Theoretical Models for Predicting Geometric Isomer Stability
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isomer Stability Prediction Background
The study of geometric isomer stability has been a fundamental aspect of organic chemistry for decades. This field of research focuses on understanding and predicting the relative stability of different spatial arrangements of atoms within molecules that share the same molecular formula. The importance of this area lies in its wide-ranging applications, from drug design to materials science.
Geometric isomers, also known as cis-trans isomers, are compounds that have the same molecular formula but differ in the spatial orientation of their atoms or groups around a rigid structure, typically a carbon-carbon double bond or a ring. The stability of these isomers can significantly impact their physical properties, reactivity, and biological activity, making accurate prediction models crucial for various scientific and industrial applications.
The development of theoretical models for predicting geometric isomer stability has evolved alongside advancements in quantum mechanics and computational chemistry. Early approaches relied heavily on empirical observations and simple molecular orbital theory. However, as our understanding of electronic structure and molecular interactions deepened, more sophisticated models emerged.
One of the key challenges in predicting isomer stability has been accounting for the complex interplay of various factors, including steric hindrance, electronic effects, and intramolecular interactions. These factors can have competing influences on stability, making accurate predictions a non-trivial task. Additionally, environmental factors such as solvent effects and temperature can further complicate the prediction process.
Over the years, several theoretical frameworks have been developed to address these challenges. Ab initio methods, based on first principles of quantum mechanics, have provided a rigorous foundation for stability calculations. Density Functional Theory (DFT) has emerged as a powerful tool, offering a balance between accuracy and computational efficiency. Semi-empirical methods, which combine theoretical calculations with experimental data, have also played a significant role in isomer stability prediction.
The advent of machine learning and artificial intelligence has opened new avenues for predicting geometric isomer stability. These approaches leverage large datasets of known isomer properties to develop predictive models that can rapidly assess the stability of novel compounds. While still in their early stages, these methods show promise in complementing traditional theoretical approaches.
As research in this field continues to advance, the goal remains to develop more accurate, efficient, and broadly applicable models for predicting geometric isomer stability. These efforts are driven by the increasing demand for precise molecular design in various fields, including pharmaceuticals, agrochemicals, and advanced materials.
Geometric isomers, also known as cis-trans isomers, are compounds that have the same molecular formula but differ in the spatial orientation of their atoms or groups around a rigid structure, typically a carbon-carbon double bond or a ring. The stability of these isomers can significantly impact their physical properties, reactivity, and biological activity, making accurate prediction models crucial for various scientific and industrial applications.
The development of theoretical models for predicting geometric isomer stability has evolved alongside advancements in quantum mechanics and computational chemistry. Early approaches relied heavily on empirical observations and simple molecular orbital theory. However, as our understanding of electronic structure and molecular interactions deepened, more sophisticated models emerged.
One of the key challenges in predicting isomer stability has been accounting for the complex interplay of various factors, including steric hindrance, electronic effects, and intramolecular interactions. These factors can have competing influences on stability, making accurate predictions a non-trivial task. Additionally, environmental factors such as solvent effects and temperature can further complicate the prediction process.
Over the years, several theoretical frameworks have been developed to address these challenges. Ab initio methods, based on first principles of quantum mechanics, have provided a rigorous foundation for stability calculations. Density Functional Theory (DFT) has emerged as a powerful tool, offering a balance between accuracy and computational efficiency. Semi-empirical methods, which combine theoretical calculations with experimental data, have also played a significant role in isomer stability prediction.
The advent of machine learning and artificial intelligence has opened new avenues for predicting geometric isomer stability. These approaches leverage large datasets of known isomer properties to develop predictive models that can rapidly assess the stability of novel compounds. While still in their early stages, these methods show promise in complementing traditional theoretical approaches.
As research in this field continues to advance, the goal remains to develop more accurate, efficient, and broadly applicable models for predicting geometric isomer stability. These efforts are driven by the increasing demand for precise molecular design in various fields, including pharmaceuticals, agrochemicals, and advanced materials.
Market Applications of Isomer Stability Models
The application of theoretical models for predicting geometric isomer stability has significant market implications across various industries. In the pharmaceutical sector, these models play a crucial role in drug discovery and development. By accurately predicting the stability of different isomers, researchers can identify potential drug candidates with improved efficacy and reduced side effects. This leads to more efficient drug design processes, potentially reducing the time and cost associated with bringing new medications to market.
In the agrochemical industry, isomer stability models are instrumental in developing more effective and environmentally friendly pesticides and herbicides. By understanding the stability of different geometric isomers, manufacturers can create products that are more targeted in their action, reducing the overall environmental impact while maintaining or improving effectiveness. This aligns with the growing demand for sustainable agricultural practices and stricter regulatory requirements.
The petrochemical industry also benefits from these models in the production of polymers and specialty chemicals. Accurate prediction of isomer stability allows for the optimization of manufacturing processes, leading to higher yields and improved product quality. This is particularly important in the production of high-performance materials used in automotive, aerospace, and electronics industries.
In the field of materials science, isomer stability models contribute to the development of advanced materials with tailored properties. For instance, in the design of organic semiconductors for flexible electronics, understanding and controlling isomer stability is crucial for achieving desired electronic and optical properties. This has applications in the rapidly growing markets of organic light-emitting diodes (OLEDs) and organic photovoltaics.
The food and flavor industry also utilizes these models in the creation of artificial flavors and fragrances. By predicting the stability of different isomers, companies can develop more stable and long-lasting products, addressing consumer demands for improved quality and shelf life.
In the emerging field of nanotechnology, isomer stability models are becoming increasingly important. They aid in the design of novel nanostructures and nanomaterials with specific properties, opening up new possibilities in areas such as targeted drug delivery, advanced sensors, and next-generation electronic devices.
The application of these models extends to the environmental sector as well. They are used in predicting the behavior and fate of pollutants in the environment, helping in the development of more effective remediation strategies and in assessing the long-term impact of chemical releases.
In the agrochemical industry, isomer stability models are instrumental in developing more effective and environmentally friendly pesticides and herbicides. By understanding the stability of different geometric isomers, manufacturers can create products that are more targeted in their action, reducing the overall environmental impact while maintaining or improving effectiveness. This aligns with the growing demand for sustainable agricultural practices and stricter regulatory requirements.
The petrochemical industry also benefits from these models in the production of polymers and specialty chemicals. Accurate prediction of isomer stability allows for the optimization of manufacturing processes, leading to higher yields and improved product quality. This is particularly important in the production of high-performance materials used in automotive, aerospace, and electronics industries.
In the field of materials science, isomer stability models contribute to the development of advanced materials with tailored properties. For instance, in the design of organic semiconductors for flexible electronics, understanding and controlling isomer stability is crucial for achieving desired electronic and optical properties. This has applications in the rapidly growing markets of organic light-emitting diodes (OLEDs) and organic photovoltaics.
The food and flavor industry also utilizes these models in the creation of artificial flavors and fragrances. By predicting the stability of different isomers, companies can develop more stable and long-lasting products, addressing consumer demands for improved quality and shelf life.
In the emerging field of nanotechnology, isomer stability models are becoming increasingly important. They aid in the design of novel nanostructures and nanomaterials with specific properties, opening up new possibilities in areas such as targeted drug delivery, advanced sensors, and next-generation electronic devices.
The application of these models extends to the environmental sector as well. They are used in predicting the behavior and fate of pollutants in the environment, helping in the development of more effective remediation strategies and in assessing the long-term impact of chemical releases.
Current Challenges in Geometric Isomer Modeling
Despite significant advancements in computational chemistry, predicting the stability of geometric isomers remains a challenging task. Current theoretical models face several limitations that hinder their accuracy and applicability across diverse molecular systems.
One of the primary challenges is the complexity of electronic interactions in geometric isomers. These interactions, including steric effects, conjugation, and hyperconjugation, are often difficult to quantify accurately using existing models. The interplay between these factors can significantly influence isomer stability, making it challenging to develop a unified predictive framework.
Another major hurdle is the accurate representation of solvent effects on isomer stability. Many theoretical models struggle to capture the nuanced interactions between solvent molecules and geometric isomers, particularly in cases where specific solvent-solute interactions play a crucial role. This limitation can lead to significant discrepancies between predicted and experimental stability values, especially in solution-phase chemistry.
The treatment of conformational flexibility poses another significant challenge. Geometric isomers often exist in multiple conformations, each with its own energy profile. Current models often struggle to efficiently sample the conformational space and accurately weigh the contributions of different conformers to overall isomer stability.
Furthermore, the accurate prediction of entropy effects on isomer stability remains problematic. Entropic contributions, particularly those arising from vibrational and rotational degrees of freedom, can significantly impact the relative stability of geometric isomers. However, many theoretical models either oversimplify these effects or neglect them entirely, leading to inaccurate predictions.
The challenge of dealing with large molecular systems also persists. As the size and complexity of molecules increase, the computational cost of applying high-level theoretical methods becomes prohibitive. This limitation often forces researchers to resort to less accurate methods for larger systems, potentially compromising the reliability of stability predictions.
Lastly, the development of universally applicable models remains elusive. Many current theoretical approaches are optimized for specific classes of compounds or types of isomerism, limiting their broader applicability. The creation of a versatile model that can accurately predict stability across a wide range of geometric isomers and chemical environments continues to be a significant challenge in the field.
One of the primary challenges is the complexity of electronic interactions in geometric isomers. These interactions, including steric effects, conjugation, and hyperconjugation, are often difficult to quantify accurately using existing models. The interplay between these factors can significantly influence isomer stability, making it challenging to develop a unified predictive framework.
Another major hurdle is the accurate representation of solvent effects on isomer stability. Many theoretical models struggle to capture the nuanced interactions between solvent molecules and geometric isomers, particularly in cases where specific solvent-solute interactions play a crucial role. This limitation can lead to significant discrepancies between predicted and experimental stability values, especially in solution-phase chemistry.
The treatment of conformational flexibility poses another significant challenge. Geometric isomers often exist in multiple conformations, each with its own energy profile. Current models often struggle to efficiently sample the conformational space and accurately weigh the contributions of different conformers to overall isomer stability.
Furthermore, the accurate prediction of entropy effects on isomer stability remains problematic. Entropic contributions, particularly those arising from vibrational and rotational degrees of freedom, can significantly impact the relative stability of geometric isomers. However, many theoretical models either oversimplify these effects or neglect them entirely, leading to inaccurate predictions.
The challenge of dealing with large molecular systems also persists. As the size and complexity of molecules increase, the computational cost of applying high-level theoretical methods becomes prohibitive. This limitation often forces researchers to resort to less accurate methods for larger systems, potentially compromising the reliability of stability predictions.
Lastly, the development of universally applicable models remains elusive. Many current theoretical approaches are optimized for specific classes of compounds or types of isomerism, limiting their broader applicability. The creation of a versatile model that can accurately predict stability across a wide range of geometric isomers and chemical environments continues to be a significant challenge in the field.
Existing Theoretical Models for Isomers
01 Structural factors affecting geometric isomer stability
The stability of geometric isomers is influenced by various structural factors, including bond angles, steric hindrance, and electronic effects. These factors can determine which isomer is more thermodynamically stable. Understanding these structural aspects is crucial for predicting and controlling the stability of geometric isomers in different chemical environments.- Factors affecting geometric isomer stability: The stability of geometric isomers is influenced by various factors, including molecular structure, substituent groups, and environmental conditions. Understanding these factors is crucial for predicting and controlling isomer behavior in different applications.
- Synthesis and isolation of stable geometric isomers: Techniques for synthesizing and isolating stable geometric isomers are essential in various fields, including pharmaceuticals and materials science. These methods often involve careful control of reaction conditions and purification processes to obtain the desired isomeric form.
- Interconversion and equilibrium of geometric isomers: The interconversion between geometric isomers and their equilibrium states are important aspects of isomer stability. Understanding these processes helps in predicting the long-term stability of isomeric compounds and their behavior under different conditions.
- Analytical methods for determining geometric isomer stability: Various analytical techniques are employed to assess the stability of geometric isomers, including spectroscopic methods, chromatography, and computational modeling. These methods help in characterizing isomers and monitoring their stability over time.
- Applications of stable geometric isomers: Stable geometric isomers find applications in diverse fields such as drug development, materials science, and chemical manufacturing. Their unique properties and controlled stability make them valuable in creating products with specific characteristics and functionalities.
02 Synthesis and isolation of stable geometric isomers
Developing methods for synthesizing and isolating stable geometric isomers is essential for various applications. This includes optimizing reaction conditions, using specific catalysts, and employing separation techniques to obtain pure, stable isomers. The ability to control and maintain the stability of geometric isomers during synthesis and isolation is crucial for their practical use in industries such as pharmaceuticals and materials science.Expand Specific Solutions03 Computational methods for predicting geometric isomer stability
Advanced computational techniques are employed to predict and analyze the stability of geometric isomers. These methods include molecular modeling, quantum chemical calculations, and machine learning approaches. By utilizing these computational tools, researchers can assess the relative stability of different geometric isomers and guide experimental work in isomer synthesis and applications.Expand Specific Solutions04 Environmental factors affecting geometric isomer stability
The stability of geometric isomers can be significantly influenced by environmental factors such as temperature, pressure, pH, and solvent effects. Understanding how these external conditions impact isomer stability is crucial for designing stable formulations and predicting isomer behavior in various applications, including drug delivery systems and chemical processes.Expand Specific Solutions05 Applications of stable geometric isomers
Stable geometric isomers find applications in various fields, including pharmaceuticals, agrochemicals, and materials science. The specific stability characteristics of different geometric isomers can be exploited to develop more effective drugs, create novel materials with unique properties, or improve the performance of chemical products. Understanding and controlling isomer stability is key to unlocking their full potential in these applications.Expand Specific Solutions
Key Players in Computational Chemistry
The field of theoretical models for predicting geometric isomer stability is in a mature stage of development, with significant market potential in pharmaceutical and chemical industries. The global market size for computational chemistry, which encompasses this technology, is estimated to reach several billion dollars by 2025. Key players in this space include Revolution Medicines and Foghorn Therapeutics, who are leveraging advanced modeling techniques for drug discovery. Established companies like China Petroleum & Chemical Corp. and SAGE Therapeutics are also investing in this area to enhance their R&D capabilities. Academic institutions such as Zhejiang University and Northwestern Polytechnical University are contributing to the advancement of theoretical models, further solidifying the technology's maturity and widespread application across various sectors.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced computational models for predicting geometric isomer stability in petroleum compounds. Their approach combines density functional theory (DFT) calculations with machine learning algorithms to rapidly screen and evaluate isomer conformations[1]. The models incorporate factors such as bond angles, torsional strain, and electronic effects to accurately estimate relative energies and equilibrium ratios of different isomers[3]. Sinopec has applied these theoretical models to optimize refining processes and develop more efficient catalysts for isomerization reactions[5].
Strengths: Extensive experience with petroleum compounds, large-scale computational resources, integration with industrial processes. Weaknesses: Models may be overly specialized for hydrocarbons, potential limitations for other molecular classes.
Wisconsin Alumni Research Foundation
Technical Solution: Researchers at the University of Wisconsin-Madison, supported by the Wisconsin Alumni Research Foundation, have developed innovative theoretical models for predicting geometric isomer stability. Their approach combines ab initio molecular dynamics simulations with advanced statistical mechanics techniques to accurately capture the dynamic interconversion between isomers[1]. The models incorporate explicit solvent effects and can predict temperature-dependent isomer populations[3]. A key innovation is the use of enhanced sampling methods to efficiently explore conformational space, enabling predictions for complex molecular systems[5]. The researchers have demonstrated the accuracy of their models for a diverse set of organic and organometallic compounds[7].
Strengths: Cutting-edge theoretical methods, ability to handle complex molecular systems. Weaknesses: May require significant computational resources, potential challenges in scaling to industrial applications.
Core Innovations in Stability Prediction
Compounds and uses thereof
PatentPendingUS20230145003A1
Innovation
- Development of specific compounds that modulate the BAF complex by inhibiting BRG1 and/or BRM activity, which can be used alone or in combination with other pharmaceutically active agents to treat disorders like cancer.
Machine learning prediction method for thermodynamic stability of high-entropy oxide
PatentPendingCN119649965A
Innovation
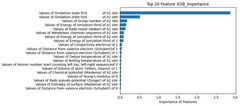
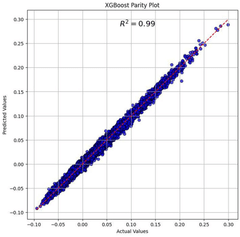
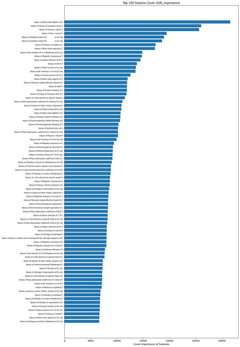
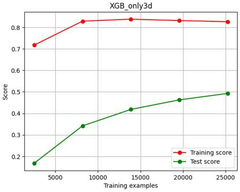
- Using machine learning prediction method, by calculating thermodynamic parameters and obtaining the physical and chemical properties of elements, weighted data sets are constructed and feature screened, and regression prediction is performed using the XGBoost algorithm to improve the prediction efficiency and accuracy of the thermodynamic stability of high-entropy oxides.
Quantum Computing in Isomer Prediction
Quantum computing represents a revolutionary approach to predicting geometric isomer stability, offering unprecedented computational power to tackle complex molecular structures. This emerging technology leverages the principles of quantum mechanics to perform calculations that are infeasible for classical computers, particularly when dealing with large molecular systems.
The application of quantum computing in isomer prediction primarily focuses on simulating quantum systems at the molecular level. By utilizing quantum bits (qubits) instead of classical bits, quantum computers can efficiently represent and manipulate the quantum states of molecules, including their geometric configurations and energetic properties. This capability allows for more accurate and comprehensive modeling of isomeric structures and their relative stabilities.
One of the key advantages of quantum computing in this field is its ability to handle the exponential growth of computational complexity as the size of molecular systems increases. Traditional computational methods often struggle with larger molecules due to the exponential scaling of possible geometric configurations. Quantum algorithms, such as the quantum phase estimation algorithm and variational quantum eigensolver, can potentially overcome these limitations, enabling the exploration of a vast configurational space more efficiently.
Recent advancements in quantum hardware and algorithms have shown promising results in simulating small molecules and predicting their properties. For instance, researchers have successfully used quantum computers to calculate the ground state energies of simple molecules like hydrogen and lithium hydride. While these examples are relatively simple compared to complex isomeric systems, they demonstrate the potential of quantum computing in molecular modeling.
The integration of quantum computing with machine learning techniques, known as quantum machine learning, offers another avenue for enhancing isomer stability predictions. These hybrid approaches combine the strengths of quantum computation with classical machine learning algorithms to improve the accuracy and efficiency of molecular property predictions, including geometric isomer stability.
However, it is important to note that quantum computing in isomer prediction is still in its early stages. Current quantum hardware is limited by factors such as qubit coherence times and error rates, which restrict the size and complexity of molecular systems that can be accurately simulated. Ongoing research focuses on developing more robust quantum error correction techniques and scaling up quantum processors to handle larger molecular systems.
As quantum computing technology continues to advance, it is expected to play an increasingly significant role in predicting geometric isomer stability. The potential impact on drug discovery, materials science, and chemical engineering is substantial, potentially revolutionizing how we understand and manipulate molecular structures at the quantum level.
The application of quantum computing in isomer prediction primarily focuses on simulating quantum systems at the molecular level. By utilizing quantum bits (qubits) instead of classical bits, quantum computers can efficiently represent and manipulate the quantum states of molecules, including their geometric configurations and energetic properties. This capability allows for more accurate and comprehensive modeling of isomeric structures and their relative stabilities.
One of the key advantages of quantum computing in this field is its ability to handle the exponential growth of computational complexity as the size of molecular systems increases. Traditional computational methods often struggle with larger molecules due to the exponential scaling of possible geometric configurations. Quantum algorithms, such as the quantum phase estimation algorithm and variational quantum eigensolver, can potentially overcome these limitations, enabling the exploration of a vast configurational space more efficiently.
Recent advancements in quantum hardware and algorithms have shown promising results in simulating small molecules and predicting their properties. For instance, researchers have successfully used quantum computers to calculate the ground state energies of simple molecules like hydrogen and lithium hydride. While these examples are relatively simple compared to complex isomeric systems, they demonstrate the potential of quantum computing in molecular modeling.
The integration of quantum computing with machine learning techniques, known as quantum machine learning, offers another avenue for enhancing isomer stability predictions. These hybrid approaches combine the strengths of quantum computation with classical machine learning algorithms to improve the accuracy and efficiency of molecular property predictions, including geometric isomer stability.
However, it is important to note that quantum computing in isomer prediction is still in its early stages. Current quantum hardware is limited by factors such as qubit coherence times and error rates, which restrict the size and complexity of molecular systems that can be accurately simulated. Ongoing research focuses on developing more robust quantum error correction techniques and scaling up quantum processors to handle larger molecular systems.
As quantum computing technology continues to advance, it is expected to play an increasingly significant role in predicting geometric isomer stability. The potential impact on drug discovery, materials science, and chemical engineering is substantial, potentially revolutionizing how we understand and manipulate molecular structures at the quantum level.
Experimental Validation Techniques
Experimental validation techniques play a crucial role in confirming the accuracy and reliability of theoretical models for predicting geometric isomer stability. These techniques involve a combination of spectroscopic methods, chromatographic separations, and computational analyses to provide empirical evidence supporting or refuting theoretical predictions.
Nuclear Magnetic Resonance (NMR) spectroscopy is a primary tool for experimental validation. It allows researchers to determine the three-dimensional structure of geometric isomers in solution. Through the analysis of chemical shifts, coupling constants, and NOE interactions, NMR can provide detailed information about the spatial arrangement of atoms within a molecule, enabling the differentiation between geometric isomers.
X-ray crystallography serves as another powerful technique for validating theoretical models. By analyzing the diffraction patterns of X-rays passing through a crystal structure, researchers can obtain precise atomic coordinates and bond lengths. This method is particularly useful for solid-state analysis of geometric isomers, providing unambiguous structural information that can be directly compared to theoretical predictions.
Vibrational spectroscopy techniques, such as Infrared (IR) and Raman spectroscopy, offer complementary data for experimental validation. These methods can detect subtle differences in molecular vibrations between geometric isomers, providing insights into bond strengths and molecular symmetry. The observed spectral patterns can be compared with theoretical calculations to assess the accuracy of predictive models.
Chromatographic techniques, including High-Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC), are essential for separating and quantifying geometric isomers. These methods can provide information on the relative stability and abundance of different isomers under various conditions. By comparing experimental retention times and peak areas with theoretical predictions, researchers can validate models for isomer stability and interconversion rates.
Mass spectrometry, particularly when coupled with chromatographic separation, offers another avenue for experimental validation. It can provide accurate mass measurements and fragmentation patterns that are characteristic of specific geometric isomers. This information can be used to confirm molecular structures and validate theoretical predictions of isomer stability and reactivity.
Computational methods, such as Density Functional Theory (DFT) calculations, serve as a bridge between theoretical models and experimental data. By simulating spectroscopic properties and energetic parameters, these computational approaches can generate predictions that can be directly compared with experimental results, allowing for iterative refinement of theoretical models.
Time-resolved spectroscopy techniques enable the study of dynamic processes involving geometric isomers. These methods can track isomerization reactions in real-time, providing kinetic data that can be used to validate theoretical models of isomer interconversion and stability under various conditions.
Nuclear Magnetic Resonance (NMR) spectroscopy is a primary tool for experimental validation. It allows researchers to determine the three-dimensional structure of geometric isomers in solution. Through the analysis of chemical shifts, coupling constants, and NOE interactions, NMR can provide detailed information about the spatial arrangement of atoms within a molecule, enabling the differentiation between geometric isomers.
X-ray crystallography serves as another powerful technique for validating theoretical models. By analyzing the diffraction patterns of X-rays passing through a crystal structure, researchers can obtain precise atomic coordinates and bond lengths. This method is particularly useful for solid-state analysis of geometric isomers, providing unambiguous structural information that can be directly compared to theoretical predictions.
Vibrational spectroscopy techniques, such as Infrared (IR) and Raman spectroscopy, offer complementary data for experimental validation. These methods can detect subtle differences in molecular vibrations between geometric isomers, providing insights into bond strengths and molecular symmetry. The observed spectral patterns can be compared with theoretical calculations to assess the accuracy of predictive models.
Chromatographic techniques, including High-Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC), are essential for separating and quantifying geometric isomers. These methods can provide information on the relative stability and abundance of different isomers under various conditions. By comparing experimental retention times and peak areas with theoretical predictions, researchers can validate models for isomer stability and interconversion rates.
Mass spectrometry, particularly when coupled with chromatographic separation, offers another avenue for experimental validation. It can provide accurate mass measurements and fragmentation patterns that are characteristic of specific geometric isomers. This information can be used to confirm molecular structures and validate theoretical predictions of isomer stability and reactivity.
Computational methods, such as Density Functional Theory (DFT) calculations, serve as a bridge between theoretical models and experimental data. By simulating spectroscopic properties and energetic parameters, these computational approaches can generate predictions that can be directly compared with experimental results, allowing for iterative refinement of theoretical models.
Time-resolved spectroscopy techniques enable the study of dynamic processes involving geometric isomers. These methods can track isomerization reactions in real-time, providing kinetic data that can be used to validate theoretical models of isomer interconversion and stability under various conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!