the three-dimensional structure of molecules and predict their potential toxic effects. This goal has become increasingly important as regulatory bodies worldwide have implemented stricter guidelines for chemical safety assessment. The ability to model geometric isomers effectively can significantly reduce the need for extensive animal testing and accelerate the drug discovery process.
Over the years, the field has witnessed a shift from simple two-dimensional representations to sophisticated three-dimensional models. Early techniques relied heavily on experimental data and empirical observations. However, with the advent of computational chemistry and machine learning, more advanced methods have emerged, allowing for more accurate predictions of isomer behavior and toxicity.
The development of quantum mechanical calculations and molecular dynamics simulations has been a game-changer in this field. These techniques have enabled researchers to model the electronic structure and conformational changes of geometric isomers with unprecedented accuracy. This has led to a better understanding of how subtle differences in spatial arrangement can lead to significant variations in toxicity profiles.
Recent advancements in artificial intelligence and deep learning have further revolutionized the modeling of geometric isomers. These technologies have made it possible to process vast amounts of structural data and identify complex patterns that were previously undetectable. This has resulted in more robust predictive models that can account for a wide range of molecular interactions and environmental factors.
The current trend in geometric isomer modeling is moving towards integrative approaches that combine multiple techniques. This includes the fusion of quantum mechanical calculations with machine learning algorithms, as well as the incorporation of experimental data to refine and validate computational models. The ultimate aim is to develop a comprehensive platform that can accurately predict the toxicological properties of geometric isomers across diverse chemical spaces.
As we look to the future, the field of geometric isomer modeling in predictive toxicology is poised for further innovation. Emerging technologies such as quantum computing and advanced neural network architectures promise to enhance the speed and accuracy of isomer modeling. These developments will be crucial in addressing the growing complexity of chemical compounds and the increasing demand for rapid, reliable toxicity assessments in various industries, including pharmaceuticals, agrochemicals, and environmental protection.
The primary objective of modeling geometric isomers in predictive toxicology is to accurately represent the three-dimensional structure of molecules and predict their potential toxic effects. This goal has become increasingly important as regulatory bodies worldwide have implemented stricter guidelines for chemical safety assessment. The ability to model geometric isomers effectively can significantly reduce the need for extensive animal testing and accelerate the drug discovery process.
Over the years, the field has witnessed a shift from simple two-dimensional representations to sophisticated three-dimensional models. Early techniques relied heavily on experimental data and empirical observations. However, with the advent of computational chemistry and machine learning, more advanced methods have emerged, allowing for more accurate predictions of isomer behavior and toxicity.
The development of quantum mechanical calculations and molecular dynamics simulations has been a game-changer in this field. These techniques have enabled researchers to model the electronic structure and conformational changes of geometric isomers with unprecedented accuracy. This has led to a better understanding of how subtle differences in spatial arrangement can lead to significant variations in toxicity profiles.
Recent advancements in artificial intelligence and deep learning have further revolutionized the modeling of geometric isomers. These technologies have made it possible to process vast amounts of structural data and identify complex patterns that were previously undetectable. This has resulted in more robust predictive models that can account for a wide range of molecular interactions and environmental factors.
The current trend in geometric isomer modeling is moving towards integrative approaches that combine multiple techniques. This includes the fusion of quantum mechanical calculations with machine learning algorithms, as well as the incorporation of experimental data to refine and validate computational models. The ultimate aim is to develop a comprehensive platform that can accurately predict the toxicological properties of geometric isomers across diverse chemical spaces.
As we look to the future, the field of geometric isomer modeling in predictive toxicology is poised for further innovation. Emerging technologies such as quantum computing and advanced neural network architectures promise to enhance the speed and accuracy of isomer modeling. These developments will be crucial in addressing the growing complexity of chemical compounds and the increasing demand for rapid, reliable toxicity assessments in various industries, including pharmaceuticals, agrochemicals, and environmental protection.
Market Demand for Predictive Toxicology Tools
The market demand for predictive toxicology tools has been steadily increasing in recent years, driven by several key factors. The pharmaceutical industry, in particular, has shown a growing interest in these tools to streamline drug discovery and development processes. By accurately predicting the toxicity of potential drug candidates early in the development pipeline, companies can significantly reduce the time and costs associated with bringing new drugs to market.
Environmental regulatory agencies have also become major consumers of predictive toxicology tools. With the increasing number of chemicals being introduced into the environment, there is a pressing need for efficient methods to assess their potential risks. These tools enable regulators to make informed decisions about chemical safety without relying solely on time-consuming and expensive animal testing.
The cosmetics industry has emerged as another significant market for predictive toxicology tools. With many countries banning animal testing for cosmetic products, companies are turning to in silico methods to ensure product safety. This shift has created a substantial demand for reliable computational models that can accurately predict the toxicity of cosmetic ingredients.
In the agrochemical sector, predictive toxicology tools are gaining traction for assessing the environmental impact of pesticides and herbicides. Farmers and agricultural companies are increasingly seeking products that are both effective and environmentally friendly, driving the need for advanced toxicity prediction methods.
The market size for predictive toxicology tools is substantial and growing. While exact figures vary, industry reports suggest that the global market for these tools is expected to expand significantly over the next decade. This growth is fueled by increasing regulatory pressures, the need for cost-effective drug development, and the push for alternatives to animal testing.
However, the market also faces challenges. The accuracy and reliability of predictive models remain a concern for many potential users. There is a demand for tools that can handle complex molecular structures, such as geometric isomers, with high precision. Additionally, there is a growing need for models that can account for species-specific differences in toxicity responses, as well as tools that can predict long-term and cumulative effects of chemical exposure.
Despite these challenges, the overall trend indicates a robust and expanding market for predictive toxicology tools. As computational power increases and machine learning techniques advance, the capabilities of these tools are expected to improve, further driving their adoption across various industries.
Environmental regulatory agencies have also become major consumers of predictive toxicology tools. With the increasing number of chemicals being introduced into the environment, there is a pressing need for efficient methods to assess their potential risks. These tools enable regulators to make informed decisions about chemical safety without relying solely on time-consuming and expensive animal testing.
The cosmetics industry has emerged as another significant market for predictive toxicology tools. With many countries banning animal testing for cosmetic products, companies are turning to in silico methods to ensure product safety. This shift has created a substantial demand for reliable computational models that can accurately predict the toxicity of cosmetic ingredients.
In the agrochemical sector, predictive toxicology tools are gaining traction for assessing the environmental impact of pesticides and herbicides. Farmers and agricultural companies are increasingly seeking products that are both effective and environmentally friendly, driving the need for advanced toxicity prediction methods.
The market size for predictive toxicology tools is substantial and growing. While exact figures vary, industry reports suggest that the global market for these tools is expected to expand significantly over the next decade. This growth is fueled by increasing regulatory pressures, the need for cost-effective drug development, and the push for alternatives to animal testing.
However, the market also faces challenges. The accuracy and reliability of predictive models remain a concern for many potential users. There is a demand for tools that can handle complex molecular structures, such as geometric isomers, with high precision. Additionally, there is a growing need for models that can account for species-specific differences in toxicity responses, as well as tools that can predict long-term and cumulative effects of chemical exposure.
Despite these challenges, the overall trend indicates a robust and expanding market for predictive toxicology tools. As computational power increases and machine learning techniques advance, the capabilities of these tools are expected to improve, further driving their adoption across various industries.
Current Challenges in Geometric Isomer Representation
The representation of geometric isomers in predictive toxicology poses several significant challenges that researchers and toxicologists are currently grappling with. One of the primary difficulties lies in accurately capturing the three-dimensional spatial arrangements of atoms within molecules, which is crucial for distinguishing between geometric isomers.
Traditional two-dimensional molecular representations often fail to adequately convey the spatial relationships that define geometric isomers. This limitation can lead to ambiguity in toxicity predictions, as the biological activity of a compound can vary dramatically between different geometric isomers. The development of robust 3D modeling techniques that can efficiently and accurately represent these spatial configurations remains an ongoing challenge.
Another significant hurdle is the integration of geometric isomer information into existing predictive toxicology models. Many current models are based on simplified molecular descriptors that do not fully account for the nuances of geometric isomerism. Adapting these models to incorporate detailed spatial information without sacrificing computational efficiency is a complex task that researchers are actively addressing.
The sheer diversity of geometric isomers presents an additional challenge. For any given molecule, there may be multiple possible geometric isomers, each with potentially distinct toxicological profiles. Developing comprehensive databases and prediction systems that can account for this variability is both time-consuming and resource-intensive.
Furthermore, the dynamic nature of molecular structures in biological systems adds another layer of complexity. Geometric isomers can interconvert under certain conditions, and modeling these transitions accurately in the context of toxicity prediction is a formidable challenge. Researchers must develop methods that can account for the potential for isomerization and its impact on toxicological outcomes.
The challenge of data scarcity also plagues the field. While experimental data on the toxicity of some geometric isomers is available, many remain unstudied. This lack of comprehensive data hampers the development and validation of predictive models, necessitating innovative approaches to data generation and extrapolation.
Lastly, the computational demands of accurately modeling geometric isomers in large-scale toxicity prediction scenarios present a significant bottleneck. Balancing the need for high-fidelity 3D representations with the practical constraints of computational resources and time remains an ongoing challenge in the field of predictive toxicology.
Traditional two-dimensional molecular representations often fail to adequately convey the spatial relationships that define geometric isomers. This limitation can lead to ambiguity in toxicity predictions, as the biological activity of a compound can vary dramatically between different geometric isomers. The development of robust 3D modeling techniques that can efficiently and accurately represent these spatial configurations remains an ongoing challenge.
Another significant hurdle is the integration of geometric isomer information into existing predictive toxicology models. Many current models are based on simplified molecular descriptors that do not fully account for the nuances of geometric isomerism. Adapting these models to incorporate detailed spatial information without sacrificing computational efficiency is a complex task that researchers are actively addressing.
The sheer diversity of geometric isomers presents an additional challenge. For any given molecule, there may be multiple possible geometric isomers, each with potentially distinct toxicological profiles. Developing comprehensive databases and prediction systems that can account for this variability is both time-consuming and resource-intensive.
Furthermore, the dynamic nature of molecular structures in biological systems adds another layer of complexity. Geometric isomers can interconvert under certain conditions, and modeling these transitions accurately in the context of toxicity prediction is a formidable challenge. Researchers must develop methods that can account for the potential for isomerization and its impact on toxicological outcomes.
The challenge of data scarcity also plagues the field. While experimental data on the toxicity of some geometric isomers is available, many remain unstudied. This lack of comprehensive data hampers the development and validation of predictive models, necessitating innovative approaches to data generation and extrapolation.
Lastly, the computational demands of accurately modeling geometric isomers in large-scale toxicity prediction scenarios present a significant bottleneck. Balancing the need for high-fidelity 3D representations with the practical constraints of computational resources and time remains an ongoing challenge in the field of predictive toxicology.
Existing Approaches for Geometric Isomer Modeling
01 3D modeling of geometric isomers
Advanced 3D modeling techniques are used to visualize and simulate geometric isomers. These methods allow for accurate representation of spatial arrangements of atoms in molecules, helping researchers and students better understand isomeric structures and their properties. The models can be rotated, zoomed, and manipulated in virtual space for comprehensive analysis.- 3D modeling of geometric isomers: Advanced 3D modeling techniques are used to visualize and analyze geometric isomers. These methods allow for accurate representation of spatial arrangements of atoms in molecules, enabling researchers to study structural differences between isomers. The models can be rotated, manipulated, and compared in virtual space, providing insights into molecular properties and behaviors.
- Computer-aided design for isomer structures: Computer-aided design (CAD) tools are employed to create and modify geometric isomer structures. These software applications offer precise control over bond angles, lengths, and atomic positions, allowing for the construction of complex isomeric molecules. CAD systems can also generate multiple isomer configurations rapidly, facilitating comparative studies and structure-property relationships.
- Machine learning algorithms for isomer prediction: Machine learning algorithms are developed to predict and classify geometric isomers based on molecular data. These AI-driven approaches can analyze large datasets of known isomers to identify patterns and relationships, enabling the prediction of new isomeric structures and their properties. This technology accelerates the discovery and design of novel compounds with desired characteristics.
- Quantum mechanical modeling of isomers: Quantum mechanical modeling techniques are applied to study geometric isomers at the electronic level. These methods provide detailed insights into the energy states, electron distributions, and molecular orbitals of isomeric structures. By incorporating quantum effects, researchers can accurately predict isomer stabilities, reactivity, and spectroscopic properties.
- Visualization and analysis tools for isomer comparison: Specialized visualization and analysis tools are developed to compare and contrast geometric isomers. These software packages offer features such as overlay analysis, difference mapping, and property calculation, allowing researchers to identify subtle structural variations and their impacts on molecular properties. Interactive displays and data visualization techniques enhance the understanding of isomeric relationships.
02 Computer-aided design of isomeric compounds
Computer-aided design tools are employed to create and modify geometric isomers. These software solutions enable chemists to construct complex molecular structures, predict their properties, and optimize their designs. The tools often incorporate databases of known compounds and can suggest potential isomeric variations based on input parameters.Expand Specific Solutions03 Machine learning for isomer prediction
Machine learning algorithms are developed to predict and classify geometric isomers. These AI-powered systems analyze large datasets of known isomeric compounds to identify patterns and relationships. The models can then be used to predict the likelihood of isomerization, suggest potential new isomers, and assist in the design of novel compounds with desired properties.Expand Specific Solutions04 Visualization techniques for isomer comparison
Specialized visualization techniques are created to compare and contrast different geometric isomers. These methods may include color-coding, overlays, and dynamic transitions to highlight structural differences between isomers. Such visualizations aid in understanding the relationship between molecular structure and properties, facilitating research and education in stereochemistry.Expand Specific Solutions05 Simulation of isomerization processes
Advanced simulation tools are developed to model the dynamic processes of isomerization. These simulations can demonstrate how molecules transition between different geometric configurations, accounting for energy barriers and environmental factors. Researchers use these tools to study reaction mechanisms, predict product distributions, and design more efficient synthetic pathways for isomeric compounds.Expand Specific Solutions
Key Players in Computational Chemistry and Toxicology
The field of modeling geometric isomers in predictive toxicology is in a growth phase, with increasing market size and technological advancements. The competitive landscape is diverse, featuring pharmaceutical giants like Pfizer and Roche, alongside specialized biotech firms such as Foghorn Therapeutics and Inspirna. Academic institutions, including the University of Queensland and École Polytechnique Fédérale de Lausanne, are also contributing significantly to research. The technology is maturing, with companies like Siemens Healthineers and DuPont applying it in various sectors. However, the complexity of isomer modeling suggests there's still room for innovation and market expansion, particularly in integrating AI and machine learning techniques.
Pfizer Inc.
Technical Solution: Pfizer has developed advanced computational methods for modeling geometric isomers in predictive toxicology. Their approach combines machine learning algorithms with molecular dynamics simulations to accurately predict the toxicological properties of different isomers[1]. The company utilizes high-throughput screening techniques to generate large datasets of isomer structures and their corresponding toxicity profiles. These datasets are then used to train deep learning models that can identify subtle structural differences between isomers and their impact on toxicity[2]. Pfizer's platform also incorporates quantum mechanical calculations to model the electronic properties of isomers, providing insights into their reactivity and potential interactions with biological targets[3].
Strengths: Comprehensive integration of multiple computational techniques, large proprietary datasets for model training. Weaknesses: High computational costs, potential limitations in predicting rare or complex isomer interactions.
The Broad Institute, Inc.
Technical Solution: The Broad Institute has developed a cutting-edge approach to modeling geometric isomers in predictive toxicology, leveraging their expertise in genomics and computational biology. Their platform integrates machine learning algorithms with systems biology approaches to predict the toxicological effects of geometric isomers at multiple biological scales[13]. The institute employs graph neural networks and attention mechanisms to capture the 3D structural information of isomers and their potential interactions with biological targets. The Broad Institute's system also incorporates transcriptomics and metabolomics data to model the downstream effects of isomers on gene expression and metabolic pathways[14]. Additionally, they have implemented advanced statistical methods, such as Bayesian networks, to quantify uncertainty in toxicity predictions and identify potential mechanisms of action[15].
Strengths: Integration of multi-omics data, strong focus on biological mechanisms and pathways. Weaknesses: May require extensive experimental data for model training, potential challenges in generalizing to diverse chemical spaces.
Innovative Methods in Isomer Representation
Methods of predicting toxicity
PatentActiveUS20170327893A1
Innovation
- A method involving contacting cells in vitro with oligomeric compounds and measuring the modulation of off-target genes' activity or amount, specifically using gapmer oligonucleotides with modified nucleosides and internucleoside linkages, to predict in vivo toxicity by identifying sentinel genes that correlate with toxicity levels.
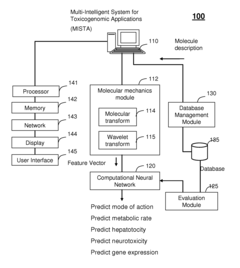
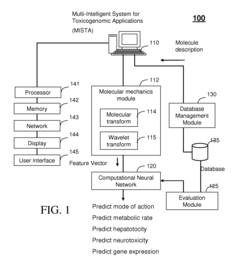
Multi-intelligent system for toxicogenomic applications (MISTA)
PatentInactiveUS20090024547A1
Innovation
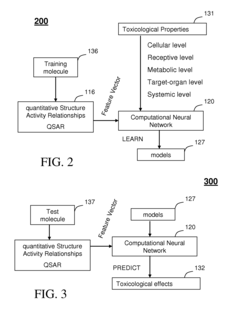
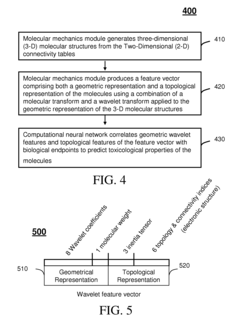
- A Multi-Intelligent System for Toxicogenomic Applications (MISTA) uses neural networks and wavelets to create an in silico toxicity-prediction platform, integrating genomics, proteomics, and other databases, employing computational modules for QSARs and wavelet analysis to rapidly and accurately predict toxicological properties of molecules, including metabolic processes, hepatotoxicity, and neurotoxicity.
Regulatory Considerations for In Silico Toxicology
Regulatory considerations play a crucial role in the development and application of in silico toxicology methods, including techniques for modeling geometric isomers in predictive toxicology. As these computational approaches gain prominence in safety assessment, regulatory agencies worldwide are adapting their guidelines to incorporate these innovative tools.
The U.S. Food and Drug Administration (FDA) has been at the forefront of embracing in silico methods for toxicology assessment. Their guidance documents now explicitly mention the use of computational models as part of an integrated approach to testing and assessment (IATA). The FDA's acceptance of these methods is contingent upon proper validation and documentation of the models used, including those for geometric isomer prediction.
Similarly, the European Medicines Agency (EMA) has issued guidelines on the use of non-animal approaches in drug development, which include in silico methods. These guidelines emphasize the importance of transparency in model development and validation, particularly when dealing with complex molecular structures like geometric isomers.
The Organization for Economic Co-operation and Development (OECD) has developed principles for the validation of (Q)SAR models, which are directly applicable to techniques for modeling geometric isomers. These principles stress the importance of a defined endpoint, an unambiguous algorithm, a defined domain of applicability, appropriate measures of goodness-of-fit, and a mechanistic interpretation if possible.
Regulatory bodies are increasingly recognizing the potential of in silico methods to reduce animal testing and improve the efficiency of toxicological assessments. However, they also emphasize the need for these methods to be scientifically robust and reliable. For geometric isomer modeling, this means demonstrating that the models can accurately predict the toxicological properties of different isomeric forms.
One of the key regulatory considerations is the validation of in silico models for geometric isomers. This involves demonstrating the model's predictive power using a diverse set of compounds, including those with known toxicological profiles. Regulatory agencies often require extensive documentation of the validation process, including statistical analyses of model performance and clear descriptions of the model's limitations and applicability domain.
Another important aspect is the integration of in silico predictions with other data sources. Regulatory bodies are increasingly advocating for a weight-of-evidence approach, where computational predictions are considered alongside experimental data and expert judgment. This is particularly relevant for geometric isomers, where subtle structural differences can have significant impacts on toxicological properties.
The U.S. Food and Drug Administration (FDA) has been at the forefront of embracing in silico methods for toxicology assessment. Their guidance documents now explicitly mention the use of computational models as part of an integrated approach to testing and assessment (IATA). The FDA's acceptance of these methods is contingent upon proper validation and documentation of the models used, including those for geometric isomer prediction.
Similarly, the European Medicines Agency (EMA) has issued guidelines on the use of non-animal approaches in drug development, which include in silico methods. These guidelines emphasize the importance of transparency in model development and validation, particularly when dealing with complex molecular structures like geometric isomers.
The Organization for Economic Co-operation and Development (OECD) has developed principles for the validation of (Q)SAR models, which are directly applicable to techniques for modeling geometric isomers. These principles stress the importance of a defined endpoint, an unambiguous algorithm, a defined domain of applicability, appropriate measures of goodness-of-fit, and a mechanistic interpretation if possible.
Regulatory bodies are increasingly recognizing the potential of in silico methods to reduce animal testing and improve the efficiency of toxicological assessments. However, they also emphasize the need for these methods to be scientifically robust and reliable. For geometric isomer modeling, this means demonstrating that the models can accurately predict the toxicological properties of different isomeric forms.
One of the key regulatory considerations is the validation of in silico models for geometric isomers. This involves demonstrating the model's predictive power using a diverse set of compounds, including those with known toxicological profiles. Regulatory agencies often require extensive documentation of the validation process, including statistical analyses of model performance and clear descriptions of the model's limitations and applicability domain.
Another important aspect is the integration of in silico predictions with other data sources. Regulatory bodies are increasingly advocating for a weight-of-evidence approach, where computational predictions are considered alongside experimental data and expert judgment. This is particularly relevant for geometric isomers, where subtle structural differences can have significant impacts on toxicological properties.
Ethical Implications of Predictive Toxicology Models
The ethical implications of predictive toxicology models, particularly those involving geometric isomers, are multifaceted and require careful consideration. These models have the potential to significantly impact public health, environmental protection, and regulatory decision-making processes.
One of the primary ethical concerns is the accuracy and reliability of these models. Predictive toxicology models for geometric isomers must be rigorously validated to ensure they provide reliable results. Inaccurate predictions could lead to harmful substances being approved for use or beneficial compounds being unnecessarily restricted, both of which have serious ethical ramifications.
The use of animal testing in developing and validating these models also raises ethical questions. While predictive models aim to reduce the need for animal testing, their development often relies on data from animal studies. Balancing the ethical concerns of animal welfare with the need for robust toxicological data is a complex issue that requires ongoing dialogue and refinement of research methodologies.
Data privacy and security present another ethical challenge. Predictive toxicology models often require large datasets, which may include sensitive information from various sources. Ensuring the proper handling, storage, and use of this data is crucial to maintain public trust and protect individual privacy rights.
The potential for bias in these models is a significant ethical concern. If the data used to train predictive models for geometric isomers is not sufficiently diverse or representative, it could lead to biased results that disproportionately affect certain populations or environments. This raises questions of fairness and equity in the application of these models.
Transparency and interpretability of predictive toxicology models are essential from an ethical standpoint. The complexity of models dealing with geometric isomers may make them difficult for non-experts to understand, potentially leading to mistrust or misuse. Ensuring that these models are as transparent and interpretable as possible is crucial for ethical implementation and public acceptance.
The ethical use of predictive toxicology models also extends to their application in regulatory frameworks. There is a need to establish clear guidelines on how these models should be used in decision-making processes, ensuring that they complement rather than replace expert judgment and empirical evidence.
Lastly, the potential for misuse or overreliance on these models raises ethical concerns. While predictive toxicology models can be powerful tools, they should not be seen as infallible or used as a substitute for comprehensive safety assessments. Balancing the benefits of these models with a cautious approach to their limitations is essential for ethical implementation in toxicology and related fields.
One of the primary ethical concerns is the accuracy and reliability of these models. Predictive toxicology models for geometric isomers must be rigorously validated to ensure they provide reliable results. Inaccurate predictions could lead to harmful substances being approved for use or beneficial compounds being unnecessarily restricted, both of which have serious ethical ramifications.
The use of animal testing in developing and validating these models also raises ethical questions. While predictive models aim to reduce the need for animal testing, their development often relies on data from animal studies. Balancing the ethical concerns of animal welfare with the need for robust toxicological data is a complex issue that requires ongoing dialogue and refinement of research methodologies.
Data privacy and security present another ethical challenge. Predictive toxicology models often require large datasets, which may include sensitive information from various sources. Ensuring the proper handling, storage, and use of this data is crucial to maintain public trust and protect individual privacy rights.
The potential for bias in these models is a significant ethical concern. If the data used to train predictive models for geometric isomers is not sufficiently diverse or representative, it could lead to biased results that disproportionately affect certain populations or environments. This raises questions of fairness and equity in the application of these models.
Transparency and interpretability of predictive toxicology models are essential from an ethical standpoint. The complexity of models dealing with geometric isomers may make them difficult for non-experts to understand, potentially leading to mistrust or misuse. Ensuring that these models are as transparent and interpretable as possible is crucial for ethical implementation and public acceptance.
The ethical use of predictive toxicology models also extends to their application in regulatory frameworks. There is a need to establish clear guidelines on how these models should be used in decision-making processes, ensuring that they complement rather than replace expert judgment and empirical evidence.
Lastly, the potential for misuse or overreliance on these models raises ethical concerns. While predictive toxicology models can be powerful tools, they should not be seen as infallible or used as a substitute for comprehensive safety assessments. Balancing the benefits of these models with a cautious approach to their limitations is essential for ethical implementation in toxicology and related fields.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!