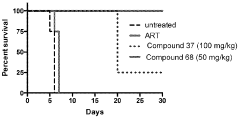
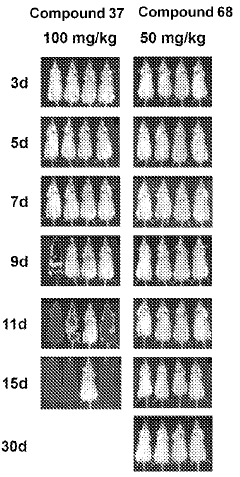
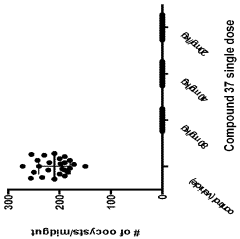
The Role of Geometric Isomers in Drug Efficacy and Toxicity
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Geometric Isomers in Pharmaceuticals: Background and Objectives
Geometric isomers, a subset of stereoisomers, have played a crucial role in the pharmaceutical industry since the early 20th century. These compounds possess identical molecular formulas and bonding arrangements but differ in the spatial orientation of their atoms or groups. The significance of geometric isomers in drug development and efficacy has become increasingly apparent over the past decades, leading to a paradigm shift in pharmaceutical research and development.
The study of geometric isomers in pharmaceuticals dates back to the 1960s when researchers began to recognize the impact of molecular geometry on drug-receptor interactions. This realization stemmed from observations that different isomers of the same compound could exhibit vastly different biological activities. The thalidomide tragedy of the late 1950s and early 1960s served as a stark reminder of the importance of understanding isomeric forms in drug development, as one isomer of thalidomide was found to be teratogenic while the other had the intended sedative effects.
As the field of medicinal chemistry advanced, the objectives of studying geometric isomers in pharmaceuticals became more refined. The primary goal has been to enhance drug efficacy while minimizing toxicity by exploiting the unique properties of specific isomers. This approach has led to the development of single-isomer drugs, which offer improved therapeutic indices compared to their racemic counterparts.
The technological advancements in analytical chemistry, particularly in chromatography and spectroscopy, have greatly facilitated the separation and characterization of geometric isomers. These tools have enabled researchers to isolate and study individual isomers with unprecedented precision, leading to a deeper understanding of structure-activity relationships in drug molecules.
In recent years, the focus has shifted towards predicting the biological activity of geometric isomers using computational methods. Molecular modeling and quantitative structure-activity relationship (QSAR) studies have become integral parts of the drug discovery process, allowing researchers to screen potential drug candidates more efficiently and predict their isomeric behavior in biological systems.
The objectives of current research in this field are multifaceted. Scientists aim to develop more efficient methods for synthesizing and purifying specific geometric isomers, improve techniques for predicting isomer-specific biological activities, and understand the mechanisms by which geometric isomers interact with their target receptors. Additionally, there is a growing interest in exploring the potential of geometric isomers in personalized medicine, where specific isomers may be tailored to individual patient profiles for optimal therapeutic outcomes.
As we look to the future, the study of geometric isomers in pharmaceuticals continues to evolve. The integration of artificial intelligence and machine learning algorithms promises to revolutionize the prediction and optimization of isomer-specific drug properties. Furthermore, emerging technologies in nanotechnology and drug delivery systems are opening new avenues for exploiting the unique characteristics of geometric isomers in targeted therapies.
The study of geometric isomers in pharmaceuticals dates back to the 1960s when researchers began to recognize the impact of molecular geometry on drug-receptor interactions. This realization stemmed from observations that different isomers of the same compound could exhibit vastly different biological activities. The thalidomide tragedy of the late 1950s and early 1960s served as a stark reminder of the importance of understanding isomeric forms in drug development, as one isomer of thalidomide was found to be teratogenic while the other had the intended sedative effects.
As the field of medicinal chemistry advanced, the objectives of studying geometric isomers in pharmaceuticals became more refined. The primary goal has been to enhance drug efficacy while minimizing toxicity by exploiting the unique properties of specific isomers. This approach has led to the development of single-isomer drugs, which offer improved therapeutic indices compared to their racemic counterparts.
The technological advancements in analytical chemistry, particularly in chromatography and spectroscopy, have greatly facilitated the separation and characterization of geometric isomers. These tools have enabled researchers to isolate and study individual isomers with unprecedented precision, leading to a deeper understanding of structure-activity relationships in drug molecules.
In recent years, the focus has shifted towards predicting the biological activity of geometric isomers using computational methods. Molecular modeling and quantitative structure-activity relationship (QSAR) studies have become integral parts of the drug discovery process, allowing researchers to screen potential drug candidates more efficiently and predict their isomeric behavior in biological systems.
The objectives of current research in this field are multifaceted. Scientists aim to develop more efficient methods for synthesizing and purifying specific geometric isomers, improve techniques for predicting isomer-specific biological activities, and understand the mechanisms by which geometric isomers interact with their target receptors. Additionally, there is a growing interest in exploring the potential of geometric isomers in personalized medicine, where specific isomers may be tailored to individual patient profiles for optimal therapeutic outcomes.
As we look to the future, the study of geometric isomers in pharmaceuticals continues to evolve. The integration of artificial intelligence and machine learning algorithms promises to revolutionize the prediction and optimization of isomer-specific drug properties. Furthermore, emerging technologies in nanotechnology and drug delivery systems are opening new avenues for exploiting the unique characteristics of geometric isomers in targeted therapies.
Market Demand for Isomer-Specific Drugs
The market demand for isomer-specific drugs has been steadily growing in recent years, driven by the increasing recognition of the importance of geometric isomers in drug efficacy and safety. Pharmaceutical companies are increasingly focusing on developing and marketing single-isomer drugs, also known as chiral drugs, due to their potential advantages over racemic mixtures.
One of the primary drivers of this demand is the improved therapeutic efficacy of single-isomer drugs. By isolating and utilizing the specific isomer responsible for the desired pharmacological effect, these drugs can potentially offer enhanced potency and reduced side effects compared to their racemic counterparts. This increased efficacy can lead to lower dosage requirements, potentially reducing the risk of adverse reactions and improving patient compliance.
The regulatory landscape has also played a significant role in shaping the market demand for isomer-specific drugs. Regulatory agencies, such as the FDA and EMA, have implemented guidelines encouraging the development of single-isomer drugs and requiring thorough investigation of individual isomers during the drug development process. This regulatory push has incentivized pharmaceutical companies to invest in isomer-specific drug development and has contributed to the growth of this market segment.
Patient safety considerations have further fueled the demand for isomer-specific drugs. The thalidomide tragedy of the 1960s, where one isomer caused severe birth defects, serves as a stark reminder of the potential risks associated with racemic mixtures. As a result, both healthcare providers and patients have become more aware of the importance of isomer-specific formulations, leading to increased demand for these products.
The market for isomer-specific drugs spans various therapeutic areas, including cardiovascular diseases, central nervous system disorders, respiratory diseases, and oncology. In particular, the oncology sector has seen significant growth in the development of chiral drugs, as the precise targeting of cancer cells often requires highly specific molecular interactions that can be achieved through single-isomer formulations.
From a commercial perspective, the development of isomer-specific drugs offers pharmaceutical companies opportunities for product differentiation and patent extension strategies. By developing single-isomer versions of existing racemic drugs, companies can potentially extend their market exclusivity and maintain competitive advantages in crowded therapeutic areas.
However, it is important to note that the development of isomer-specific drugs also presents challenges. The separation and purification of specific isomers can be technically complex and costly, potentially impacting the overall drug development timeline and expenses. Additionally, not all racemic drugs necessarily benefit from isomer separation, as in some cases, both isomers may contribute to the therapeutic effect or have complementary activities.
One of the primary drivers of this demand is the improved therapeutic efficacy of single-isomer drugs. By isolating and utilizing the specific isomer responsible for the desired pharmacological effect, these drugs can potentially offer enhanced potency and reduced side effects compared to their racemic counterparts. This increased efficacy can lead to lower dosage requirements, potentially reducing the risk of adverse reactions and improving patient compliance.
The regulatory landscape has also played a significant role in shaping the market demand for isomer-specific drugs. Regulatory agencies, such as the FDA and EMA, have implemented guidelines encouraging the development of single-isomer drugs and requiring thorough investigation of individual isomers during the drug development process. This regulatory push has incentivized pharmaceutical companies to invest in isomer-specific drug development and has contributed to the growth of this market segment.
Patient safety considerations have further fueled the demand for isomer-specific drugs. The thalidomide tragedy of the 1960s, where one isomer caused severe birth defects, serves as a stark reminder of the potential risks associated with racemic mixtures. As a result, both healthcare providers and patients have become more aware of the importance of isomer-specific formulations, leading to increased demand for these products.
The market for isomer-specific drugs spans various therapeutic areas, including cardiovascular diseases, central nervous system disorders, respiratory diseases, and oncology. In particular, the oncology sector has seen significant growth in the development of chiral drugs, as the precise targeting of cancer cells often requires highly specific molecular interactions that can be achieved through single-isomer formulations.
From a commercial perspective, the development of isomer-specific drugs offers pharmaceutical companies opportunities for product differentiation and patent extension strategies. By developing single-isomer versions of existing racemic drugs, companies can potentially extend their market exclusivity and maintain competitive advantages in crowded therapeutic areas.
However, it is important to note that the development of isomer-specific drugs also presents challenges. The separation and purification of specific isomers can be technically complex and costly, potentially impacting the overall drug development timeline and expenses. Additionally, not all racemic drugs necessarily benefit from isomer separation, as in some cases, both isomers may contribute to the therapeutic effect or have complementary activities.
Current Challenges in Geometric Isomer Separation
The separation of geometric isomers remains a significant challenge in pharmaceutical research and development. Despite advancements in analytical techniques, the isolation and purification of these structurally similar compounds continue to pose difficulties for researchers and manufacturers alike.
One of the primary challenges in geometric isomer separation is the similarity in physicochemical properties between the isomers. These compounds often exhibit nearly identical molecular weights, boiling points, and solubilities, making traditional separation methods less effective. This similarity necessitates the development of highly selective and sensitive separation techniques to achieve the required purity levels for pharmaceutical applications.
Chromatographic methods, particularly high-performance liquid chromatography (HPLC), have been widely employed for geometric isomer separation. However, achieving adequate resolution and peak separation remains challenging, especially for complex mixtures or when dealing with compounds that have subtle structural differences. The selection of appropriate stationary phases and mobile phase compositions is critical and often requires extensive method development and optimization.
Another significant hurdle is the potential for interconversion between geometric isomers during the separation process. Environmental factors such as temperature, pH, and exposure to light can induce isomerization, compromising the purity of the isolated compounds. This instability necessitates careful control of separation conditions and often requires the development of specialized stabilization techniques to maintain isomeric integrity throughout the purification process.
Scale-up of geometric isomer separation processes from laboratory to industrial scale presents additional challenges. Maintaining separation efficiency and purity levels while increasing throughput and reducing costs is a delicate balance that requires significant process engineering efforts. The need for specialized equipment and the potential for increased production costs can impact the economic viability of drug candidates that require geometric isomer separation.
Furthermore, the regulatory landscape surrounding geometric isomers in pharmaceuticals adds another layer of complexity to the separation challenge. Stringent purity requirements and the need for comprehensive characterization of isomeric impurities demand highly accurate and reproducible separation methods. This regulatory scrutiny necessitates the development of robust analytical protocols capable of detecting and quantifying even trace amounts of isomeric impurities.
Emerging technologies, such as supercritical fluid chromatography (SFC) and simulated moving bed (SMB) chromatography, offer promising solutions for some geometric isomer separation challenges. However, these techniques often require significant investment in equipment and expertise, limiting their widespread adoption in the pharmaceutical industry.
One of the primary challenges in geometric isomer separation is the similarity in physicochemical properties between the isomers. These compounds often exhibit nearly identical molecular weights, boiling points, and solubilities, making traditional separation methods less effective. This similarity necessitates the development of highly selective and sensitive separation techniques to achieve the required purity levels for pharmaceutical applications.
Chromatographic methods, particularly high-performance liquid chromatography (HPLC), have been widely employed for geometric isomer separation. However, achieving adequate resolution and peak separation remains challenging, especially for complex mixtures or when dealing with compounds that have subtle structural differences. The selection of appropriate stationary phases and mobile phase compositions is critical and often requires extensive method development and optimization.
Another significant hurdle is the potential for interconversion between geometric isomers during the separation process. Environmental factors such as temperature, pH, and exposure to light can induce isomerization, compromising the purity of the isolated compounds. This instability necessitates careful control of separation conditions and often requires the development of specialized stabilization techniques to maintain isomeric integrity throughout the purification process.
Scale-up of geometric isomer separation processes from laboratory to industrial scale presents additional challenges. Maintaining separation efficiency and purity levels while increasing throughput and reducing costs is a delicate balance that requires significant process engineering efforts. The need for specialized equipment and the potential for increased production costs can impact the economic viability of drug candidates that require geometric isomer separation.
Furthermore, the regulatory landscape surrounding geometric isomers in pharmaceuticals adds another layer of complexity to the separation challenge. Stringent purity requirements and the need for comprehensive characterization of isomeric impurities demand highly accurate and reproducible separation methods. This regulatory scrutiny necessitates the development of robust analytical protocols capable of detecting and quantifying even trace amounts of isomeric impurities.
Emerging technologies, such as supercritical fluid chromatography (SFC) and simulated moving bed (SMB) chromatography, offer promising solutions for some geometric isomer separation challenges. However, these techniques often require significant investment in equipment and expertise, limiting their widespread adoption in the pharmaceutical industry.
Existing Methods for Geometric Isomer Analysis
01 Efficacy differences between geometric isomers
Geometric isomers can exhibit different levels of efficacy due to their spatial arrangements. These differences can affect binding to target molecules, metabolic processes, and overall therapeutic effects. Understanding these variations is crucial for drug development and optimization.- Efficacy differences between geometric isomers: Geometric isomers can exhibit different levels of efficacy due to their spatial arrangements. This difference in structure can affect how the compounds interact with biological targets, potentially leading to variations in potency, binding affinity, and overall therapeutic effect. Understanding these differences is crucial for drug development and optimization.
- Toxicity profiles of geometric isomers: The toxicity of geometric isomers can vary significantly despite having the same molecular formula. This is due to differences in their three-dimensional structure, which can affect how they interact with biological systems. Some isomers may be more toxic than others, necessitating careful evaluation during drug development and safety assessments.
- Analytical methods for distinguishing geometric isomers: Various analytical techniques are employed to distinguish between geometric isomers and assess their purity. These methods include chromatography, spectroscopy, and crystallography. Accurate identification and quantification of isomers are essential for evaluating their efficacy and toxicity profiles in pharmaceutical and chemical applications.
- Synthesis and separation of geometric isomers: Developing efficient methods for synthesizing and separating geometric isomers is crucial for studying their individual properties. Techniques such as stereoselective synthesis, isomerization, and advanced separation methods are employed to obtain pure isomers for further analysis of their efficacy and toxicity.
- Structure-activity relationships of geometric isomers: Understanding the structure-activity relationships of geometric isomers is vital for predicting their biological effects. This involves studying how small changes in spatial arrangement can lead to significant differences in efficacy and toxicity. Such knowledge aids in the rational design of drugs and the optimization of chemical compounds for various applications.
02 Toxicity profiles of geometric isomers
Geometric isomers may have distinct toxicity profiles despite having the same molecular formula. This can result in varying side effects, bioaccumulation, and environmental impacts. Assessing the toxicity of individual isomers is essential for safety evaluations and regulatory compliance.Expand Specific Solutions03 Analytical methods for distinguishing geometric isomers
Advanced analytical techniques are employed to differentiate and quantify geometric isomers. These methods include spectroscopic techniques, chromatography, and computational modeling. Accurate identification and characterization of isomers are crucial for assessing their efficacy and toxicity profiles.Expand Specific Solutions04 Isomer-specific drug design and development
Pharmaceutical research focuses on developing isomer-specific drugs to optimize efficacy and minimize toxicity. This approach involves selecting the most beneficial geometric isomer or creating racemic mixtures with desired properties. Understanding structure-activity relationships is key to this process.Expand Specific Solutions05 Regulatory considerations for geometric isomers
Regulatory agencies require comprehensive data on the efficacy and toxicity of individual geometric isomers in drug applications. This includes information on stereochemistry, purity, and potential differences in biological activity. Compliance with these requirements is essential for drug approval and market authorization.Expand Specific Solutions
Key Players in Isomer-Based Pharmaceutical Research
The field of geometric isomers in drug efficacy and toxicity is in a mature stage of development, with a significant market size and established research. Companies like Genentech, Vertex Pharmaceuticals, and Roche are at the forefront, leveraging advanced technologies to explore the impact of molecular structure on drug performance. The competitive landscape is characterized by a mix of large pharmaceutical companies and specialized biotech firms, each contributing to the growing body of knowledge in this area. As the understanding of structure-activity relationships deepens, there is potential for breakthrough innovations in drug design and development, driving continued investment and research in this critical aspect of pharmaceutical science.
Genentech, Inc.
Technical Solution: Genentech, a subsidiary of Roche, has developed a unique approach to exploiting geometric isomerism in biopharmaceuticals. Their technology focuses on engineering protein therapeutics with specific three-dimensional structures, which is critical for biological activity. Genentech's platform includes advanced protein folding prediction algorithms and directed evolution techniques to optimize the spatial arrangement of amino acids in therapeutic proteins[7]. They have also pioneered the use of site-specific conjugation methods to attach small molecules to proteins in precise geometric orientations, enhancing drug efficacy and stability[8]. Genentech's approach has been particularly successful in developing antibody-drug conjugates, where the geometric configuration of the conjugated drug molecule significantly impacts its therapeutic index[9].
Strengths: Expertise in protein engineering and antibody-drug conjugates; advanced computational biology capabilities. Weaknesses: High development and production costs; potential immunogenicity issues with engineered proteins.
Vertex Pharmaceuticals, Inc.
Technical Solution: Vertex Pharmaceuticals has developed a sophisticated approach to leveraging geometric isomerism in drug design, particularly in their cystic fibrosis treatments. Their technology platform focuses on structure-based drug design, which allows them to precisely engineer molecules with specific geometric configurations. This approach has led to the development of CFTR modulators like ivacaftor and tezacaftor, where the spatial arrangement of atoms plays a crucial role in drug efficacy[1]. Vertex's method involves using advanced computational modeling to predict how different geometric isomers will interact with target proteins, allowing them to optimize drug candidates for maximum efficacy and minimal toxicity[2]. They also employ high-throughput screening techniques to rapidly assess the performance of various geometric isomers, accelerating the drug discovery process[3].
Strengths: Highly targeted approach leading to breakthrough treatments; advanced computational modeling capabilities. Weaknesses: High development costs; potential for unexpected side effects due to complex molecular interactions.
Breakthrough Technologies in Isomer Characterization
Erbb/BTK inhibitors
PatentPendingEP4356975A2
Innovation
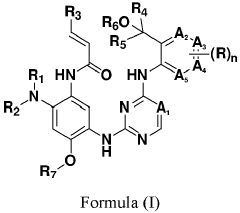
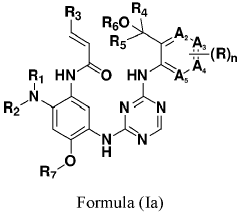
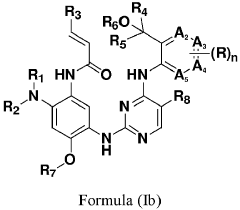
- Development of compounds represented by Formula (I) and its pharmaceutically acceptable salts, esters, hydrates, and stereoisomers, which are used in pharmaceutical compositions to inhibit ErbB family kinases and BTK, particularly targeting mutant forms to enhance therapeutic efficacy.
Compounds and methods for the treatment of malaria
PatentInactiveIN202118043692A
Innovation
- Development of specific compounds, such as those represented by Formula I and listed in Table 1, which offer new structural features and functional groups to target malaria parasites effectively, including those resistant to existing drugs.
Regulatory Framework for Isomeric Drug Approval
The regulatory framework for isomeric drug approval has evolved significantly in recent years, reflecting the growing understanding of the importance of geometric isomers in drug efficacy and toxicity. Regulatory agencies worldwide, including the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have implemented stringent guidelines to ensure the safety and efficacy of isomeric drugs.
These agencies now require pharmaceutical companies to provide comprehensive data on the stereochemistry of their drug candidates. This includes detailed information on the synthesis, purification, and characterization of individual isomers, as well as their biological activities. The FDA's policy on stereoisomeric drugs, established in 1992, mandates that new drug applications must address the stereoisomeric composition of the drug substance and its potential impact on safety and efficacy.
For drugs containing chiral centers, regulatory bodies often demand the development and testing of single enantiomers rather than racemic mixtures. This approach, known as "chiral switching," has led to the development of several successful single-enantiomer drugs, such as esomeprazole (Nexium) and escitalopram (Lexapro). These single-isomer drugs often demonstrate improved efficacy and reduced side effects compared to their racemic counterparts.
The regulatory framework also emphasizes the importance of stability testing for isomeric drugs. Manufacturers must demonstrate that the stereochemical purity of the drug remains consistent throughout its shelf life, as isomerization during storage could potentially alter the drug's therapeutic profile. This requirement has led to advancements in analytical techniques for monitoring stereochemical purity, including high-performance liquid chromatography (HPLC) and circular dichroism spectroscopy.
In addition to pre-market approval requirements, regulatory agencies have established post-market surveillance programs to monitor the long-term safety and efficacy of isomeric drugs. These programs aim to identify any unexpected differences in the performance of geometric isomers that may not have been apparent during clinical trials.
The regulatory landscape for isomeric drugs continues to evolve, with ongoing discussions about the need for more specific guidelines on the development and approval of drugs with multiple chiral centers. As our understanding of the role of geometric isomers in drug efficacy and toxicity deepens, it is likely that regulatory frameworks will continue to adapt, ensuring that the benefits of stereochemical optimization are fully realized in drug development.
These agencies now require pharmaceutical companies to provide comprehensive data on the stereochemistry of their drug candidates. This includes detailed information on the synthesis, purification, and characterization of individual isomers, as well as their biological activities. The FDA's policy on stereoisomeric drugs, established in 1992, mandates that new drug applications must address the stereoisomeric composition of the drug substance and its potential impact on safety and efficacy.
For drugs containing chiral centers, regulatory bodies often demand the development and testing of single enantiomers rather than racemic mixtures. This approach, known as "chiral switching," has led to the development of several successful single-enantiomer drugs, such as esomeprazole (Nexium) and escitalopram (Lexapro). These single-isomer drugs often demonstrate improved efficacy and reduced side effects compared to their racemic counterparts.
The regulatory framework also emphasizes the importance of stability testing for isomeric drugs. Manufacturers must demonstrate that the stereochemical purity of the drug remains consistent throughout its shelf life, as isomerization during storage could potentially alter the drug's therapeutic profile. This requirement has led to advancements in analytical techniques for monitoring stereochemical purity, including high-performance liquid chromatography (HPLC) and circular dichroism spectroscopy.
In addition to pre-market approval requirements, regulatory agencies have established post-market surveillance programs to monitor the long-term safety and efficacy of isomeric drugs. These programs aim to identify any unexpected differences in the performance of geometric isomers that may not have been apparent during clinical trials.
The regulatory landscape for isomeric drugs continues to evolve, with ongoing discussions about the need for more specific guidelines on the development and approval of drugs with multiple chiral centers. As our understanding of the role of geometric isomers in drug efficacy and toxicity deepens, it is likely that regulatory frameworks will continue to adapt, ensuring that the benefits of stereochemical optimization are fully realized in drug development.
Ethical Implications of Isomer-Based Therapies
The ethical implications of isomer-based therapies are multifaceted and require careful consideration as the field of drug development continues to advance. One primary concern is the potential for unintended consequences when manipulating geometric isomers. While certain isomers may exhibit enhanced therapeutic effects, others could lead to unforeseen toxicity or adverse reactions. This raises questions about the responsibility of pharmaceutical companies and regulatory bodies in ensuring comprehensive safety assessments for all isomeric forms of a drug.
Another ethical consideration is the issue of equitable access to isomer-specific treatments. As research progresses, it is likely that some geometric isomers will prove more effective than others for certain conditions. This could lead to the development of more targeted and efficacious drugs, but also potentially increase their cost. The resulting disparity in access to these advanced therapies could exacerbate existing healthcare inequalities, particularly in regions with limited resources or underdeveloped healthcare systems.
The use of geometric isomers in drug development also raises concerns about informed consent and patient autonomy. As the complexity of drug mechanisms increases, it becomes more challenging to fully explain the risks and benefits to patients. This could potentially compromise their ability to make truly informed decisions about their treatment options. Healthcare providers and researchers must strive to balance the promise of improved therapies with the ethical obligation to ensure patient understanding and consent.
Furthermore, the potential for isomer-based therapies to be used for enhancement purposes rather than strictly medical treatments presents additional ethical challenges. As our understanding of the subtle differences between geometric isomers grows, there may be opportunities to develop drugs that enhance cognitive function, physical performance, or other desirable traits. This blurs the line between treatment and enhancement, raising questions about the appropriate limits of medical intervention and the potential for creating new forms of social inequality.
Lastly, the environmental impact of producing and disposing of isomer-specific drugs must be considered. The manufacturing processes for these compounds may require more resources or generate more waste than traditional drug production methods. Additionally, the release of these highly specific chemical compounds into the environment could have unforeseen consequences on ecosystems and wildlife. Balancing the potential benefits of isomer-based therapies with their environmental costs is an ethical imperative that must be addressed as this field continues to evolve.
Another ethical consideration is the issue of equitable access to isomer-specific treatments. As research progresses, it is likely that some geometric isomers will prove more effective than others for certain conditions. This could lead to the development of more targeted and efficacious drugs, but also potentially increase their cost. The resulting disparity in access to these advanced therapies could exacerbate existing healthcare inequalities, particularly in regions with limited resources or underdeveloped healthcare systems.
The use of geometric isomers in drug development also raises concerns about informed consent and patient autonomy. As the complexity of drug mechanisms increases, it becomes more challenging to fully explain the risks and benefits to patients. This could potentially compromise their ability to make truly informed decisions about their treatment options. Healthcare providers and researchers must strive to balance the promise of improved therapies with the ethical obligation to ensure patient understanding and consent.
Furthermore, the potential for isomer-based therapies to be used for enhancement purposes rather than strictly medical treatments presents additional ethical challenges. As our understanding of the subtle differences between geometric isomers grows, there may be opportunities to develop drugs that enhance cognitive function, physical performance, or other desirable traits. This blurs the line between treatment and enhancement, raising questions about the appropriate limits of medical intervention and the potential for creating new forms of social inequality.
Lastly, the environmental impact of producing and disposing of isomer-specific drugs must be considered. The manufacturing processes for these compounds may require more resources or generate more waste than traditional drug production methods. Additionally, the release of these highly specific chemical compounds into the environment could have unforeseen consequences on ecosystems and wildlife. Balancing the potential benefits of isomer-based therapies with their environmental costs is an ethical imperative that must be addressed as this field continues to evolve.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!