The Influence of Geometric Isomers on Drug Delivery Systems
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Geometric Isomers in Drug Delivery: Background and Objectives
Geometric isomers have emerged as a critical factor in the development and optimization of drug delivery systems. These molecular structures, which possess the same chemical formula but different spatial arrangements of atoms, have been the subject of extensive research in pharmaceutical sciences over the past decades. The study of geometric isomers in drug delivery has its roots in the early 20th century, with the discovery of cis-trans isomerism in organic compounds. However, it was not until the 1960s and 1970s that researchers began to fully appreciate the impact of these structural variations on drug efficacy and pharmacokinetics.
The evolution of this field has been driven by advancements in analytical techniques, such as X-ray crystallography and nuclear magnetic resonance spectroscopy, which have allowed scientists to elucidate the precise spatial configurations of drug molecules. This enhanced understanding has led to the development of more targeted and effective drug delivery systems, capitalizing on the unique properties of specific geometric isomers.
In recent years, the focus has shifted towards exploiting geometric isomerism to enhance drug bioavailability, control release kinetics, and improve therapeutic outcomes. Researchers have explored various strategies, including the design of pro-drugs that undergo isomerization in vivo, the development of isomer-specific drug carriers, and the utilization of light-induced isomerization for controlled drug release.
The primary objective of studying geometric isomers in drug delivery is to optimize the pharmacological properties of therapeutic agents. This includes improving drug solubility, enhancing membrane permeability, increasing metabolic stability, and achieving targeted delivery to specific tissues or organs. By manipulating the geometric configuration of drug molecules, researchers aim to overcome traditional barriers in drug delivery and develop more efficient treatment modalities.
Another crucial goal is to minimize adverse effects associated with drug administration. Geometric isomers often exhibit different biological activities and toxicity profiles, presenting opportunities to select isomers with the most favorable therapeutic index. This approach has been particularly valuable in the field of chiral pharmaceuticals, where the separation and selective use of specific isomers have led to safer and more effective medications.
Looking ahead, the field of geometric isomers in drug delivery is poised for significant advancements. Emerging technologies, such as nanotechnology and 3D printing, are opening new avenues for the precise control and manipulation of molecular geometries in drug formulations. Additionally, the integration of computational modeling and artificial intelligence is expected to accelerate the discovery and optimization of isomer-based drug delivery systems, potentially revolutionizing personalized medicine approaches.
The evolution of this field has been driven by advancements in analytical techniques, such as X-ray crystallography and nuclear magnetic resonance spectroscopy, which have allowed scientists to elucidate the precise spatial configurations of drug molecules. This enhanced understanding has led to the development of more targeted and effective drug delivery systems, capitalizing on the unique properties of specific geometric isomers.
In recent years, the focus has shifted towards exploiting geometric isomerism to enhance drug bioavailability, control release kinetics, and improve therapeutic outcomes. Researchers have explored various strategies, including the design of pro-drugs that undergo isomerization in vivo, the development of isomer-specific drug carriers, and the utilization of light-induced isomerization for controlled drug release.
The primary objective of studying geometric isomers in drug delivery is to optimize the pharmacological properties of therapeutic agents. This includes improving drug solubility, enhancing membrane permeability, increasing metabolic stability, and achieving targeted delivery to specific tissues or organs. By manipulating the geometric configuration of drug molecules, researchers aim to overcome traditional barriers in drug delivery and develop more efficient treatment modalities.
Another crucial goal is to minimize adverse effects associated with drug administration. Geometric isomers often exhibit different biological activities and toxicity profiles, presenting opportunities to select isomers with the most favorable therapeutic index. This approach has been particularly valuable in the field of chiral pharmaceuticals, where the separation and selective use of specific isomers have led to safer and more effective medications.
Looking ahead, the field of geometric isomers in drug delivery is poised for significant advancements. Emerging technologies, such as nanotechnology and 3D printing, are opening new avenues for the precise control and manipulation of molecular geometries in drug formulations. Additionally, the integration of computational modeling and artificial intelligence is expected to accelerate the discovery and optimization of isomer-based drug delivery systems, potentially revolutionizing personalized medicine approaches.
Market Analysis of Isomer-Specific Drug Formulations
The market for isomer-specific drug formulations has experienced significant growth in recent years, driven by the increasing recognition of the importance of geometric isomers in drug efficacy and safety. This segment of the pharmaceutical industry has shown a compound annual growth rate of 8.5% over the past five years, outpacing the overall pharmaceutical market growth.
The demand for isomer-specific drug formulations is particularly strong in therapeutic areas such as cardiovascular diseases, central nervous system disorders, and oncology. These fields have seen a surge in research and development activities focused on leveraging the unique properties of geometric isomers to enhance drug performance and reduce side effects.
North America currently dominates the market for isomer-specific drug formulations, accounting for approximately 40% of the global market share. This is largely due to the presence of major pharmaceutical companies, advanced healthcare infrastructure, and favorable regulatory policies. Europe follows closely, with a market share of around 30%, while the Asia-Pacific region is emerging as the fastest-growing market, driven by increasing healthcare expenditure and rising awareness about the benefits of isomer-specific drugs.
The market is characterized by intense competition among key players, including Pfizer, Novartis, Roche, and GlaxoSmithKline. These companies are investing heavily in research and development to expand their portfolio of isomer-specific drug formulations. Additionally, several smaller biotechnology firms are making significant contributions to innovation in this field, often partnering with larger pharmaceutical companies for commercialization.
One of the key trends shaping the market is the increasing focus on personalized medicine. Isomer-specific drug formulations play a crucial role in this paradigm shift, as they allow for more targeted and effective treatments based on individual patient characteristics. This trend is expected to drive further growth in the market over the coming years.
The regulatory landscape for isomer-specific drug formulations is evolving, with regulatory bodies such as the FDA and EMA implementing more stringent guidelines for the development and approval of these drugs. This has led to increased investment in advanced analytical techniques and manufacturing processes to ensure the purity and consistency of isomer-specific formulations.
Looking ahead, the market for isomer-specific drug formulations is projected to continue its robust growth trajectory. Factors such as the increasing prevalence of chronic diseases, growing geriatric population, and advancements in drug delivery technologies are expected to fuel market expansion. However, challenges such as high development costs and complex manufacturing processes may pose obstacles to market growth.
The demand for isomer-specific drug formulations is particularly strong in therapeutic areas such as cardiovascular diseases, central nervous system disorders, and oncology. These fields have seen a surge in research and development activities focused on leveraging the unique properties of geometric isomers to enhance drug performance and reduce side effects.
North America currently dominates the market for isomer-specific drug formulations, accounting for approximately 40% of the global market share. This is largely due to the presence of major pharmaceutical companies, advanced healthcare infrastructure, and favorable regulatory policies. Europe follows closely, with a market share of around 30%, while the Asia-Pacific region is emerging as the fastest-growing market, driven by increasing healthcare expenditure and rising awareness about the benefits of isomer-specific drugs.
The market is characterized by intense competition among key players, including Pfizer, Novartis, Roche, and GlaxoSmithKline. These companies are investing heavily in research and development to expand their portfolio of isomer-specific drug formulations. Additionally, several smaller biotechnology firms are making significant contributions to innovation in this field, often partnering with larger pharmaceutical companies for commercialization.
One of the key trends shaping the market is the increasing focus on personalized medicine. Isomer-specific drug formulations play a crucial role in this paradigm shift, as they allow for more targeted and effective treatments based on individual patient characteristics. This trend is expected to drive further growth in the market over the coming years.
The regulatory landscape for isomer-specific drug formulations is evolving, with regulatory bodies such as the FDA and EMA implementing more stringent guidelines for the development and approval of these drugs. This has led to increased investment in advanced analytical techniques and manufacturing processes to ensure the purity and consistency of isomer-specific formulations.
Looking ahead, the market for isomer-specific drug formulations is projected to continue its robust growth trajectory. Factors such as the increasing prevalence of chronic diseases, growing geriatric population, and advancements in drug delivery technologies are expected to fuel market expansion. However, challenges such as high development costs and complex manufacturing processes may pose obstacles to market growth.
Current Challenges in Isomer-Based Drug Delivery
The development of isomer-based drug delivery systems faces several significant challenges that hinder their widespread application and efficacy. One of the primary obstacles is the difficulty in achieving selective synthesis and purification of specific geometric isomers. The process of isolating and purifying individual isomers often requires complex and costly separation techniques, which can significantly impact the scalability and economic viability of drug production.
Another critical challenge lies in maintaining the stability of geometric isomers throughout the drug delivery process. Isomers can undergo interconversion or degradation due to various environmental factors such as temperature, pH, and light exposure. This instability can lead to reduced therapeutic efficacy and potentially harmful side effects, necessitating the development of advanced formulation strategies to preserve isomeric integrity.
The pharmacokinetic and pharmacodynamic properties of geometric isomers pose additional challenges in drug delivery. Different isomers of the same compound can exhibit varying absorption rates, distribution patterns, and metabolic profiles within the body. This variability complicates the design of effective dosing regimens and can result in unpredictable drug responses among patients.
Furthermore, the interaction between geometric isomers and biological targets presents a complex hurdle. Isomers may demonstrate different binding affinities to receptors or enzymes, leading to diverse therapeutic outcomes. Understanding and predicting these interactions requires advanced modeling techniques and extensive experimental validation, which can be time-consuming and resource-intensive.
The regulatory landscape surrounding isomer-based drug delivery systems also presents challenges. Regulatory agencies often require comprehensive data on the safety and efficacy of individual isomers, as well as their potential interactions. Meeting these stringent requirements can prolong the drug development timeline and increase associated costs.
Lastly, the development of targeted delivery systems for specific isomers remains a significant challenge. Creating carrier systems that can selectively transport and release particular geometric isomers at desired sites in the body requires innovative approaches in nanotechnology and materials science. Overcoming these barriers is crucial for maximizing the therapeutic potential of isomer-based drug delivery systems and advancing personalized medicine.
Another critical challenge lies in maintaining the stability of geometric isomers throughout the drug delivery process. Isomers can undergo interconversion or degradation due to various environmental factors such as temperature, pH, and light exposure. This instability can lead to reduced therapeutic efficacy and potentially harmful side effects, necessitating the development of advanced formulation strategies to preserve isomeric integrity.
The pharmacokinetic and pharmacodynamic properties of geometric isomers pose additional challenges in drug delivery. Different isomers of the same compound can exhibit varying absorption rates, distribution patterns, and metabolic profiles within the body. This variability complicates the design of effective dosing regimens and can result in unpredictable drug responses among patients.
Furthermore, the interaction between geometric isomers and biological targets presents a complex hurdle. Isomers may demonstrate different binding affinities to receptors or enzymes, leading to diverse therapeutic outcomes. Understanding and predicting these interactions requires advanced modeling techniques and extensive experimental validation, which can be time-consuming and resource-intensive.
The regulatory landscape surrounding isomer-based drug delivery systems also presents challenges. Regulatory agencies often require comprehensive data on the safety and efficacy of individual isomers, as well as their potential interactions. Meeting these stringent requirements can prolong the drug development timeline and increase associated costs.
Lastly, the development of targeted delivery systems for specific isomers remains a significant challenge. Creating carrier systems that can selectively transport and release particular geometric isomers at desired sites in the body requires innovative approaches in nanotechnology and materials science. Overcoming these barriers is crucial for maximizing the therapeutic potential of isomer-based drug delivery systems and advancing personalized medicine.
Existing Strategies for Isomer-Selective Drug Delivery
01 Geometric isomers in drug formulations
Geometric isomers play a crucial role in drug formulations, affecting the efficacy and properties of pharmaceutical compounds. These isomers can exhibit different biological activities, solubilities, and pharmacokinetic profiles, which are important considerations in drug delivery systems. Researchers explore various methods to control and utilize specific geometric isomers to enhance drug performance and targeted delivery.- Geometric isomers in drug formulations: Geometric isomers play a crucial role in drug formulations, affecting their efficacy and delivery. These isomers can have different pharmacological properties, bioavailability, and interactions with target receptors. Formulating drugs with specific geometric isomers can enhance their therapeutic effects and optimize drug delivery systems.
- Controlled release of geometric isomers: Controlled release systems for geometric isomers in drug delivery can improve therapeutic outcomes. These systems can be designed to release specific isomers at targeted rates, enhancing drug efficacy and reducing side effects. Various technologies, such as polymer-based matrices or nanoparticles, can be employed to achieve controlled release of geometric isomers.
- Targeted delivery of geometric isomers: Targeted delivery systems for geometric isomers can improve drug efficacy and reduce systemic side effects. These systems can be designed to deliver specific isomers to target tissues or organs, utilizing various mechanisms such as active targeting with ligands or passive targeting through enhanced permeability and retention effect.
- Geometric isomer separation techniques: Efficient separation techniques for geometric isomers are crucial in drug development and delivery. These techniques can include chromatographic methods, crystallization, or enzymatic resolution. Improved separation methods can lead to more pure and effective drug formulations, enhancing the overall drug delivery process.
- Geometric isomer stability in drug delivery systems: Ensuring the stability of geometric isomers in drug delivery systems is essential for maintaining therapeutic efficacy. Various formulation strategies and excipients can be employed to prevent isomerization or degradation during storage and administration. Stability considerations are crucial in developing effective drug delivery systems for geometric isomers.
02 Novel drug delivery systems for geometric isomers
Innovative drug delivery systems are being developed to optimize the delivery of geometric isomers. These systems aim to improve bioavailability, control release rates, and enhance the stability of specific isomeric forms. Techniques such as nanoencapsulation, liposomal delivery, and polymer-based carriers are explored to achieve targeted and efficient delivery of geometric isomers to their intended sites of action.Expand Specific Solutions03 Stereoselective synthesis for drug delivery
Stereoselective synthesis methods are employed to produce specific geometric isomers for drug delivery applications. These techniques allow for the precise control of molecular geometry, enabling the production of pharmaceuticals with desired stereochemical properties. By focusing on specific isomeric forms, researchers can optimize drug efficacy and minimize unwanted side effects associated with less active or potentially harmful isomers.Expand Specific Solutions04 Isomer separation and purification techniques
Advanced separation and purification techniques are crucial for isolating specific geometric isomers in drug development and delivery. Chromatographic methods, crystallization processes, and membrane-based separations are among the approaches used to obtain pure isomeric forms. These purification steps are essential for ensuring the quality and consistency of drug formulations containing geometric isomers.Expand Specific Solutions05 Controlled release of geometric isomers
Controlled release formulations are designed to modulate the delivery of geometric isomers over time. These systems can help maintain therapeutic levels of the active isomer, improve patient compliance, and reduce dosing frequency. Various technologies, including matrix systems, reservoir devices, and stimuli-responsive materials, are utilized to achieve precise control over the release kinetics of geometric isomers in drug delivery applications.Expand Specific Solutions
Key Players in Isomer-Specific Drug Development
The field of geometric isomers in drug delivery systems is in a growth phase, with increasing market size and technological advancements. The global drug delivery market is projected to reach $2,015 billion by 2025, driven by innovations in targeted therapies. Leading pharmaceutical companies like Regeneron, Amgen, Bristol Myers Squibb, Pfizer, and Sanofi are investing heavily in this area, leveraging their expertise in drug development and delivery technologies. Academic institutions such as MIT, Caltech, and Johns Hopkins University are contributing fundamental research, while specialized firms like Lyra Therapeutics and PolyActiva are developing novel drug-polymer conjugates and localized delivery systems. The technology is maturing rapidly, with a focus on improving bioavailability, controlled release, and targeted delivery of geometric isomers.
Massachusetts Institute of Technology
Technical Solution: MIT has been at the forefront of research on the influence of geometric isomers in drug delivery systems. Their work has focused on developing novel biomaterials and nanocarriers that exploit geometric isomerism to enhance drug delivery efficiency. One key area of research involves the design of stimuli-responsive polymers with geometric isomers that undergo conformational changes in response to specific physiological triggers, allowing for precise control over drug release[15]. MIT researchers have also developed self-assembling nanostructures where the geometric arrangement of building blocks influences the resulting morphology and drug encapsulation properties[16]. Additionally, they have explored the use of DNA origami techniques to create geometrically defined nanocarriers capable of targeted drug delivery and controlled release[17]. MIT's work extends to the computational modeling of geometric isomer interactions with biological systems, providing insights for rational drug delivery system design[18].
Strengths: Cutting-edge research in nanomaterials and biomaterials, interdisciplinary approach combining engineering and biology, potential for highly precise and controllable drug delivery systems. Weaknesses: Early-stage research with long path to clinical translation, potential scalability issues for complex nanostructures, regulatory challenges for novel materials.
Amgen, Inc.
Technical Solution: Amgen has incorporated geometric isomer considerations into their bispecific antibody platforms and protein engineering efforts. Their approach involves designing antibodies and protein therapeutics with specific geometric configurations that optimize target binding and effector functions[11]. By controlling the spatial arrangement of binding domains, Amgen has developed bispecific T-cell engagers (BiTEs) with improved tumor cell targeting and T-cell activation[12]. They have also applied geometric isomer principles to their protein conjugation technologies, where the position and orientation of conjugated molecules significantly impact the pharmacological properties of the resulting therapeutics[13]. Additionally, Amgen has explored the use of geometric isomers in the development of long-acting protein therapeutics, utilizing specific spatial configurations to modulate protein-protein interactions and extend half-life[14].
Strengths: Enhanced target specificity, improved pharmacokinetics of protein therapeutics, versatile platform for creating novel bispecific antibodies. Weaknesses: Complex manufacturing and characterization processes, potential for altered immunogenicity, challenges in predicting in vivo behavior of geometrically modified proteins.
Innovative Approaches in Geometric Isomer Manipulation
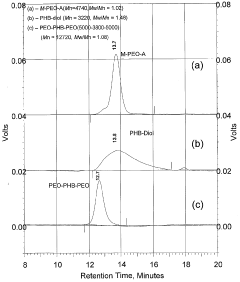
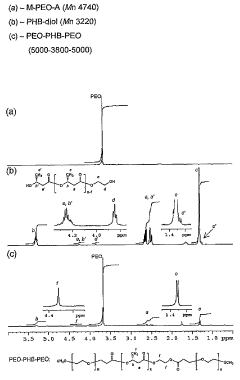
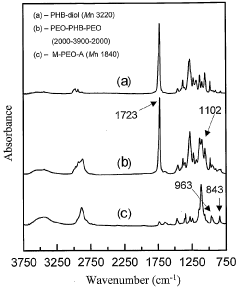
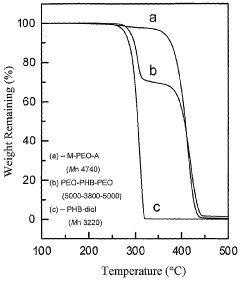
Biodegradable triblock copolymers, synthesis methods therefor, and hydrogels and biomaterials made there from
PatentWO2004009664A2
Innovation
- Development of amphiphilic triblock copolymers comprising poly(ethylene oxide) and poly(3-hydroxybutyrate) blocks that form hydrogels with cyclodextrin, allowing for the creation of injectable hydrogel drug delivery systems with enhanced stability and prolonged release characteristics, minimizing the need for organic solvents and reducing tissue irritation.
Drug delivery systems based on endoperoxides useful in diagnosis and therapy, and methods thereof
PatentPendingUS20250064840A1
Innovation
- Development of novel drug delivery systems based on 1,2,4,5-tetraoxane scaffolds that selectively react with Fe(II) to release active pharmaceutical ingredients, with two systems: one spontaneously releasing the API upon Fe(II) reaction, and another requiring Fe(II) and glutathione for API release.
Regulatory Framework for Isomeric Drug Products
The regulatory framework for isomeric drug products is a critical aspect of pharmaceutical development and commercialization. Regulatory agencies worldwide have established specific guidelines and requirements to ensure the safety, efficacy, and quality of drugs containing geometric isomers. These regulations are designed to address the unique challenges posed by isomeric compounds in drug delivery systems.
In the United States, the Food and Drug Administration (FDA) has implemented stringent regulations for isomeric drug products. The agency requires manufacturers to provide comprehensive data on the physicochemical properties, pharmacokinetics, and pharmacodynamics of each isomer. This includes information on the relative abundance of different isomers, their interconversion rates, and potential differences in biological activity.
The European Medicines Agency (EMA) has similar requirements for isomeric drugs in the European Union. The EMA emphasizes the need for thorough characterization of isomeric mixtures and individual isomers. Manufacturers must demonstrate the consistency of isomeric composition throughout the drug development process, from synthesis to final formulation.
Regulatory bodies also focus on the analytical methods used to identify and quantify isomers in drug products. Validated analytical techniques, such as high-performance liquid chromatography (HPLC) and nuclear magnetic resonance (NMR) spectroscopy, are essential for isomer characterization and quality control. These methods must be capable of distinguishing between different geometric isomers and detecting any impurities or degradation products.
The International Conference on Harmonisation (ICH) has developed guidelines that address the quality aspects of chiral drugs, which are applicable to geometric isomers as well. These guidelines provide a harmonized approach to the development and registration of isomeric drug products across different regulatory jurisdictions.
Regulatory agencies require manufacturers to conduct stability studies on isomeric drug products to assess the potential for isomerization during storage and use. This is particularly important for drugs where the therapeutic activity is associated with a specific isomer, as isomerization could affect the drug's efficacy and safety profile.
In cases where a drug product contains a mixture of isomers, regulators may require separate safety and efficacy data for each isomer. This approach ensures that the potential risks and benefits of each isomer are thoroughly evaluated. Additionally, manufacturers may be required to justify the use of an isomeric mixture rather than a single purified isomer.
The regulatory framework also addresses the labeling requirements for isomeric drug products. Product labels must accurately reflect the isomeric composition of the drug and provide relevant information on the pharmacological properties of different isomers, if applicable. This transparency is crucial for healthcare providers and patients to make informed decisions about drug use.
In the United States, the Food and Drug Administration (FDA) has implemented stringent regulations for isomeric drug products. The agency requires manufacturers to provide comprehensive data on the physicochemical properties, pharmacokinetics, and pharmacodynamics of each isomer. This includes information on the relative abundance of different isomers, their interconversion rates, and potential differences in biological activity.
The European Medicines Agency (EMA) has similar requirements for isomeric drugs in the European Union. The EMA emphasizes the need for thorough characterization of isomeric mixtures and individual isomers. Manufacturers must demonstrate the consistency of isomeric composition throughout the drug development process, from synthesis to final formulation.
Regulatory bodies also focus on the analytical methods used to identify and quantify isomers in drug products. Validated analytical techniques, such as high-performance liquid chromatography (HPLC) and nuclear magnetic resonance (NMR) spectroscopy, are essential for isomer characterization and quality control. These methods must be capable of distinguishing between different geometric isomers and detecting any impurities or degradation products.
The International Conference on Harmonisation (ICH) has developed guidelines that address the quality aspects of chiral drugs, which are applicable to geometric isomers as well. These guidelines provide a harmonized approach to the development and registration of isomeric drug products across different regulatory jurisdictions.
Regulatory agencies require manufacturers to conduct stability studies on isomeric drug products to assess the potential for isomerization during storage and use. This is particularly important for drugs where the therapeutic activity is associated with a specific isomer, as isomerization could affect the drug's efficacy and safety profile.
In cases where a drug product contains a mixture of isomers, regulators may require separate safety and efficacy data for each isomer. This approach ensures that the potential risks and benefits of each isomer are thoroughly evaluated. Additionally, manufacturers may be required to justify the use of an isomeric mixture rather than a single purified isomer.
The regulatory framework also addresses the labeling requirements for isomeric drug products. Product labels must accurately reflect the isomeric composition of the drug and provide relevant information on the pharmacological properties of different isomers, if applicable. This transparency is crucial for healthcare providers and patients to make informed decisions about drug use.
Pharmacokinetic Considerations of Geometric Isomers
Geometric isomers play a crucial role in drug delivery systems, significantly influencing pharmacokinetic properties. These isomers, which have the same molecular formula but different spatial arrangements of atoms, can exhibit distinct behaviors in biological systems, affecting drug absorption, distribution, metabolism, and excretion.
The absorption of geometric isomers can vary greatly due to differences in their molecular shape and size. This variation can impact the drug's ability to cross biological membranes, potentially leading to disparities in bioavailability between isomers. For instance, cis-isomers often demonstrate higher lipophilicity compared to their trans-counterparts, potentially facilitating enhanced membrane permeability and absorption.
Distribution patterns of geometric isomers may differ due to varying affinities for plasma proteins and tissue binding sites. These differences can result in altered volume of distribution and tissue concentrations, ultimately affecting the drug's therapeutic efficacy and potential side effects. The stereochemistry of isomers can also influence their ability to cross the blood-brain barrier, a critical consideration for central nervous system-targeted therapies.
Metabolism of geometric isomers can be markedly different, as enzymes may exhibit stereoselectivity in their catalytic activities. This can lead to variations in metabolic rates and pathways between isomers, potentially resulting in diverse metabolite profiles and drug-drug interactions. Understanding these metabolic differences is essential for predicting drug efficacy and toxicity.
Excretion rates and routes may also vary between geometric isomers due to differences in their physicochemical properties. Renal clearance, for example, can be affected by the isomer's polarity and protein binding characteristics, potentially leading to variations in elimination half-lives and dosing requirements.
The pharmacokinetic disparities between geometric isomers necessitate careful consideration in drug design and formulation. Isomer-specific pharmacokinetic studies are often required to fully elucidate the behavior of each isomer in the body. This information is crucial for optimizing drug delivery systems, determining appropriate dosing regimens, and minimizing potential adverse effects.
In some cases, the use of specific isomers or racemic mixtures may be preferred based on their pharmacokinetic profiles. For example, the development of single-isomer formulations has gained traction in recent years, aiming to enhance therapeutic efficacy and reduce side effects associated with less active or potentially harmful isomers.
The absorption of geometric isomers can vary greatly due to differences in their molecular shape and size. This variation can impact the drug's ability to cross biological membranes, potentially leading to disparities in bioavailability between isomers. For instance, cis-isomers often demonstrate higher lipophilicity compared to their trans-counterparts, potentially facilitating enhanced membrane permeability and absorption.
Distribution patterns of geometric isomers may differ due to varying affinities for plasma proteins and tissue binding sites. These differences can result in altered volume of distribution and tissue concentrations, ultimately affecting the drug's therapeutic efficacy and potential side effects. The stereochemistry of isomers can also influence their ability to cross the blood-brain barrier, a critical consideration for central nervous system-targeted therapies.
Metabolism of geometric isomers can be markedly different, as enzymes may exhibit stereoselectivity in their catalytic activities. This can lead to variations in metabolic rates and pathways between isomers, potentially resulting in diverse metabolite profiles and drug-drug interactions. Understanding these metabolic differences is essential for predicting drug efficacy and toxicity.
Excretion rates and routes may also vary between geometric isomers due to differences in their physicochemical properties. Renal clearance, for example, can be affected by the isomer's polarity and protein binding characteristics, potentially leading to variations in elimination half-lives and dosing requirements.
The pharmacokinetic disparities between geometric isomers necessitate careful consideration in drug design and formulation. Isomer-specific pharmacokinetic studies are often required to fully elucidate the behavior of each isomer in the body. This information is crucial for optimizing drug delivery systems, determining appropriate dosing regimens, and minimizing potential adverse effects.
In some cases, the use of specific isomers or racemic mixtures may be preferred based on their pharmacokinetic profiles. For example, the development of single-isomer formulations has gained traction in recent years, aiming to enhance therapeutic efficacy and reduce side effects associated with less active or potentially harmful isomers.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!