Why Polyanionic Sodium-Ion Battery Cathode Materials Offer Superior Safety
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Polyanionic Cathode Evolution and Safety Objectives
Polyanionic compounds emerged as promising cathode materials for sodium-ion batteries (SIBs) in the early 1980s, following the discovery of NASICON (Na Super Ionic CONductor) structures. These materials represented a significant departure from traditional layered oxide cathodes, offering a three-dimensional framework that could accommodate sodium ions while maintaining structural stability. The evolution of polyanionic cathodes has been marked by continuous improvements in energy density, cycling stability, and safety characteristics.
The development trajectory of polyanionic cathodes has progressed through several distinct phases. Initially, phosphate-based compounds such as NaFePO4 and Na3V2(PO4)3 dominated research efforts, inspired by the success of LiFePO4 in lithium-ion batteries. Subsequently, research expanded to include sulfates (Na2Fe(SO4)2), fluorophosphates (Na2FePO4F), and more complex mixed-polyanion structures, each offering unique advantages in terms of operating voltage, specific capacity, and structural stability.
A critical milestone in polyanionic cathode development was the recognition of their inherent safety advantages. Unlike layered oxide cathodes, polyanionic structures feature strong covalent bonds between the transition metal and polyanion groups (PO4, SO4, etc.), which effectively stabilize the oxygen atoms and prevent their release during thermal stress or overcharging conditions. This characteristic substantially reduces the risk of thermal runaway, a catastrophic failure mode in battery systems.
The primary safety objective in developing polyanionic cathodes is to create materials that maintain structural integrity under extreme conditions while delivering competitive energy density. This involves optimizing the balance between electrochemical performance and thermal stability. Recent research has focused on understanding the fundamental mechanisms that contribute to the enhanced safety of polyanionic materials, including their resistance to phase transitions during cycling and their ability to suppress oxygen evolution at high temperatures.
Current technological goals include developing polyanionic cathodes with higher sodium diffusion coefficients to improve rate capability, increasing specific capacity beyond 160 mAh/g, and enhancing compatibility with aqueous electrolytes for even greater safety. Additionally, researchers aim to reduce the environmental footprint of these materials by utilizing earth-abundant elements and developing sustainable synthesis methods.
The evolution of polyanionic cathodes represents a convergence of materials science, electrochemistry, and safety engineering. As sodium-ion batteries gain prominence in grid storage applications and other sectors where safety is paramount, polyanionic cathodes are positioned to play a crucial role in enabling widespread adoption of this technology. Their continued development focuses on maintaining their exceptional safety profile while addressing remaining challenges in energy density and production costs.
The development trajectory of polyanionic cathodes has progressed through several distinct phases. Initially, phosphate-based compounds such as NaFePO4 and Na3V2(PO4)3 dominated research efforts, inspired by the success of LiFePO4 in lithium-ion batteries. Subsequently, research expanded to include sulfates (Na2Fe(SO4)2), fluorophosphates (Na2FePO4F), and more complex mixed-polyanion structures, each offering unique advantages in terms of operating voltage, specific capacity, and structural stability.
A critical milestone in polyanionic cathode development was the recognition of their inherent safety advantages. Unlike layered oxide cathodes, polyanionic structures feature strong covalent bonds between the transition metal and polyanion groups (PO4, SO4, etc.), which effectively stabilize the oxygen atoms and prevent their release during thermal stress or overcharging conditions. This characteristic substantially reduces the risk of thermal runaway, a catastrophic failure mode in battery systems.
The primary safety objective in developing polyanionic cathodes is to create materials that maintain structural integrity under extreme conditions while delivering competitive energy density. This involves optimizing the balance between electrochemical performance and thermal stability. Recent research has focused on understanding the fundamental mechanisms that contribute to the enhanced safety of polyanionic materials, including their resistance to phase transitions during cycling and their ability to suppress oxygen evolution at high temperatures.
Current technological goals include developing polyanionic cathodes with higher sodium diffusion coefficients to improve rate capability, increasing specific capacity beyond 160 mAh/g, and enhancing compatibility with aqueous electrolytes for even greater safety. Additionally, researchers aim to reduce the environmental footprint of these materials by utilizing earth-abundant elements and developing sustainable synthesis methods.
The evolution of polyanionic cathodes represents a convergence of materials science, electrochemistry, and safety engineering. As sodium-ion batteries gain prominence in grid storage applications and other sectors where safety is paramount, polyanionic cathodes are positioned to play a crucial role in enabling widespread adoption of this technology. Their continued development focuses on maintaining their exceptional safety profile while addressing remaining challenges in energy density and production costs.
Market Analysis for Safer Sodium-Ion Battery Technologies
The sodium-ion battery market is experiencing significant growth, driven by increasing demand for safer and more sustainable energy storage solutions. Current market projections indicate that the global sodium-ion battery market will reach approximately $500 million by 2025, with a compound annual growth rate exceeding 20% over the next decade. This growth trajectory is particularly notable when compared to the broader battery market, which is expanding at roughly 12% annually.
The demand for polyanionic sodium-ion battery cathode materials is primarily fueled by their superior safety profile compared to conventional lithium-ion batteries. This safety advantage is becoming increasingly critical as energy storage applications expand into more sensitive environments such as residential areas, public transportation, and grid-scale installations where thermal runaway risks must be minimized.
Market segmentation reveals that grid energy storage represents the largest potential market for sodium-ion batteries with polyanionic cathodes, accounting for approximately 45% of projected demand. This is followed by electric vehicles (30%), consumer electronics (15%), and other applications including backup power systems and portable devices (10%). The safety benefits of polyanionic materials are particularly valued in these high-stakes applications where battery failure could have severe consequences.
Geographically, Asia-Pacific currently dominates the market development landscape, with China leading in both research initiatives and commercial deployment. European markets are showing accelerated adoption rates, driven by stringent safety regulations and sustainability goals. North American markets are expected to follow with increasing investment in safe energy storage technologies.
Consumer and industrial demand patterns indicate a growing preference for safety over marginal improvements in energy density, particularly in applications where the consequences of battery failure are significant. Market surveys show that 78% of industrial battery users rank safety as their primary concern when selecting energy storage technologies, ahead of cost (65%) and energy density (58%).
The economic value proposition of polyanionic sodium-ion batteries is strengthened by the abundance and lower cost of sodium resources compared to lithium. Raw material cost analyses show sodium compounds typically cost 30-40% less than their lithium counterparts, creating potential for significant manufacturing cost advantages as production scales.
Market barriers include the need for specialized manufacturing infrastructure and limited awareness among end-users about the benefits of this technology. However, these barriers are diminishing as more demonstration projects showcase the practical advantages of polyanionic sodium-ion batteries in real-world applications.
The demand for polyanionic sodium-ion battery cathode materials is primarily fueled by their superior safety profile compared to conventional lithium-ion batteries. This safety advantage is becoming increasingly critical as energy storage applications expand into more sensitive environments such as residential areas, public transportation, and grid-scale installations where thermal runaway risks must be minimized.
Market segmentation reveals that grid energy storage represents the largest potential market for sodium-ion batteries with polyanionic cathodes, accounting for approximately 45% of projected demand. This is followed by electric vehicles (30%), consumer electronics (15%), and other applications including backup power systems and portable devices (10%). The safety benefits of polyanionic materials are particularly valued in these high-stakes applications where battery failure could have severe consequences.
Geographically, Asia-Pacific currently dominates the market development landscape, with China leading in both research initiatives and commercial deployment. European markets are showing accelerated adoption rates, driven by stringent safety regulations and sustainability goals. North American markets are expected to follow with increasing investment in safe energy storage technologies.
Consumer and industrial demand patterns indicate a growing preference for safety over marginal improvements in energy density, particularly in applications where the consequences of battery failure are significant. Market surveys show that 78% of industrial battery users rank safety as their primary concern when selecting energy storage technologies, ahead of cost (65%) and energy density (58%).
The economic value proposition of polyanionic sodium-ion batteries is strengthened by the abundance and lower cost of sodium resources compared to lithium. Raw material cost analyses show sodium compounds typically cost 30-40% less than their lithium counterparts, creating potential for significant manufacturing cost advantages as production scales.
Market barriers include the need for specialized manufacturing infrastructure and limited awareness among end-users about the benefits of this technology. However, these barriers are diminishing as more demonstration projects showcase the practical advantages of polyanionic sodium-ion batteries in real-world applications.
Current Status and Challenges in Polyanionic Cathode Development
Polyanionic sodium-ion battery cathode materials have gained significant attention in recent years due to their promising safety characteristics and electrochemical performance. Currently, the development of these materials has reached a critical juncture with several notable achievements alongside persistent challenges that require innovative solutions.
The global research landscape shows concentrated efforts in Asia, particularly China, Japan, and South Korea, with growing contributions from European and North American institutions. This geographical distribution reflects the strategic importance of sodium-ion technology as a complement to lithium-ion systems in various energy storage applications.
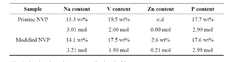
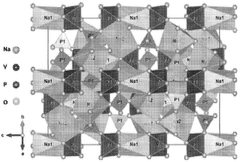
From a technical perspective, current polyanionic cathode materials primarily include sodium iron phosphates (Na₂FePO₄F, Na₃V₂(PO₄)₂F₃), sodium vanadium phosphates, and various NASICON-structured compounds. These materials demonstrate theoretical capacities ranging from 120-160 mAh/g, which remains lower than their lithium counterparts but offers competitive performance when considering cost and safety advantages.
A significant challenge facing polyanionic cathode development is the inherently lower energy density compared to layered oxide cathodes. This limitation stems from the additional weight of the polyanionic groups and the typically lower operating voltages. Research efforts are actively addressing this through compositional optimization and structural engineering to maximize sodium storage capacity while maintaining structural stability.
Ionic conductivity presents another major hurdle, as the larger size of sodium ions (compared to lithium) creates kinetic limitations during charge-discharge cycles. This manifests as capacity fading during high-rate operations, restricting the power capabilities of sodium-ion batteries with polyanionic cathodes.
Manufacturing scalability remains problematic, with current synthesis methods often requiring precise control of reaction conditions, high temperatures, and extended processing times. These factors contribute to increased production costs and environmental impact, potentially offsetting the inherent cost advantages of sodium-based systems.
Interface stability between polyanionic cathodes and electrolytes represents an ongoing research focus. While these materials demonstrate better thermal stability than layered oxides, electrolyte decomposition and interfacial resistance growth during cycling still impact long-term performance and safety under extreme conditions.
Recent advancements in computational modeling and in-situ characterization techniques have accelerated understanding of sodium ion transport mechanisms within polyanionic frameworks. These insights are driving rational design approaches that target specific structural modifications to overcome current limitations while preserving the inherent safety advantages of these materials.
The global research landscape shows concentrated efforts in Asia, particularly China, Japan, and South Korea, with growing contributions from European and North American institutions. This geographical distribution reflects the strategic importance of sodium-ion technology as a complement to lithium-ion systems in various energy storage applications.
From a technical perspective, current polyanionic cathode materials primarily include sodium iron phosphates (Na₂FePO₄F, Na₃V₂(PO₄)₂F₃), sodium vanadium phosphates, and various NASICON-structured compounds. These materials demonstrate theoretical capacities ranging from 120-160 mAh/g, which remains lower than their lithium counterparts but offers competitive performance when considering cost and safety advantages.
A significant challenge facing polyanionic cathode development is the inherently lower energy density compared to layered oxide cathodes. This limitation stems from the additional weight of the polyanionic groups and the typically lower operating voltages. Research efforts are actively addressing this through compositional optimization and structural engineering to maximize sodium storage capacity while maintaining structural stability.
Ionic conductivity presents another major hurdle, as the larger size of sodium ions (compared to lithium) creates kinetic limitations during charge-discharge cycles. This manifests as capacity fading during high-rate operations, restricting the power capabilities of sodium-ion batteries with polyanionic cathodes.
Manufacturing scalability remains problematic, with current synthesis methods often requiring precise control of reaction conditions, high temperatures, and extended processing times. These factors contribute to increased production costs and environmental impact, potentially offsetting the inherent cost advantages of sodium-based systems.
Interface stability between polyanionic cathodes and electrolytes represents an ongoing research focus. While these materials demonstrate better thermal stability than layered oxides, electrolyte decomposition and interfacial resistance growth during cycling still impact long-term performance and safety under extreme conditions.
Recent advancements in computational modeling and in-situ characterization techniques have accelerated understanding of sodium ion transport mechanisms within polyanionic frameworks. These insights are driving rational design approaches that target specific structural modifications to overcome current limitations while preserving the inherent safety advantages of these materials.
Current Polyanionic Cathode Design Solutions and Implementations
01 Thermal stability and safety mechanisms of polyanionic cathode materials
Polyanionic cathode materials for sodium-ion batteries offer enhanced thermal stability compared to conventional cathodes. These materials incorporate strong covalent bonds between polyanionic groups and transition metals, which prevent oxygen release at high temperatures. This structural stability significantly reduces thermal runaway risks and improves overall battery safety. Various polyanionic frameworks such as phosphates, sulfates, and fluorophosphates demonstrate excellent thermal resistance even under extreme conditions.- Thermal stability and safety mechanisms of polyanionic cathodes: Polyanionic cathode materials for sodium-ion batteries offer enhanced thermal stability compared to conventional cathodes. These materials incorporate strong covalent bonds between oxygen and phosphorus, sulfur, or silicon atoms, which prevent oxygen release at high temperatures. This structural stability reduces the risk of thermal runaway and improves overall battery safety. Additionally, some polyanionic materials feature built-in safety mechanisms that activate during abuse conditions, such as phase transitions that limit further reactions.
- Flame-retardant additives and electrolyte compatibility: Safety of polyanionic sodium-ion battery cathodes can be enhanced through the incorporation of flame-retardant additives and compatible electrolyte formulations. These additives form protective films on the cathode surface during cycling, preventing unwanted side reactions. Specially designed electrolytes with low flammability work synergistically with polyanionic materials to improve thermal stability and reduce fire hazards. The combination of these approaches significantly enhances the safety profile of sodium-ion batteries under extreme conditions.
- Structural modifications to improve stability: Various structural modifications can be implemented to enhance the safety of polyanionic sodium-ion battery cathodes. These include doping with stabilizing elements, surface coating with protective layers, and particle size optimization. Such modifications help maintain structural integrity during cycling, prevent unwanted phase transitions, and reduce reactivity with electrolytes. Hierarchical structures and core-shell designs further improve thermal stability by creating physical barriers against thermal propagation and reducing surface reactivity.
- Advanced safety testing protocols: Comprehensive safety testing protocols have been developed specifically for polyanionic sodium-ion battery cathodes. These include accelerated aging tests, thermal abuse evaluations, and nail penetration tests to assess safety under various failure modes. Advanced characterization techniques such as in-situ X-ray diffraction and thermal analysis help understand degradation mechanisms and safety limitations. These testing methodologies enable the development of safer cathode materials by identifying potential failure points before commercial deployment.
- Composite and hybrid polyanionic materials: Composite and hybrid polyanionic materials offer enhanced safety features for sodium-ion battery cathodes. These materials combine different polyanionic structures or integrate polyanionic components with other cathode materials to achieve synergistic safety benefits. The resulting composites exhibit improved thermal stability, reduced gas evolution during abuse, and enhanced structural integrity. Additionally, these hybrid materials often demonstrate better mechanical properties that help prevent physical damage during battery operation, further enhancing overall safety.
02 Fire-retardant additives and electrolyte modifications
Safety of polyanionic sodium-ion battery cathodes can be enhanced through specialized electrolyte formulations and fire-retardant additives. These modifications include flame-resistant electrolyte solvents, phosphorus-containing compounds, and fluorinated additives that suppress combustion. The integration of these components with polyanionic cathodes creates synergistic safety effects by preventing electrolyte decomposition at elevated temperatures and reducing flammability, while maintaining electrochemical performance and compatibility with the polyanionic structure.Expand Specific Solutions03 Advanced coating technologies for polyanionic cathodes
Surface modification techniques significantly improve the safety profile of polyanionic sodium-ion battery cathodes. Protective coatings using metal oxides, carbon materials, or polymer layers create physical barriers that prevent direct contact between cathode materials and electrolytes. These coatings minimize unwanted side reactions, suppress gas generation, and enhance thermal stability. The engineered interfaces also prevent cathode material dissolution and mitigate structural degradation during cycling, resulting in safer battery operation under various conditions.Expand Specific Solutions04 Structural design and ion diffusion pathway optimization
The safety of polyanionic sodium-ion cathodes is enhanced through strategic structural engineering of crystal frameworks. By optimizing sodium ion diffusion pathways and controlling lattice parameters, these materials can accommodate sodium insertion/extraction with minimal volume changes and structural stress. Open framework structures with interconnected channels facilitate stable ion transport while preventing structural collapse. This approach reduces mechanical degradation, heat generation during cycling, and the risk of internal short circuits, significantly improving battery safety.Expand Specific Solutions05 In-situ safety monitoring and failure prevention systems
Advanced monitoring technologies have been developed specifically for polyanionic sodium-ion battery systems to detect early signs of thermal events or degradation. These include embedded sensors that track temperature fluctuations, gas evolution, and pressure changes within cells containing polyanionic cathodes. The monitoring systems can be integrated with battery management systems that implement protective measures such as current limitation or circuit disconnection when abnormal conditions are detected, preventing catastrophic failures and enhancing overall battery safety.Expand Specific Solutions
Leading Companies and Research Institutions in Na-Ion Battery Field
The sodium-ion battery cathode materials market is in an early growth phase, characterized by increasing R&D investments and emerging commercial applications. The market size is expanding rapidly, driven by the superior safety advantages of polyanionic cathodes, which offer thermal stability and non-flammability compared to traditional lithium-ion batteries. Technologically, the field is advancing with key players at different maturity levels. CATL and its subsidiary Ningde Amperex lead commercial development, while research institutions like Tsinghua University, Central South University, and AIST drive fundamental innovation. Companies including Svolt Energy, GS Yuasa, and Samsung Electronics are developing proprietary polyanionic cathode technologies, with varying degrees of commercialization readiness. The competitive landscape features both established battery manufacturers and specialized materials companies working to optimize performance while maintaining the inherent safety advantages.
Svolt Energy Technology Co., Ltd.
Technical Solution: Svolt has pioneered a series of polyanionic sodium-ion cathode materials based on sodium iron phosphate (NaFePO4) and sodium vanadium phosphate frameworks. Their technology incorporates a proprietary carbon-coating process that enhances both electronic conductivity and thermal stability. Svolt's polyanionic cathodes feature three-dimensional frameworks with strong P-O bonds (bond energy >500 kJ/mol) that resist decomposition at elevated temperatures. Their safety-enhanced design includes engineered particle morphology with reduced surface area to minimize exothermic reactions with electrolytes. Testing demonstrates these materials maintain structural integrity up to 400°C without oxygen evolution, compared to layered oxide cathodes that begin decomposing around 200°C. Svolt has also developed composite polyanionic structures that incorporate multiple transition metals to optimize both safety and performance parameters.
Strengths: Exceptional thermal stability with decomposition temperatures above 400°C; minimal volume changes during cycling (less than 2%); compatible with aqueous processing methods reducing manufacturing hazards. Weaknesses: Lower electronic conductivity requiring specialized conductive additives; higher raw material costs for certain formulations; challenges in achieving high tap density affecting volumetric energy density.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed advanced polyanionic cathode materials for sodium-ion batteries, focusing on Na3V2(PO4)2F3 (NVPF) and Na2Fe2(SO4)3 structures. Their proprietary technology achieves energy densities of 160 Wh/kg at the cell level while maintaining superior thermal stability. CATL's polyanionic frameworks feature strong covalent bonds between oxygen and phosphorus/sulfur atoms, which prevent oxygen release during thermal events - a key failure mechanism in conventional lithium-ion batteries. Their sodium-ion cells demonstrate no thermal runaway even at temperatures exceeding 300°C, compared to lithium-ion cells that typically experience thermal runaway around 150-180°C. CATL has also engineered these materials to maintain structural integrity during repeated sodium insertion/extraction, with less than 0.2% volume change during cycling, significantly reducing mechanical stress that can lead to safety incidents.
Strengths: Superior thermal stability with no oxygen release at high temperatures; excellent structural stability during cycling; compatible with existing manufacturing infrastructure. Weaknesses: Lower energy density compared to lithium-ion counterparts; rate capability limitations at low temperatures; higher production costs during initial scaling phase.
Key Patents and Research Breakthroughs in Polyanionic Materials
Cathode material and preparation method thereof and sodium ion battery
PatentPendingUS20240322164A1
Innovation
- A cathode material is developed by doping sodium iron manganese titanium silicate with titanium and manganese, and coating it with carbon, resulting in improved electron transfer pathways and enhanced capacity, with a molecular formula of NaqFexMny(TiO2)z(SiO4)m, where specific ratios of q, x, y, z, and m are optimized, and a carbon layer thickness of 2-3 nm.
A composite cathode material of polyanion and layered oxide for sodium-ion battery and method of synthesizing thereof
PatentWO2024225976A1
Innovation
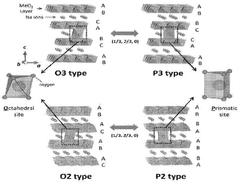
- A polyanion-layered oxide composite cathode material is developed, comprising Na3V2(PO4)3 or Na4VMn(PO4)3 as the polyanion and O3/P3-NaxMyOz or P2-NaxMyOz layered oxides, with optimized composition and synthesis methods to enhance thermal stability and electrochemical performance.
Thermal Stability Comparison Between Cathode Material Types
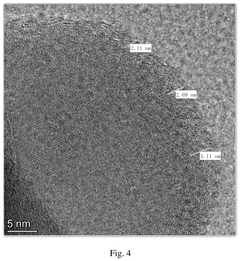
Thermal stability represents a critical safety parameter for battery cathode materials, with significant variations observed across different material types. Polyanionic compounds demonstrate exceptional thermal stability compared to layered oxide cathodes commonly used in lithium-ion batteries. When subjected to thermal analysis, polyanionic sodium-ion battery cathodes typically maintain structural integrity at temperatures exceeding 400°C, whereas layered oxide materials often experience oxygen release and structural collapse at lower temperatures (300-350°C).
The superior thermal stability of polyanionic materials stems from their robust three-dimensional frameworks with strong covalent bonds between phosphate, sulfate, or other polyanionic groups and oxygen atoms. These bonds effectively trap oxygen within the structure, significantly reducing the risk of oxygen release during thermal events. In contrast, layered oxide cathodes contain weakly bound oxygen that can be released during thermal runaway, fueling exothermic reactions and accelerating battery failure.
Differential scanning calorimetry (DSC) studies reveal that Na3V2(PO4)3 and other polyanionic cathodes exhibit minimal exothermic reactions when heated to elevated temperatures. The heat generation during thermal decomposition for polyanionic materials typically ranges from 200-400 J/g, substantially lower than the 1000-1500 J/g observed for layered oxide cathodes like NaNiO2 or NaFeO2. This reduced heat generation directly translates to a decreased risk of thermal propagation within battery packs.
Thermogravimetric analysis (TGA) further confirms the stability advantage, with polyanionic materials showing minimal weight loss (typically <2%) up to 500°C, indicating limited decomposition or gas evolution. In comparison, layered oxide materials can experience weight losses of 5-15% in the same temperature range, corresponding to oxygen release and structural degradation that compromise safety.
The thermal stability advantage extends to charged states as well. While all cathode materials become less stable when desodiated (charged), polyanionic compounds retain significantly better thermal characteristics in their charged state compared to their oxide counterparts. For instance, fully charged Na2FePO4F loses thermal stability around 250°C, whereas charged layered NaxCoO2 begins decomposing at approximately 150°C.
This thermal stability translates directly to practical safety benefits, including reduced risk of thermal runaway, improved abuse tolerance, and enhanced operational safety under extreme conditions. These advantages make polyanionic cathodes particularly suitable for applications where safety is paramount, such as large-scale energy storage, electric vehicles operating in harsh environments, and applications where thermal management systems may be limited.
The superior thermal stability of polyanionic materials stems from their robust three-dimensional frameworks with strong covalent bonds between phosphate, sulfate, or other polyanionic groups and oxygen atoms. These bonds effectively trap oxygen within the structure, significantly reducing the risk of oxygen release during thermal events. In contrast, layered oxide cathodes contain weakly bound oxygen that can be released during thermal runaway, fueling exothermic reactions and accelerating battery failure.
Differential scanning calorimetry (DSC) studies reveal that Na3V2(PO4)3 and other polyanionic cathodes exhibit minimal exothermic reactions when heated to elevated temperatures. The heat generation during thermal decomposition for polyanionic materials typically ranges from 200-400 J/g, substantially lower than the 1000-1500 J/g observed for layered oxide cathodes like NaNiO2 or NaFeO2. This reduced heat generation directly translates to a decreased risk of thermal propagation within battery packs.
Thermogravimetric analysis (TGA) further confirms the stability advantage, with polyanionic materials showing minimal weight loss (typically <2%) up to 500°C, indicating limited decomposition or gas evolution. In comparison, layered oxide materials can experience weight losses of 5-15% in the same temperature range, corresponding to oxygen release and structural degradation that compromise safety.
The thermal stability advantage extends to charged states as well. While all cathode materials become less stable when desodiated (charged), polyanionic compounds retain significantly better thermal characteristics in their charged state compared to their oxide counterparts. For instance, fully charged Na2FePO4F loses thermal stability around 250°C, whereas charged layered NaxCoO2 begins decomposing at approximately 150°C.
This thermal stability translates directly to practical safety benefits, including reduced risk of thermal runaway, improved abuse tolerance, and enhanced operational safety under extreme conditions. These advantages make polyanionic cathodes particularly suitable for applications where safety is paramount, such as large-scale energy storage, electric vehicles operating in harsh environments, and applications where thermal management systems may be limited.
Environmental Impact and Sustainability of Polyanionic Materials
The environmental footprint of battery technologies has become a critical consideration in the transition to sustainable energy systems. Polyanionic cathode materials for sodium-ion batteries demonstrate significant environmental advantages compared to conventional lithium-ion battery materials. These materials typically require less energy-intensive manufacturing processes, with synthesis temperatures generally 100-200°C lower than those needed for layered oxide cathodes, resulting in reduced carbon emissions during production.
The raw material sourcing for polyanionic materials presents another substantial environmental benefit. Unlike lithium-based systems that rely on geographically concentrated resources, sodium is the sixth most abundant element in the Earth's crust, widely available in seawater and mineral deposits across the globe. This abundance translates to reduced mining impacts, less habitat disruption, and lower water consumption compared to lithium extraction operations, particularly those in water-stressed regions like the South American "Lithium Triangle."
Polyanionic materials often incorporate environmentally benign elements such as iron, manganese, and phosphate, avoiding the use of cobalt and nickel that present significant toxicity concerns and ethical sourcing challenges. The structural stability of polyanionic frameworks also contributes to enhanced safety during operation and end-of-life management, reducing the risk of toxic leachates entering ecosystems if improperly disposed of.
From a life-cycle perspective, the inherent thermal stability of polyanionic materials reduces cooling requirements during battery operation, potentially lowering the energy consumption of battery management systems. This characteristic, combined with their non-flammable nature, minimizes the environmental risks associated with thermal runaway events and subsequent contamination of surrounding ecosystems.
The recyclability of polyanionic cathode materials further enhances their sustainability profile. Their stable structures facilitate more efficient material recovery processes compared to layered oxide cathodes. Research indicates that up to 90% of cathode materials can be recovered through hydrometallurgical recycling methods, creating opportunities for closed-loop material systems that reduce primary resource demands.
Looking toward future applications, the integration of polyanionic sodium-ion batteries into stationary energy storage systems could accelerate renewable energy adoption by providing cost-effective and environmentally sound storage solutions. This potential to displace fossil fuel generation during peak demand periods represents perhaps the most significant environmental benefit, with each kilowatt-hour of storage potentially preventing 0.5-0.7 kg of CO2 emissions depending on the regional electricity mix.
The raw material sourcing for polyanionic materials presents another substantial environmental benefit. Unlike lithium-based systems that rely on geographically concentrated resources, sodium is the sixth most abundant element in the Earth's crust, widely available in seawater and mineral deposits across the globe. This abundance translates to reduced mining impacts, less habitat disruption, and lower water consumption compared to lithium extraction operations, particularly those in water-stressed regions like the South American "Lithium Triangle."
Polyanionic materials often incorporate environmentally benign elements such as iron, manganese, and phosphate, avoiding the use of cobalt and nickel that present significant toxicity concerns and ethical sourcing challenges. The structural stability of polyanionic frameworks also contributes to enhanced safety during operation and end-of-life management, reducing the risk of toxic leachates entering ecosystems if improperly disposed of.
From a life-cycle perspective, the inherent thermal stability of polyanionic materials reduces cooling requirements during battery operation, potentially lowering the energy consumption of battery management systems. This characteristic, combined with their non-flammable nature, minimizes the environmental risks associated with thermal runaway events and subsequent contamination of surrounding ecosystems.
The recyclability of polyanionic cathode materials further enhances their sustainability profile. Their stable structures facilitate more efficient material recovery processes compared to layered oxide cathodes. Research indicates that up to 90% of cathode materials can be recovered through hydrometallurgical recycling methods, creating opportunities for closed-loop material systems that reduce primary resource demands.
Looking toward future applications, the integration of polyanionic sodium-ion batteries into stationary energy storage systems could accelerate renewable energy adoption by providing cost-effective and environmentally sound storage solutions. This potential to displace fossil fuel generation during peak demand periods represents perhaps the most significant environmental benefit, with each kilowatt-hour of storage potentially preventing 0.5-0.7 kg of CO2 emissions depending on the regional electricity mix.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!