Advanced Applications of Gel Electrophoresis in Medicine
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Gel Electrophoresis Evolution and Objectives
Gel electrophoresis has evolved significantly since its inception in the 1930s, becoming a cornerstone technique in molecular biology and medical diagnostics. Initially developed for protein separation, the method has undergone substantial refinements to address the growing demands of medical research and clinical applications.
The evolution of gel electrophoresis in medicine can be traced through several key milestones. In the 1950s, the introduction of paper electrophoresis marked a significant advancement, allowing for the separation of serum proteins. This technique quickly found applications in diagnosing various blood disorders. The 1960s saw the development of polyacrylamide gel electrophoresis (PAGE), which offered superior resolution for protein separation and became instrumental in studying complex protein mixtures.
A major breakthrough came in the 1970s with the advent of agarose gel electrophoresis for DNA separation. This technique revolutionized genetic research and laid the foundation for modern molecular diagnostics. The subsequent development of pulsed-field gel electrophoresis (PFGE) in the 1980s enabled the separation of large DNA molecules, crucial for genome mapping and the study of bacterial pathogens.
Recent advancements have focused on improving sensitivity, resolution, and automation. The introduction of capillary electrophoresis in the 1990s allowed for high-throughput analysis with minimal sample requirements. Two-dimensional gel electrophoresis has become a powerful tool in proteomics, enabling the simultaneous analysis of thousands of proteins.
The objectives of gel electrophoresis in medicine have expanded alongside these technological advancements. Initially aimed at basic protein and DNA separation, the technique now serves a multitude of purposes in clinical diagnostics, forensic medicine, and biomedical research. Current objectives include the development of point-of-care diagnostic devices, the integration of gel electrophoresis with other analytical techniques for comprehensive biomolecule analysis, and the application of microfluidic technologies for miniaturized, high-performance separations.
Looking forward, the goals for gel electrophoresis in medicine are centered on enhancing its capabilities for personalized medicine. This includes developing ultra-sensitive methods for detecting biomarkers in complex biological samples, improving the analysis of circulating tumor DNA for early cancer detection, and refining techniques for rapid pathogen identification in infectious diseases. Additionally, there is a push towards creating more sustainable and environmentally friendly electrophoresis methods, aligning with the broader goals of green chemistry in laboratory practices.
The evolution of gel electrophoresis in medicine can be traced through several key milestones. In the 1950s, the introduction of paper electrophoresis marked a significant advancement, allowing for the separation of serum proteins. This technique quickly found applications in diagnosing various blood disorders. The 1960s saw the development of polyacrylamide gel electrophoresis (PAGE), which offered superior resolution for protein separation and became instrumental in studying complex protein mixtures.
A major breakthrough came in the 1970s with the advent of agarose gel electrophoresis for DNA separation. This technique revolutionized genetic research and laid the foundation for modern molecular diagnostics. The subsequent development of pulsed-field gel electrophoresis (PFGE) in the 1980s enabled the separation of large DNA molecules, crucial for genome mapping and the study of bacterial pathogens.
Recent advancements have focused on improving sensitivity, resolution, and automation. The introduction of capillary electrophoresis in the 1990s allowed for high-throughput analysis with minimal sample requirements. Two-dimensional gel electrophoresis has become a powerful tool in proteomics, enabling the simultaneous analysis of thousands of proteins.
The objectives of gel electrophoresis in medicine have expanded alongside these technological advancements. Initially aimed at basic protein and DNA separation, the technique now serves a multitude of purposes in clinical diagnostics, forensic medicine, and biomedical research. Current objectives include the development of point-of-care diagnostic devices, the integration of gel electrophoresis with other analytical techniques for comprehensive biomolecule analysis, and the application of microfluidic technologies for miniaturized, high-performance separations.
Looking forward, the goals for gel electrophoresis in medicine are centered on enhancing its capabilities for personalized medicine. This includes developing ultra-sensitive methods for detecting biomarkers in complex biological samples, improving the analysis of circulating tumor DNA for early cancer detection, and refining techniques for rapid pathogen identification in infectious diseases. Additionally, there is a push towards creating more sustainable and environmentally friendly electrophoresis methods, aligning with the broader goals of green chemistry in laboratory practices.
Medical Demand for Gel Electrophoresis
Gel electrophoresis has become an indispensable tool in modern medicine, with its applications extending far beyond basic research laboratories. The medical demand for this technique has grown exponentially in recent years, driven by advancements in personalized medicine, genetic testing, and molecular diagnostics.
In clinical settings, gel electrophoresis plays a crucial role in diagnosing various genetic disorders. It enables the separation and analysis of DNA fragments, allowing healthcare professionals to identify mutations or abnormalities associated with specific diseases. This capability has revolutionized prenatal testing, offering expectant parents valuable insights into potential genetic risks for their unborn children.
The technique's versatility extends to cancer diagnostics and treatment monitoring. Oncologists rely on gel electrophoresis to detect cancer-specific biomarkers in patient samples, facilitating early detection and precise staging of tumors. Furthermore, it aids in monitoring treatment efficacy by tracking changes in tumor-specific DNA or protein profiles over time, enabling personalized treatment adjustments.
In the realm of infectious diseases, gel electrophoresis has become an essential tool for identifying pathogens and determining antibiotic resistance. It allows for rapid and accurate identification of bacterial and viral strains, crucial in outbreak situations where timely diagnosis can prevent widespread transmission. Additionally, the technique helps in tracking the evolution of antibiotic-resistant bacteria, informing treatment strategies and public health policies.
The pharmaceutical industry heavily relies on gel electrophoresis for drug development and quality control. It is used to analyze the purity and integrity of therapeutic proteins, ensuring the safety and efficacy of biopharmaceuticals. This application is particularly vital in the production of monoclonal antibodies and other biologics, which are increasingly important in treating various diseases.
Forensic medicine has also benefited significantly from gel electrophoresis. The technique is fundamental in DNA profiling for criminal investigations and paternity testing. Its ability to separate and visualize DNA fragments with high resolution makes it an invaluable tool in forensic laboratories worldwide.
As precision medicine continues to evolve, the demand for more sophisticated and high-throughput gel electrophoresis techniques is growing. There is an increasing need for automated systems that can process large numbers of samples quickly and accurately, particularly in large-scale genetic screening programs and epidemiological studies.
The integration of gel electrophoresis with other analytical techniques, such as mass spectrometry and next-generation sequencing, is expanding its capabilities and applications in medicine. This synergy is opening new avenues for biomarker discovery and comprehensive molecular profiling, further driving the demand for advanced gel electrophoresis systems in medical research and clinical practice.
In clinical settings, gel electrophoresis plays a crucial role in diagnosing various genetic disorders. It enables the separation and analysis of DNA fragments, allowing healthcare professionals to identify mutations or abnormalities associated with specific diseases. This capability has revolutionized prenatal testing, offering expectant parents valuable insights into potential genetic risks for their unborn children.
The technique's versatility extends to cancer diagnostics and treatment monitoring. Oncologists rely on gel electrophoresis to detect cancer-specific biomarkers in patient samples, facilitating early detection and precise staging of tumors. Furthermore, it aids in monitoring treatment efficacy by tracking changes in tumor-specific DNA or protein profiles over time, enabling personalized treatment adjustments.
In the realm of infectious diseases, gel electrophoresis has become an essential tool for identifying pathogens and determining antibiotic resistance. It allows for rapid and accurate identification of bacterial and viral strains, crucial in outbreak situations where timely diagnosis can prevent widespread transmission. Additionally, the technique helps in tracking the evolution of antibiotic-resistant bacteria, informing treatment strategies and public health policies.
The pharmaceutical industry heavily relies on gel electrophoresis for drug development and quality control. It is used to analyze the purity and integrity of therapeutic proteins, ensuring the safety and efficacy of biopharmaceuticals. This application is particularly vital in the production of monoclonal antibodies and other biologics, which are increasingly important in treating various diseases.
Forensic medicine has also benefited significantly from gel electrophoresis. The technique is fundamental in DNA profiling for criminal investigations and paternity testing. Its ability to separate and visualize DNA fragments with high resolution makes it an invaluable tool in forensic laboratories worldwide.
As precision medicine continues to evolve, the demand for more sophisticated and high-throughput gel electrophoresis techniques is growing. There is an increasing need for automated systems that can process large numbers of samples quickly and accurately, particularly in large-scale genetic screening programs and epidemiological studies.
The integration of gel electrophoresis with other analytical techniques, such as mass spectrometry and next-generation sequencing, is expanding its capabilities and applications in medicine. This synergy is opening new avenues for biomarker discovery and comprehensive molecular profiling, further driving the demand for advanced gel electrophoresis systems in medical research and clinical practice.
Current Challenges in Gel Electrophoresis
Despite the widespread use and established protocols of gel electrophoresis in medicine, several challenges persist that limit its advanced applications. One of the primary issues is the resolution and sensitivity of the technique, particularly when dealing with complex biological samples. As medical research delves into more intricate molecular interactions and rare biomarkers, the need for higher resolution separation becomes crucial.
The time-consuming nature of gel electrophoresis poses another significant challenge, especially in clinical settings where rapid diagnostics are essential. The lengthy process of sample preparation, gel casting, running the electrophoresis, and subsequent analysis can delay critical medical decisions. This time factor becomes particularly problematic when dealing with time-sensitive conditions or in emergency medical situations.
Reproducibility and standardization across different laboratories and clinical settings remain ongoing concerns. Variations in gel composition, running conditions, and even environmental factors can lead to inconsistencies in results, potentially affecting diagnostic accuracy and research outcomes. This lack of standardization hampers the widespread adoption of gel electrophoresis-based techniques in routine clinical practice.
The limited automation in gel electrophoresis procedures presents another hurdle. While some steps have been automated, the overall process still requires significant manual intervention, increasing the potential for human error and reducing throughput. This limitation becomes more pronounced in high-volume clinical laboratories or large-scale research projects.
Quantification and data analysis of gel electrophoresis results also pose challenges. Traditional methods of band intensity measurement can be subjective and lack the precision required for advanced medical applications. The need for more sophisticated, automated image analysis tools is evident, especially for applications requiring precise quantification of multiple analytes.
Environmental and safety concerns associated with some of the reagents used in gel electrophoresis, particularly in DNA analysis, present additional challenges. The use of potentially harmful staining agents and UV light for visualization raises safety issues and limits the widespread adoption of these techniques in point-of-care settings.
Lastly, the integration of gel electrophoresis with other analytical techniques remains a challenge. While combining electrophoresis with mass spectrometry or other advanced analytical methods can provide powerful insights, the practical implementation of these integrated approaches in clinical settings is still limited by technical and logistical constraints.
The time-consuming nature of gel electrophoresis poses another significant challenge, especially in clinical settings where rapid diagnostics are essential. The lengthy process of sample preparation, gel casting, running the electrophoresis, and subsequent analysis can delay critical medical decisions. This time factor becomes particularly problematic when dealing with time-sensitive conditions or in emergency medical situations.
Reproducibility and standardization across different laboratories and clinical settings remain ongoing concerns. Variations in gel composition, running conditions, and even environmental factors can lead to inconsistencies in results, potentially affecting diagnostic accuracy and research outcomes. This lack of standardization hampers the widespread adoption of gel electrophoresis-based techniques in routine clinical practice.
The limited automation in gel electrophoresis procedures presents another hurdle. While some steps have been automated, the overall process still requires significant manual intervention, increasing the potential for human error and reducing throughput. This limitation becomes more pronounced in high-volume clinical laboratories or large-scale research projects.
Quantification and data analysis of gel electrophoresis results also pose challenges. Traditional methods of band intensity measurement can be subjective and lack the precision required for advanced medical applications. The need for more sophisticated, automated image analysis tools is evident, especially for applications requiring precise quantification of multiple analytes.
Environmental and safety concerns associated with some of the reagents used in gel electrophoresis, particularly in DNA analysis, present additional challenges. The use of potentially harmful staining agents and UV light for visualization raises safety issues and limits the widespread adoption of these techniques in point-of-care settings.
Lastly, the integration of gel electrophoresis with other analytical techniques remains a challenge. While combining electrophoresis with mass spectrometry or other advanced analytical methods can provide powerful insights, the practical implementation of these integrated approaches in clinical settings is still limited by technical and logistical constraints.
Cutting-edge Gel Electrophoresis Techniques
01 Gel composition and preparation
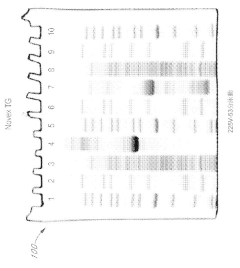
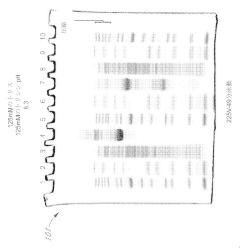
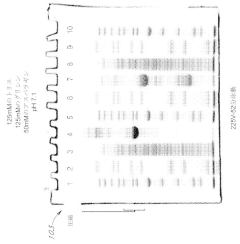
Various gel compositions and preparation methods are used in gel electrophoresis. These include specific formulations of agarose, polyacrylamide, and other polymers to create gels with desired properties for different applications. The composition and preparation of the gel can significantly affect the separation and resolution of molecules during electrophoresis.- Gel composition and preparation: Various gel compositions and preparation methods are used in gel electrophoresis. These include specific formulations of agarose, polyacrylamide, and other polymers to create gels with desired properties for different applications. The composition and preparation of the gel can significantly affect the separation and resolution of molecules during electrophoresis.
- Electrophoresis apparatus design: Innovations in electrophoresis apparatus design focus on improving efficiency, resolution, and ease of use. These designs may include novel electrode configurations, buffer systems, or integrated cooling mechanisms. Some apparatus designs also incorporate features for automated sample loading or real-time monitoring of the electrophoresis process.
- Detection and analysis methods: Advanced detection and analysis methods are developed to enhance the sensitivity and accuracy of gel electrophoresis results. These may include fluorescence-based detection, image analysis software, or integration with mass spectrometry. Some methods focus on real-time monitoring of the separation process or automated data interpretation.
- Microfluidic and miniaturized systems: Miniaturized gel electrophoresis systems, often integrated into microfluidic devices, are developed for applications requiring small sample volumes or high-throughput analysis. These systems may incorporate novel fabrication techniques, integrated sample preparation steps, or parallelized separation channels.
- Specialized applications and modifications: Modifications to traditional gel electrophoresis techniques are developed for specialized applications. These may include pulsed-field gel electrophoresis for large DNA molecules, two-dimensional gel electrophoresis for protein separation, or adaptations for specific types of biomolecules or environmental conditions.
02 Electrophoresis apparatus design
Innovations in electrophoresis apparatus design focus on improving efficiency, resolution, and ease of use. These designs may include novel electrode configurations, buffer systems, or integrated cooling mechanisms. Some apparatuses are designed for specific applications or to handle multiple samples simultaneously.Expand Specific Solutions03 Detection and analysis methods
Advanced detection and analysis methods are developed to improve the sensitivity and accuracy of gel electrophoresis results. These may include fluorescence-based detection, image analysis software, or integration with mass spectrometry. Some methods allow for real-time monitoring of the electrophoresis process.Expand Specific Solutions04 Microfluidic and miniaturized systems
Miniaturized and microfluidic gel electrophoresis systems are developed to reduce sample and reagent consumption, increase throughput, and enable integration with other analytical techniques. These systems often incorporate novel fabrication methods and materials to achieve desired performance characteristics.Expand Specific Solutions05 Application-specific modifications
Modifications to gel electrophoresis techniques are developed for specific applications, such as DNA sequencing, protein analysis, or clinical diagnostics. These modifications may involve changes in gel composition, running conditions, or sample preparation methods to optimize separation and analysis for particular types of molecules or samples.Expand Specific Solutions
Key Players in Gel Electrophoresis Industry
The field of advanced gel electrophoresis applications in medicine is in a growth phase, with increasing market size and technological advancements. The global market for this technology is expanding due to its crucial role in various medical applications, including diagnostics and research. Companies like Life Technologies Corp., Genzyme Ltd, and Applied Biosystems LLC are at the forefront, driving innovation and market growth. The technology's maturity is evolving rapidly, with continuous improvements in resolution, speed, and automation. Academic institutions such as Jilin University and The Regents of the University of California are contributing significantly to research and development, further enhancing the technology's capabilities and applications in medical sciences.
Jilin University
Technical Solution: Researchers at Jilin University have developed novel applications of gel electrophoresis for medical diagnostics. They have pioneered the use of microchip gel electrophoresis for rapid separation and detection of disease biomarkers[7]. This miniaturized platform allows for high-throughput analysis of multiple samples with minimal reagent consumption. The university has also explored the integration of nanomaterials into gel matrices to enhance separation efficiency and sensitivity[8]. Additionally, they have developed specialized staining techniques for visualizing specific proteins and nucleic acids in complex biological samples, improving the diagnostic capabilities of gel electrophoresis in clinical settings[9].
Strengths: Innovative miniaturization and nanomaterial integration; Enhanced sensitivity for biomarker detection. Weaknesses: Technologies may be in early stages of development; Potential challenges in scaling up for widespread clinical use.
The Regents of the University of California
Technical Solution: The University of California system has made significant contributions to advancing gel electrophoresis applications in medicine. Researchers have developed novel gel formulations with improved resolution for separating large biomolecules, such as high molecular weight proteins and DNA fragments[10]. They have also pioneered the use of capillary gel electrophoresis for rapid, high-resolution analysis of genetic mutations associated with various diseases[11]. The university has explored the integration of microfluidic technologies with gel electrophoresis, enabling the development of point-of-care diagnostic devices for rapid disease detection[12]. Additionally, they have applied advanced imaging techniques to enhance the visualization and quantification of separated biomolecules in complex clinical samples.
Strengths: Diverse range of innovations across multiple aspects of gel electrophoresis; Strong focus on clinical applications. Weaknesses: Some technologies may require further validation for clinical use; Potential intellectual property complexities due to multiple research groups.
Innovative Gel Electrophoresis Applications
Electrophoresis gels with extended shelf life and high performance
PatentActiveJP2018530758A
Innovation
- Formulations of polyacrylamide gels with a near-neutral pH (6.5 to 7.5) using gel amine buffers, primary gel ampholytes, and conjugated gel ampholytes such as threonine and serine, which maintain gel stability and improve separation efficiency.
Electrophoresis controllers and methods for controlling electrophoresis process
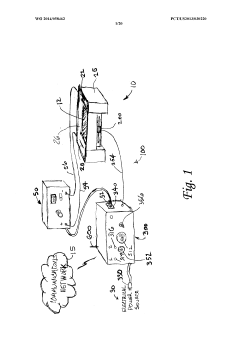
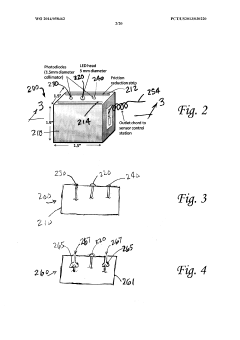
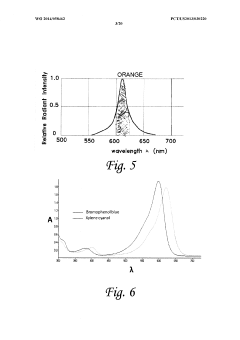
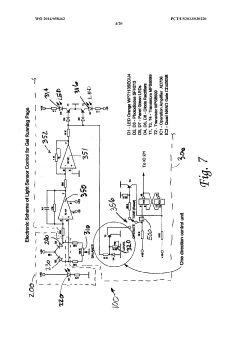
PatentWO2014058462A1
Innovation
- An electrophoresis controller with a sensor and light source that emits light into the gel matrix, detecting the migration of a tracking dye and automatically shutting off the power supply when the dye reaches a predetermined point, accompanied by alerts to the user.
Regulatory Framework for Medical Gel Electrophoresis
The regulatory framework for medical gel electrophoresis is a complex and evolving landscape that ensures the safety, efficacy, and quality of this advanced diagnostic technique in healthcare settings. In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing the development, manufacturing, and marketing of gel electrophoresis systems and related products used in medical applications.
Under the FDA's regulatory purview, gel electrophoresis systems are typically classified as Class II medical devices, which require a 510(k) premarket notification submission. This process involves demonstrating that the new device is substantially equivalent to a legally marketed predicate device in terms of safety and effectiveness. Manufacturers must provide detailed information on the device's intended use, technological characteristics, and performance data to support their claims.
The Clinical Laboratory Improvement Amendments (CLIA) of 1988 also significantly impact the use of gel electrophoresis in medical settings. CLIA regulations establish quality standards for all laboratory testing performed on human specimens for health assessment or disease diagnosis, treatment, and prevention. Laboratories using gel electrophoresis must comply with CLIA requirements, including personnel qualifications, quality control procedures, and proficiency testing.
Internationally, regulatory bodies such as the European Medicines Agency (EMA) and Japan's Pharmaceuticals and Medical Devices Agency (PMDA) have established their own frameworks for overseeing medical gel electrophoresis. The European Union's In Vitro Diagnostic Regulation (IVDR), which came into full effect in May 2022, has introduced more stringent requirements for the classification and conformity assessment of in vitro diagnostic devices, including gel electrophoresis systems used in medical diagnostics.
Regulatory compliance also extends to the reagents and consumables used in gel electrophoresis. These materials must meet strict quality and safety standards, often requiring separate approvals or certifications. Good Manufacturing Practices (GMP) guidelines are typically applied to ensure consistent production of high-quality reagents and gels.
As the field of medical gel electrophoresis continues to advance, regulatory frameworks are adapting to address emerging technologies and applications. For instance, the integration of artificial intelligence and machine learning in gel electrophoresis analysis has prompted discussions on how to regulate these software-enhanced systems effectively. Regulatory bodies are working to develop guidelines that balance innovation with patient safety and data integrity.
Under the FDA's regulatory purview, gel electrophoresis systems are typically classified as Class II medical devices, which require a 510(k) premarket notification submission. This process involves demonstrating that the new device is substantially equivalent to a legally marketed predicate device in terms of safety and effectiveness. Manufacturers must provide detailed information on the device's intended use, technological characteristics, and performance data to support their claims.
The Clinical Laboratory Improvement Amendments (CLIA) of 1988 also significantly impact the use of gel electrophoresis in medical settings. CLIA regulations establish quality standards for all laboratory testing performed on human specimens for health assessment or disease diagnosis, treatment, and prevention. Laboratories using gel electrophoresis must comply with CLIA requirements, including personnel qualifications, quality control procedures, and proficiency testing.
Internationally, regulatory bodies such as the European Medicines Agency (EMA) and Japan's Pharmaceuticals and Medical Devices Agency (PMDA) have established their own frameworks for overseeing medical gel electrophoresis. The European Union's In Vitro Diagnostic Regulation (IVDR), which came into full effect in May 2022, has introduced more stringent requirements for the classification and conformity assessment of in vitro diagnostic devices, including gel electrophoresis systems used in medical diagnostics.
Regulatory compliance also extends to the reagents and consumables used in gel electrophoresis. These materials must meet strict quality and safety standards, often requiring separate approvals or certifications. Good Manufacturing Practices (GMP) guidelines are typically applied to ensure consistent production of high-quality reagents and gels.
As the field of medical gel electrophoresis continues to advance, regulatory frameworks are adapting to address emerging technologies and applications. For instance, the integration of artificial intelligence and machine learning in gel electrophoresis analysis has prompted discussions on how to regulate these software-enhanced systems effectively. Regulatory bodies are working to develop guidelines that balance innovation with patient safety and data integrity.
Ethical Considerations in Genetic Analysis
The application of gel electrophoresis in medical genetics has revolutionized our understanding of human health and disease. However, this powerful tool also raises significant ethical considerations that must be carefully addressed. One primary concern is the potential for genetic discrimination based on the results of electrophoretic analyses. As these techniques become more advanced and accessible, there is a risk that individuals could face prejudice or unfair treatment in areas such as employment or insurance based on their genetic predispositions.
Privacy and confidentiality of genetic information obtained through gel electrophoresis are paramount ethical issues. The sensitive nature of this data necessitates robust safeguards to protect individuals from unauthorized access or misuse of their genetic profiles. This becomes increasingly challenging in an era of digital health records and large-scale genomic databases.
Informed consent is another critical ethical consideration. Patients must fully understand the implications of genetic testing, including the potential for uncovering incidental findings or variants of unknown significance. The complexity of genetic information often requires specialized genetic counseling to ensure that individuals can make informed decisions about testing and its consequences.
The use of gel electrophoresis in prenatal genetic testing raises additional ethical dilemmas. While it can provide valuable information about potential genetic disorders, it also introduces questions about selective reproduction and the societal implications of such choices. This intersects with broader debates about disability rights and the value placed on genetic diversity.
Equitable access to advanced gel electrophoresis techniques in medicine is an important ethical consideration. As these technologies become more sophisticated, there is a risk of exacerbating health disparities if they are not made widely available across different socioeconomic groups and geographic regions.
Lastly, the rapid advancement of gel electrophoresis applications in medicine outpaces the development of ethical and regulatory frameworks. This gap necessitates ongoing dialogue between scientists, ethicists, policymakers, and the public to ensure that the benefits of these technologies are realized while minimizing potential harms and respecting individual rights and societal values.
Privacy and confidentiality of genetic information obtained through gel electrophoresis are paramount ethical issues. The sensitive nature of this data necessitates robust safeguards to protect individuals from unauthorized access or misuse of their genetic profiles. This becomes increasingly challenging in an era of digital health records and large-scale genomic databases.
Informed consent is another critical ethical consideration. Patients must fully understand the implications of genetic testing, including the potential for uncovering incidental findings or variants of unknown significance. The complexity of genetic information often requires specialized genetic counseling to ensure that individuals can make informed decisions about testing and its consequences.
The use of gel electrophoresis in prenatal genetic testing raises additional ethical dilemmas. While it can provide valuable information about potential genetic disorders, it also introduces questions about selective reproduction and the societal implications of such choices. This intersects with broader debates about disability rights and the value placed on genetic diversity.
Equitable access to advanced gel electrophoresis techniques in medicine is an important ethical consideration. As these technologies become more sophisticated, there is a risk of exacerbating health disparities if they are not made widely available across different socioeconomic groups and geographic regions.
Lastly, the rapid advancement of gel electrophoresis applications in medicine outpaces the development of ethical and regulatory frameworks. This gap necessitates ongoing dialogue between scientists, ethicists, policymakers, and the public to ensure that the benefits of these technologies are realized while minimizing potential harms and respecting individual rights and societal values.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!