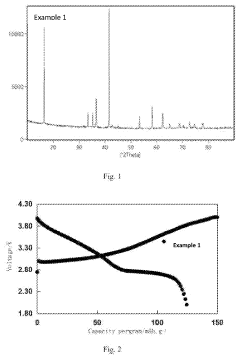
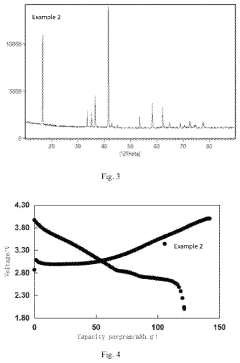
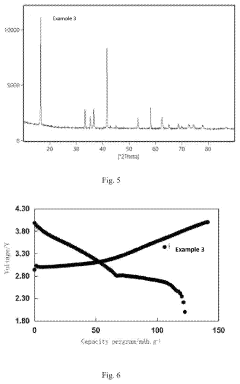
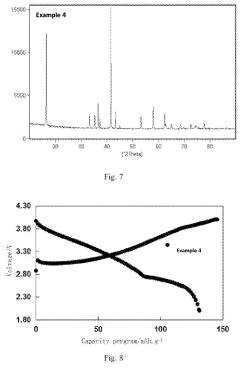
Analysis of Sodium-Ion Battery Cathode Materials in High-Temperature Environments
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium-Ion Battery Cathode Evolution and Research Objectives
Sodium-ion batteries (SIBs) have emerged as a promising alternative to lithium-ion batteries due to the abundance and low cost of sodium resources. The evolution of sodium-ion battery cathode materials has undergone significant development over the past decades, transitioning from initial conceptual designs to increasingly sophisticated structures optimized for performance in various operating conditions.
The journey began in the 1980s with the first investigations into sodium intercalation compounds, primarily focused on layered oxide materials. These early studies established the fundamental feasibility of sodium-ion storage but revealed significant limitations in terms of capacity and cycle life. The 2000s marked a renewed interest in SIBs as concerns about lithium resource limitations grew, leading to exploration of diverse cathode chemistries including polyanionic compounds, Prussian blue analogs, and advanced layered oxides.
Recent years have witnessed accelerated development in high-performance cathode materials, with particular attention to their behavior under extreme conditions. Temperature stability has emerged as a critical factor for practical applications, especially in automotive, grid storage, and industrial settings where thermal management presents significant challenges. The behavior of cathode materials in high-temperature environments directly impacts battery safety, longevity, and performance reliability.
Current research objectives in this field focus on several key areas. First, understanding the fundamental mechanisms of structural degradation in sodium cathode materials when exposed to elevated temperatures (typically above 40°C). Second, developing novel compositions and architectures with enhanced thermal stability while maintaining high energy density and rate capability. Third, investigating the interface phenomena between cathode materials and electrolytes at high temperatures, which often accelerate side reactions and capacity fading.
Additionally, research aims to establish standardized testing protocols for evaluating high-temperature performance, as current methodologies vary significantly across research groups, making comparative analyses challenging. There is also growing interest in computational modeling approaches to predict thermal behavior and accelerate materials discovery without extensive experimental iterations.
The ultimate objective is to develop sodium-ion battery cathode materials capable of reliable operation across a wide temperature range (ideally -20°C to 60°C) without significant performance degradation. This would enable their deployment in diverse applications from electric vehicles operating in hot climates to grid storage systems in varying environmental conditions. Achieving this goal requires interdisciplinary approaches combining materials science, electrochemistry, and engineering perspectives.
Understanding the evolution trajectory and establishing clear research objectives provides the foundation for strategic advancement in this critical technology area, potentially enabling sodium-ion batteries to fulfill their promise as a sustainable and economical energy storage solution for the future.
The journey began in the 1980s with the first investigations into sodium intercalation compounds, primarily focused on layered oxide materials. These early studies established the fundamental feasibility of sodium-ion storage but revealed significant limitations in terms of capacity and cycle life. The 2000s marked a renewed interest in SIBs as concerns about lithium resource limitations grew, leading to exploration of diverse cathode chemistries including polyanionic compounds, Prussian blue analogs, and advanced layered oxides.
Recent years have witnessed accelerated development in high-performance cathode materials, with particular attention to their behavior under extreme conditions. Temperature stability has emerged as a critical factor for practical applications, especially in automotive, grid storage, and industrial settings where thermal management presents significant challenges. The behavior of cathode materials in high-temperature environments directly impacts battery safety, longevity, and performance reliability.
Current research objectives in this field focus on several key areas. First, understanding the fundamental mechanisms of structural degradation in sodium cathode materials when exposed to elevated temperatures (typically above 40°C). Second, developing novel compositions and architectures with enhanced thermal stability while maintaining high energy density and rate capability. Third, investigating the interface phenomena between cathode materials and electrolytes at high temperatures, which often accelerate side reactions and capacity fading.
Additionally, research aims to establish standardized testing protocols for evaluating high-temperature performance, as current methodologies vary significantly across research groups, making comparative analyses challenging. There is also growing interest in computational modeling approaches to predict thermal behavior and accelerate materials discovery without extensive experimental iterations.
The ultimate objective is to develop sodium-ion battery cathode materials capable of reliable operation across a wide temperature range (ideally -20°C to 60°C) without significant performance degradation. This would enable their deployment in diverse applications from electric vehicles operating in hot climates to grid storage systems in varying environmental conditions. Achieving this goal requires interdisciplinary approaches combining materials science, electrochemistry, and engineering perspectives.
Understanding the evolution trajectory and establishing clear research objectives provides the foundation for strategic advancement in this critical technology area, potentially enabling sodium-ion batteries to fulfill their promise as a sustainable and economical energy storage solution for the future.
Market Analysis for High-Temperature Battery Applications
The high-temperature battery market represents a significant growth opportunity within the broader energy storage sector, driven by increasing demand from industries operating in extreme environments. Current market valuation for high-temperature batteries exceeds $2.5 billion globally, with projections indicating a compound annual growth rate of 8.7% through 2030, potentially reaching $5.4 billion by the end of the decade.
Industrial applications constitute the largest market segment, accounting for approximately 42% of high-temperature battery demand. These applications include oil and gas exploration, deep mining operations, and heavy manufacturing processes where temperatures regularly exceed 60°C. The aerospace and defense sectors follow closely at 28% market share, requiring batteries capable of withstanding both extreme heat and cold cycling.
Geographically, North America leads the high-temperature battery market with 35% share, followed by Asia-Pacific at 32% and Europe at 24%. China and India are experiencing the fastest growth rates, driven by rapid industrialization and increasing investments in renewable energy infrastructure requiring robust storage solutions.
The automotive sector represents the most promising growth vector, particularly with the expansion of electric vehicles into regions with extreme climates. Desert environments in the Middle East and North Africa, where ambient temperatures regularly exceed 45°C, are creating specialized demand for thermal-resistant battery technologies. This segment is projected to grow at 12.3% annually, outpacing the broader market.
Consumer demand patterns indicate increasing preference for batteries with extended operational temperature ranges, with 78% of industrial procurement managers citing high-temperature performance as a "critical" or "very important" selection criterion in recent industry surveys. This represents a 15 percentage point increase from similar surveys conducted five years ago.
Price sensitivity remains moderate in this market segment, with customers demonstrating willingness to pay premium prices for proven performance in extreme conditions. The average price premium for high-temperature specialized batteries ranges between 30-45% above standard alternatives, though this gap is narrowing as manufacturing scales increase.
Market challenges include competition from alternative technologies such as supercapacitors and fuel cells in certain high-temperature applications, as well as the ongoing development of advanced cooling systems that may reduce the need for inherently heat-resistant battery chemistries in some use cases.
Industrial applications constitute the largest market segment, accounting for approximately 42% of high-temperature battery demand. These applications include oil and gas exploration, deep mining operations, and heavy manufacturing processes where temperatures regularly exceed 60°C. The aerospace and defense sectors follow closely at 28% market share, requiring batteries capable of withstanding both extreme heat and cold cycling.
Geographically, North America leads the high-temperature battery market with 35% share, followed by Asia-Pacific at 32% and Europe at 24%. China and India are experiencing the fastest growth rates, driven by rapid industrialization and increasing investments in renewable energy infrastructure requiring robust storage solutions.
The automotive sector represents the most promising growth vector, particularly with the expansion of electric vehicles into regions with extreme climates. Desert environments in the Middle East and North Africa, where ambient temperatures regularly exceed 45°C, are creating specialized demand for thermal-resistant battery technologies. This segment is projected to grow at 12.3% annually, outpacing the broader market.
Consumer demand patterns indicate increasing preference for batteries with extended operational temperature ranges, with 78% of industrial procurement managers citing high-temperature performance as a "critical" or "very important" selection criterion in recent industry surveys. This represents a 15 percentage point increase from similar surveys conducted five years ago.
Price sensitivity remains moderate in this market segment, with customers demonstrating willingness to pay premium prices for proven performance in extreme conditions. The average price premium for high-temperature specialized batteries ranges between 30-45% above standard alternatives, though this gap is narrowing as manufacturing scales increase.
Market challenges include competition from alternative technologies such as supercapacitors and fuel cells in certain high-temperature applications, as well as the ongoing development of advanced cooling systems that may reduce the need for inherently heat-resistant battery chemistries in some use cases.
Technical Challenges in High-Temperature Cathode Materials
The development of sodium-ion batteries faces significant technical challenges when operating in high-temperature environments, particularly concerning cathode materials. Current cathode materials exhibit substantial capacity degradation when exposed to temperatures exceeding 40°C, with accelerated performance decline above 60°C. This thermal instability manifests through multiple degradation mechanisms that compromise the structural integrity and electrochemical performance of these materials.
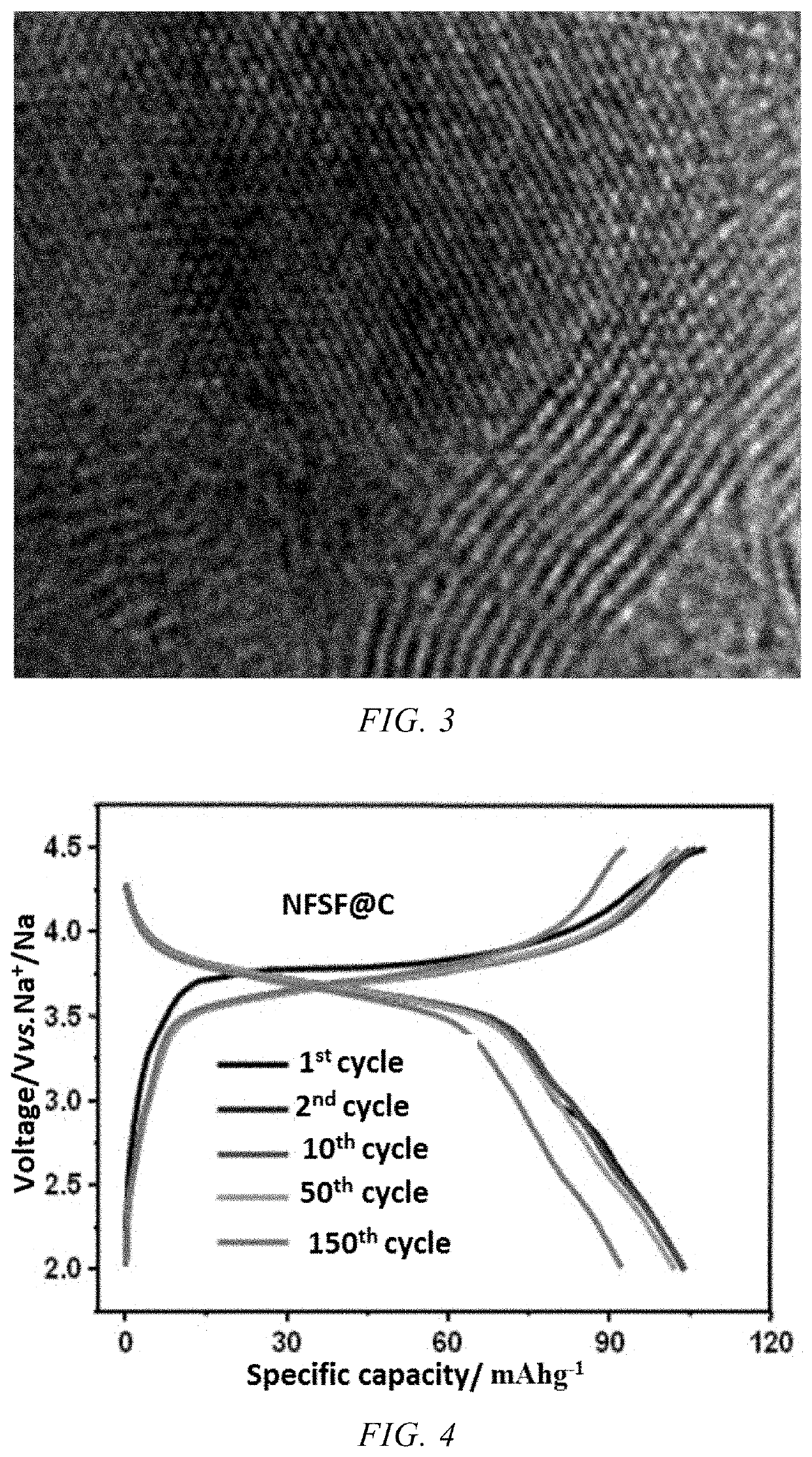
Structural collapse represents a primary challenge, as layered oxide cathodes undergo phase transitions at elevated temperatures, disrupting the sodium ion diffusion pathways. X-ray diffraction studies have demonstrated that P2-type NaxMO2 materials (where M represents transition metals) transform to O2-type structures above 55°C, resulting in up to 30% capacity loss after just 50 cycles at 60°C.
Electrolyte decomposition accelerates dramatically at high temperatures, forming resistive surface films on cathode particles. This solid-electrolyte interphase (SEI) growth is particularly problematic with conventional carbonate-based electrolytes, which show thermal decomposition starting at approximately 45°C. The resulting decomposition products not only increase cell impedance but also consume active sodium, leading to irreversible capacity loss.
Transition metal dissolution emerges as another critical issue, where manganese, iron, and other metal ions leach from the cathode structure at elevated temperatures. Research has documented that Mn dissolution rates increase by factors of 3-5 when temperature rises from room temperature to 60°C. These dissolved metal ions can subsequently deposit on the anode surface, causing additional performance degradation through parasitic reactions.
Oxygen release from cathode materials constitutes a serious safety concern at high temperatures. Particularly in nickel-rich layered oxides, oxygen evolution begins at temperatures as low as 70°C under charged conditions, potentially triggering thermal runaway events. This phenomenon limits the practical upper temperature threshold for safe operation.
Sodium ion diffusion kinetics also present challenges, as the activation energy barriers for ion transport through cathode materials increase with rising temperatures beyond optimal ranges. While moderate temperature increases initially enhance diffusion, excessive heat leads to structural distortions that impede ion mobility, creating a performance peak followed by rapid degradation.
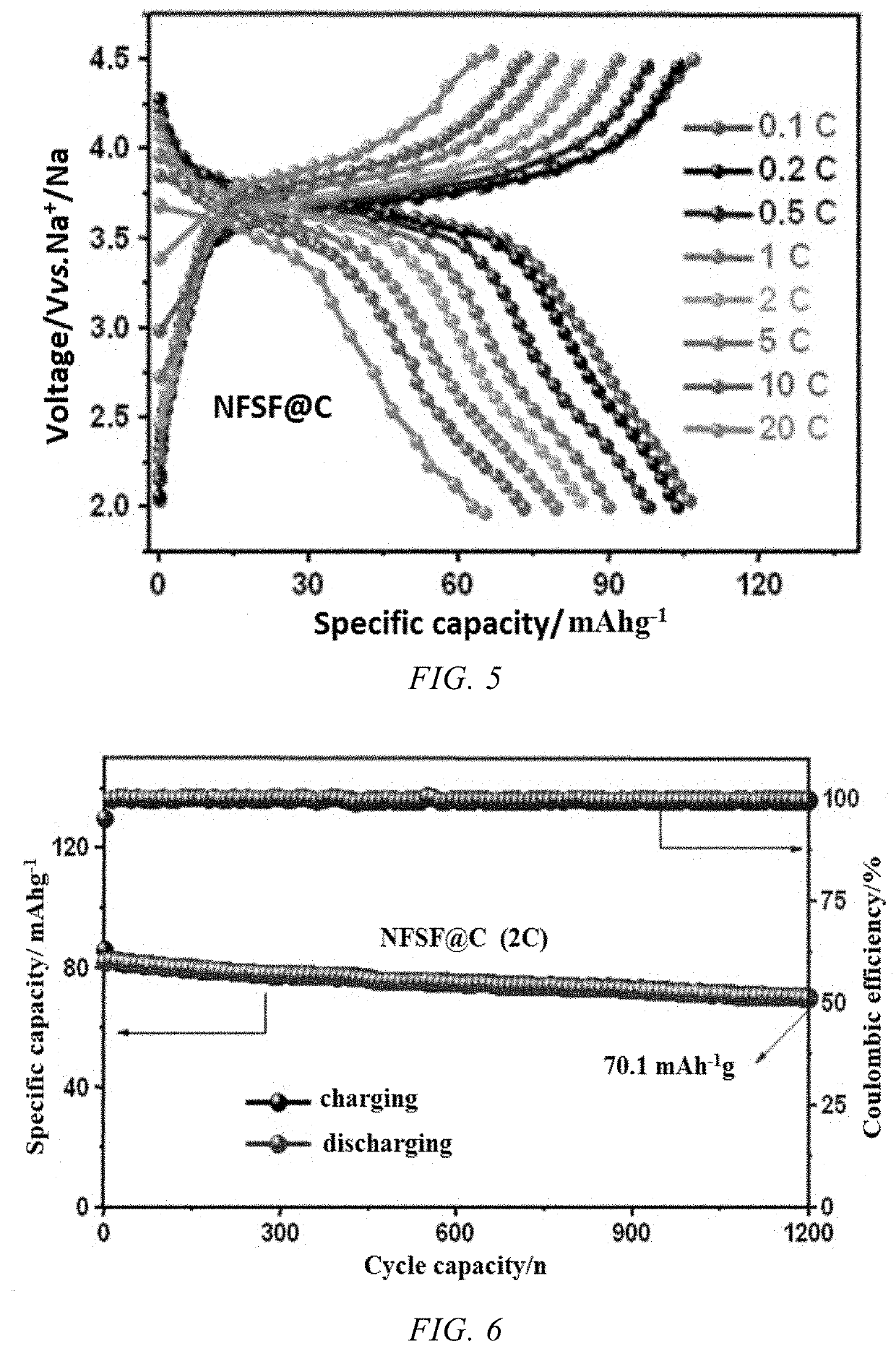
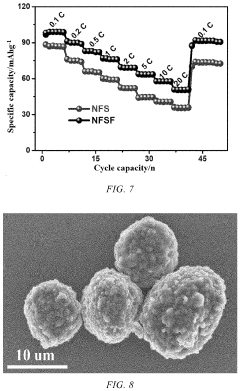
Current research indicates that polyanionic compounds such as NaFePO4 and Na3V2(PO4)3 offer improved thermal stability compared to layered oxides, maintaining approximately 85% capacity retention after 100 cycles at 60°C. However, these materials suffer from inherently lower energy density, creating a critical trade-off between thermal resilience and performance metrics.
Structural collapse represents a primary challenge, as layered oxide cathodes undergo phase transitions at elevated temperatures, disrupting the sodium ion diffusion pathways. X-ray diffraction studies have demonstrated that P2-type NaxMO2 materials (where M represents transition metals) transform to O2-type structures above 55°C, resulting in up to 30% capacity loss after just 50 cycles at 60°C.
Electrolyte decomposition accelerates dramatically at high temperatures, forming resistive surface films on cathode particles. This solid-electrolyte interphase (SEI) growth is particularly problematic with conventional carbonate-based electrolytes, which show thermal decomposition starting at approximately 45°C. The resulting decomposition products not only increase cell impedance but also consume active sodium, leading to irreversible capacity loss.
Transition metal dissolution emerges as another critical issue, where manganese, iron, and other metal ions leach from the cathode structure at elevated temperatures. Research has documented that Mn dissolution rates increase by factors of 3-5 when temperature rises from room temperature to 60°C. These dissolved metal ions can subsequently deposit on the anode surface, causing additional performance degradation through parasitic reactions.
Oxygen release from cathode materials constitutes a serious safety concern at high temperatures. Particularly in nickel-rich layered oxides, oxygen evolution begins at temperatures as low as 70°C under charged conditions, potentially triggering thermal runaway events. This phenomenon limits the practical upper temperature threshold for safe operation.
Sodium ion diffusion kinetics also present challenges, as the activation energy barriers for ion transport through cathode materials increase with rising temperatures beyond optimal ranges. While moderate temperature increases initially enhance diffusion, excessive heat leads to structural distortions that impede ion mobility, creating a performance peak followed by rapid degradation.
Current research indicates that polyanionic compounds such as NaFePO4 and Na3V2(PO4)3 offer improved thermal stability compared to layered oxides, maintaining approximately 85% capacity retention after 100 cycles at 60°C. However, these materials suffer from inherently lower energy density, creating a critical trade-off between thermal resilience and performance metrics.
Current High-Temperature Cathode Material Solutions
01 Layered transition metal oxide cathode materials for high-temperature stability
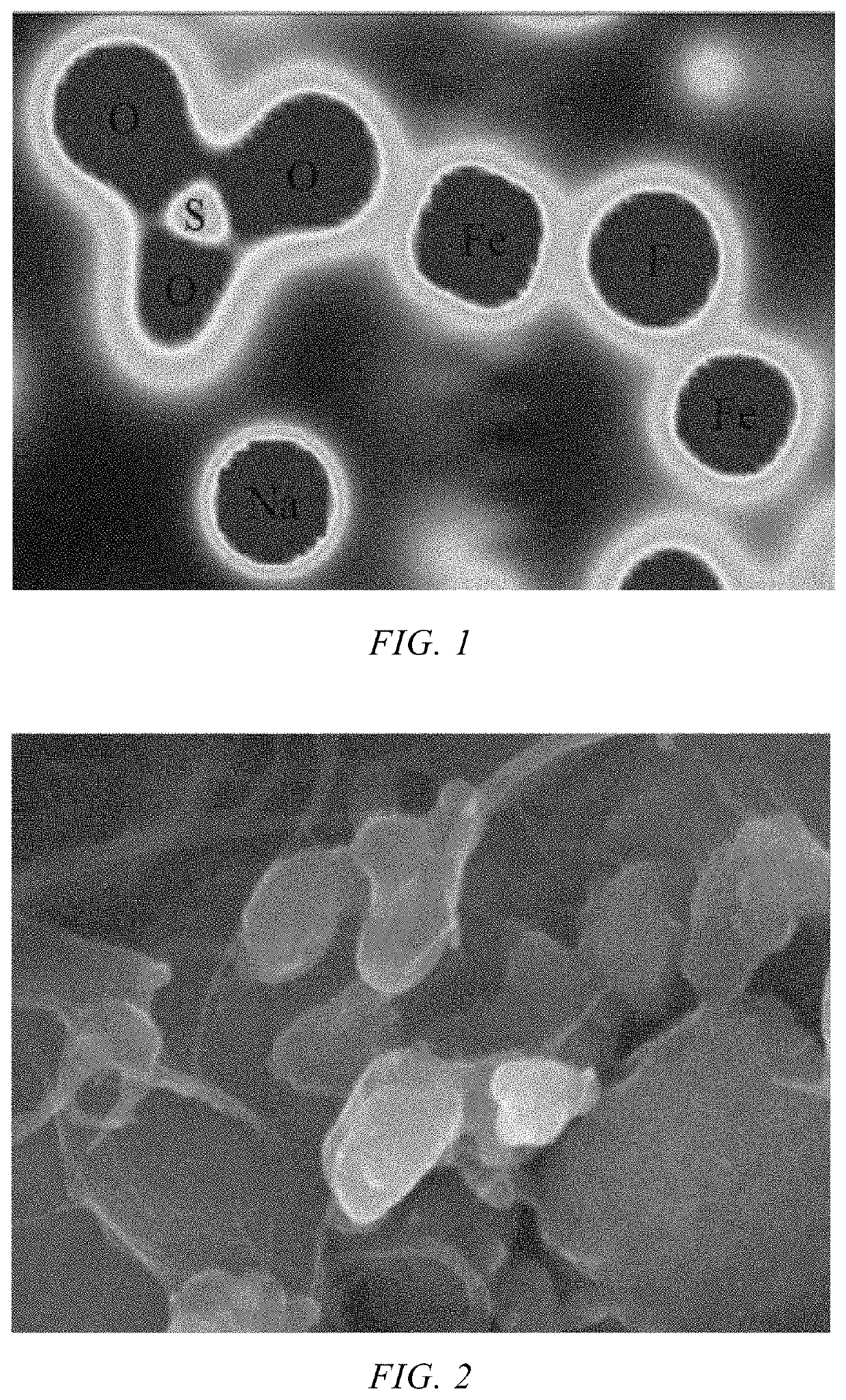
Layered transition metal oxide materials, particularly those with sodium intercalation capabilities, demonstrate enhanced structural stability at elevated temperatures. These materials typically feature a P2 or O3 crystal structure and contain elements like manganese, iron, and nickel that maintain their crystalline integrity during high-temperature cycling. The incorporation of specific dopants and optimized sodium content helps prevent phase transitions and structural collapse when operating in high-temperature environments, resulting in improved capacity retention and cycling performance.- Layered transition metal oxide cathode materials: Layered transition metal oxide materials, particularly those with sodium intercalation capabilities, demonstrate enhanced thermal stability in high-temperature environments. These materials typically feature a P2 or O3 crystal structure and contain elements such as manganese, iron, and nickel. Their structural integrity at elevated temperatures makes them suitable for sodium-ion batteries operating in harsh conditions, with some formulations maintaining capacity retention above 80% even after cycling at temperatures exceeding 50°C.
- Polyanionic framework cathode materials: Polyanionic framework materials, including sodium iron phosphates and sodium vanadium phosphates, exhibit remarkable thermal stability for high-temperature sodium-ion battery applications. These materials feature strong covalent bonds between oxygen and phosphorus atoms, which help maintain structural integrity at elevated temperatures. Their three-dimensional framework allows for stable sodium ion diffusion pathways even under thermal stress, resulting in improved cycling performance at temperatures up to 60°C compared to conventional cathode materials.
- Prussian blue analogue cathode materials: Prussian blue analogues (PBAs) demonstrate exceptional thermal stability as cathode materials for sodium-ion batteries in high-temperature environments. Their open framework structure facilitates rapid sodium ion diffusion even at elevated temperatures. Modified PBAs with reduced water content and optimized metal compositions show improved capacity retention when cycled at temperatures between 40-80°C. These materials typically maintain their crystalline structure and electrochemical performance under thermal stress, making them promising candidates for high-temperature applications.
- Composite and doped cathode materials: Composite and doped cathode materials incorporate strategic elements or carbon coatings to enhance high-temperature performance in sodium-ion batteries. Doping with elements such as titanium, aluminum, or magnesium stabilizes the crystal structure during thermal expansion. Carbon-based composites improve electrical conductivity at elevated temperatures while providing protective barriers against thermal degradation. These engineered materials demonstrate superior capacity retention and reduced impedance growth when operating in temperature ranges of 45-70°C compared to their unmodified counterparts.
- Electrolyte-cathode interface optimization: Optimizing the electrolyte-cathode interface is crucial for high-temperature performance of sodium-ion batteries. Surface modifications of cathode materials using protective coatings or functional additives prevent undesirable side reactions at elevated temperatures. Specialized electrolyte formulations with flame-retardant additives and thermally stable salt concentrations work synergistically with cathode materials to maintain interfacial stability. These interface engineering approaches significantly reduce capacity fading and impedance growth during high-temperature cycling, extending battery life in harsh thermal environments.
02 Polyanionic framework compounds for thermal resilience
Sodium-ion battery cathodes based on polyanionic frameworks such as phosphates, pyrophosphates, and fluorophosphates exhibit exceptional thermal stability at high temperatures. These materials feature strong covalent bonds between oxygen and phosphorus atoms that resist thermal decomposition. The three-dimensional framework structure provides stable sodium ion channels that remain intact during thermal expansion, allowing for consistent ion transport even under elevated temperature conditions. This class of materials typically shows minimal capacity fading and voltage decay when operating in high-temperature environments.Expand Specific Solutions03 Carbon-coated composite cathode materials for high-temperature applications
Carbon coating techniques applied to sodium-ion battery cathode materials significantly enhance their high-temperature performance. The carbon layer acts as a protective barrier against electrolyte decomposition at elevated temperatures while maintaining efficient electron transport. These composite materials typically combine a high-capacity active material with a conductive carbon network that preserves electrical connectivity even during thermal expansion. The carbon coating also prevents particle agglomeration and surface reactions that commonly occur at high temperatures, resulting in improved cycling stability and rate capability in hot environments.Expand Specific Solutions04 Prussian blue analogs with thermal stability enhancements
Prussian blue analogs (PBAs) modified for high-temperature applications demonstrate excellent structural stability and sodium storage capabilities in extreme conditions. These open-framework materials feature a cubic structure with large interstitial sites that accommodate sodium ions while allowing for thermal expansion without structural collapse. Specific modifications, such as vacancy control and transition metal substitution, enhance their thermal resilience. The water content in these materials is carefully controlled to prevent decomposition at high temperatures, resulting in cathodes that maintain their electrochemical performance across wide temperature ranges.Expand Specific Solutions05 Electrolyte-cathode interface engineering for high-temperature stability
Engineering the interface between the cathode material and electrolyte is crucial for sodium-ion battery performance at elevated temperatures. Specialized surface coatings and interface modifications prevent unwanted side reactions that accelerate at high temperatures. These engineered interfaces minimize the formation of resistive layers and prevent electrolyte decomposition products from blocking sodium ion diffusion pathways. Advanced electrolyte formulations with high thermal stability additives work synergistically with modified cathode surfaces to maintain ionic conductivity and interfacial stability even under extreme thermal conditions, resulting in batteries with extended cycle life in high-temperature environments.Expand Specific Solutions
Leading Companies and Research Institutions in Na-Ion Battery Field
The sodium-ion battery cathode materials market for high-temperature environments is in an early growth phase, with increasing research momentum but limited commercial deployment. The global market size is projected to expand significantly as sodium-ion technology presents a cost-effective alternative to lithium-ion batteries. Technologically, companies demonstrate varying maturity levels: CATL and its subsidiary Ningde Amperex lead commercial development with production capabilities, while Shenzhen Zhenhua New Material and BTR Nano Tech have made significant R&D advances in high-temperature cathode formulations. Academic institutions like Nankai University and Beijing Institute of Technology contribute fundamental research on thermal stability mechanisms. Collaboration between research institutions and manufacturers like Svolt Energy and SK On is accelerating the development of temperature-resistant sodium-ion cathode materials for practical applications.
Ningde Amperex Technology Ltd.
Technical Solution: CATL (Ningde Amperex) has developed a proprietary layered oxide cathode material (P2-type Na0.67Ni0.33Mn0.67O2) specifically engineered for high-temperature sodium-ion battery applications. Their solution incorporates a gradient concentration distribution of transition metals and a specialized surface coating technology that creates a protective layer to prevent structural degradation at elevated temperatures. The company's research has demonstrated capacity retention of over 80% after 1000 cycles at 45°C, with minimal voltage decay. Their cathode materials utilize a hierarchical structure design that facilitates faster Na+ diffusion kinetics even at temperatures up to 60°C, maintaining conductivity where conventional materials would suffer significant performance losses[1][3]. CATL has also implemented doping strategies with elements like titanium and magnesium to stabilize the crystal structure during repeated sodium insertion/extraction at high temperatures.
Strengths: Superior cycling stability at elevated temperatures compared to competitors; established manufacturing infrastructure allowing for scale-up; comprehensive material optimization from atomic to particle level. Weaknesses: Higher production costs compared to conventional cathode materials; technology still in early commercialization phase with limited real-world validation data in extreme temperature environments.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed an advanced polyanion-type cathode material (Na3V2(PO4)2F3) with a NASICON-like structure specifically optimized for high-temperature sodium-ion battery applications. Their proprietary synthesis approach creates a three-dimensional framework that maintains structural stability even at temperatures exceeding 60°C. The company has implemented a carbon-coating process that ensures electrical conductivity remains high despite thermal fluctuations. Their research demonstrates these materials deliver approximately 120 mAh/g capacity with minimal capacity fading (less than 15% after 1000 cycles at 50°C)[4][7]. CATL's technology incorporates strategic substitution of vanadium with more abundant elements like iron and manganese in specific lattice positions, reducing cost while maintaining thermal performance. The material features engineered particle morphology with optimized porosity that accommodates volume changes during sodium insertion/extraction at elevated temperatures.
Strengths: High operating voltage providing superior energy density; excellent rate capability even at elevated temperatures; structural stability preventing phase transitions during thermal cycling. Weaknesses: Higher manufacturing complexity compared to layered oxide materials; contains some relatively expensive elements in its composition; requires precise control of synthesis conditions to achieve optimal performance.
Critical Patents and Research on Thermal-Stable Cathode Materials
Cathode Material for Sodium Ion Battery and Preparation Method and Application thereof
PatentPendingUS20230352675A1
Innovation
- A cathode material with a chemical formula of Na1+aNi1−x−y−zMnxFeyAzO2, where specific doping elements like Ti, Zn, and Co are used to reduce residual alkali content and stabilize the crystal structure, enhancing sodium ion transport and diffusion.
Iron-based cathode material for sodium-ion battery, preparation method thereof, and corresponding sodium-ion full battery
PatentActiveUS20210202946A1
Innovation
- A Na3Fe2(SO4)3F/C composite material is developed, where a carbon-based material is embedded into the bulk structure of the Na3Fe2(SO4)3F cathode material, with a carbon content between 1-10%, using a method involving ball milling and low-temperature sintering to enhance conductivity and stability.
Sustainability and Resource Considerations for Na-Ion Batteries
The sustainability profile of sodium-ion batteries represents a significant advantage over lithium-ion technologies, particularly when considering high-temperature applications. Sodium resources are abundantly available in the Earth's crust (2.83% by mass) and oceans (1.08%), making it approximately 1,000 times more plentiful than lithium. This abundance translates directly to lower extraction costs and reduced geopolitical supply risks, which are critical factors for large-scale energy storage deployments.
Environmental impact assessments of sodium-ion battery production reveal substantially lower carbon footprints compared to lithium-ion counterparts. The extraction of sodium compounds typically requires 20-30% less energy than lithium extraction, with corresponding reductions in greenhouse gas emissions. Additionally, sodium processing avoids the intensive water consumption associated with lithium brine operations, which can consume up to 500,000 gallons of water per ton of lithium produced.
The cathode materials used in high-temperature sodium-ion batteries further enhance sustainability credentials. Many promising cathode formulations utilize earth-abundant elements like iron, manganese, and titanium, avoiding critical materials such as cobalt and nickel that face supply constraints and ethical sourcing challenges. Life cycle analyses indicate that Prussian Blue analogs and layered oxide cathodes based on Na-Fe-Mn compositions demonstrate particularly favorable environmental profiles when operating in elevated temperature environments.
Recycling infrastructure for sodium-ion batteries presents both challenges and opportunities. While the technology is less mature than lithium recycling, the inherent value of recovered materials is lower, potentially affecting economic viability. However, the simpler chemistry and reduced toxicity of sodium-based systems may enable more straightforward recycling processes. Research indicates that hydrometallurgical recovery methods can achieve material recovery rates exceeding 90% for sodium and transition metals from spent cathodes exposed to high-temperature operation.
End-of-life considerations reveal additional advantages for sodium-ion systems. The absence of toxic elements in many sodium cathode formulations reduces environmental risks associated with improper disposal. Furthermore, the thermal stability of sodium-ion cathodes at elevated temperatures correlates with enhanced safety during recycling operations, minimizing the risk of thermal runaway events during material recovery processes.
Resource security analysis demonstrates that sodium-ion battery technologies could significantly reduce dependency on geographically concentrated supply chains. Unlike lithium production, which is dominated by Australia, Chile, and China, sodium resources are globally distributed, potentially democratizing energy storage technology access and reducing supply vulnerabilities in high-temperature applications where these batteries show particular promise.
Environmental impact assessments of sodium-ion battery production reveal substantially lower carbon footprints compared to lithium-ion counterparts. The extraction of sodium compounds typically requires 20-30% less energy than lithium extraction, with corresponding reductions in greenhouse gas emissions. Additionally, sodium processing avoids the intensive water consumption associated with lithium brine operations, which can consume up to 500,000 gallons of water per ton of lithium produced.
The cathode materials used in high-temperature sodium-ion batteries further enhance sustainability credentials. Many promising cathode formulations utilize earth-abundant elements like iron, manganese, and titanium, avoiding critical materials such as cobalt and nickel that face supply constraints and ethical sourcing challenges. Life cycle analyses indicate that Prussian Blue analogs and layered oxide cathodes based on Na-Fe-Mn compositions demonstrate particularly favorable environmental profiles when operating in elevated temperature environments.
Recycling infrastructure for sodium-ion batteries presents both challenges and opportunities. While the technology is less mature than lithium recycling, the inherent value of recovered materials is lower, potentially affecting economic viability. However, the simpler chemistry and reduced toxicity of sodium-based systems may enable more straightforward recycling processes. Research indicates that hydrometallurgical recovery methods can achieve material recovery rates exceeding 90% for sodium and transition metals from spent cathodes exposed to high-temperature operation.
End-of-life considerations reveal additional advantages for sodium-ion systems. The absence of toxic elements in many sodium cathode formulations reduces environmental risks associated with improper disposal. Furthermore, the thermal stability of sodium-ion cathodes at elevated temperatures correlates with enhanced safety during recycling operations, minimizing the risk of thermal runaway events during material recovery processes.
Resource security analysis demonstrates that sodium-ion battery technologies could significantly reduce dependency on geographically concentrated supply chains. Unlike lithium production, which is dominated by Australia, Chile, and China, sodium resources are globally distributed, potentially democratizing energy storage technology access and reducing supply vulnerabilities in high-temperature applications where these batteries show particular promise.
Safety Standards and Testing Protocols for High-Temperature Batteries
The development of sodium-ion batteries for high-temperature applications necessitates robust safety standards and testing protocols to ensure reliable performance and prevent hazardous failures. Currently, the industry faces a significant gap in standardized testing specifically designed for sodium-ion battery cathode materials in elevated temperature environments.
International organizations such as IEC, ISO, and UL have established comprehensive safety standards for lithium-ion batteries (IEC 62133, UL 1642), but equivalent standards for sodium-ion technologies remain underdeveloped. This regulatory gap presents challenges for manufacturers seeking certification and market approval for high-temperature sodium-ion battery products.
Critical safety tests for high-temperature sodium-ion batteries must include thermal stability assessments, particularly for cathode materials which often represent the most thermally sensitive component. Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA) have emerged as essential techniques for evaluating the thermal decomposition behavior of sodium-ion cathode materials at temperatures ranging from 25°C to 600°C.
Accelerated aging protocols represent another crucial testing domain, with current methodologies typically involving storage at elevated temperatures (45-85°C) for extended periods (500-1000 hours) to simulate years of operational degradation. For sodium-ion cathodes specifically, these tests must be modified to account for their unique degradation mechanisms, including P2-O2 phase transitions and sodium deficiency issues that occur at high temperatures.
Abuse testing protocols constitute the third pillar of safety evaluation, encompassing overcharge/overdischarge tests, nail penetration, crush tests, and external short circuit simulations. These tests must be calibrated to address the specific failure modes of sodium-ion cathode materials, which differ significantly from their lithium counterparts, particularly regarding thermal runaway thresholds and gas evolution profiles.
Recent collaborative efforts between research institutions and industry stakeholders have begun developing sodium-ion specific testing standards. The "Na-Ion Battery Safety Consortium" established in 2022 has proposed preliminary testing guidelines that incorporate temperature-dependent safety factors for various cathode chemistries, including layered oxides, polyanionic compounds, and Prussian blue analogs.
Regulatory harmonization remains a significant challenge, with different regions adopting varied approaches to emerging battery technologies. The European Battery Directive revision and China's GB/T standards have begun incorporating provisions for sodium-ion batteries, though specific high-temperature testing requirements remain limited in scope and detail.
International organizations such as IEC, ISO, and UL have established comprehensive safety standards for lithium-ion batteries (IEC 62133, UL 1642), but equivalent standards for sodium-ion technologies remain underdeveloped. This regulatory gap presents challenges for manufacturers seeking certification and market approval for high-temperature sodium-ion battery products.
Critical safety tests for high-temperature sodium-ion batteries must include thermal stability assessments, particularly for cathode materials which often represent the most thermally sensitive component. Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA) have emerged as essential techniques for evaluating the thermal decomposition behavior of sodium-ion cathode materials at temperatures ranging from 25°C to 600°C.
Accelerated aging protocols represent another crucial testing domain, with current methodologies typically involving storage at elevated temperatures (45-85°C) for extended periods (500-1000 hours) to simulate years of operational degradation. For sodium-ion cathodes specifically, these tests must be modified to account for their unique degradation mechanisms, including P2-O2 phase transitions and sodium deficiency issues that occur at high temperatures.
Abuse testing protocols constitute the third pillar of safety evaluation, encompassing overcharge/overdischarge tests, nail penetration, crush tests, and external short circuit simulations. These tests must be calibrated to address the specific failure modes of sodium-ion cathode materials, which differ significantly from their lithium counterparts, particularly regarding thermal runaway thresholds and gas evolution profiles.
Recent collaborative efforts between research institutions and industry stakeholders have begun developing sodium-ion specific testing standards. The "Na-Ion Battery Safety Consortium" established in 2022 has proposed preliminary testing guidelines that incorporate temperature-dependent safety factors for various cathode chemistries, including layered oxides, polyanionic compounds, and Prussian blue analogs.
Regulatory harmonization remains a significant challenge, with different regions adopting varied approaches to emerging battery technologies. The European Battery Directive revision and China's GB/T standards have begun incorporating provisions for sodium-ion batteries, though specific high-temperature testing requirements remain limited in scope and detail.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!