Sodium-Ion Battery Cathode Materials Interface Stability with Solid Electrolytes
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium-Ion Battery Cathode Interface Evolution
The evolution of cathode-electrolyte interfaces in sodium-ion batteries represents a critical aspect of their overall performance and longevity. When examining solid electrolyte implementations specifically, the interface undergoes dynamic changes during cycling that significantly impact battery functionality. Initially, upon cell assembly, a pristine interface exists between cathode materials and solid electrolytes, characterized by direct physical contact with minimal chemical interaction.
During the first few cycles, a nascent solid electrolyte interphase (SEI) layer begins to form as electrochemical reactions initiate decomposition of electrolyte components at the cathode surface. This early-stage interface is typically thin and inconsistent, with composition heavily dependent on the specific cathode material and solid electrolyte pairing. Common cathode materials such as layered oxides (NaxMO2), polyanionic compounds, and Prussian blue analogs each demonstrate distinctive interface evolution patterns.
As cycling continues, the interface layer grows and stabilizes, developing a more complex structure. For layered oxide cathodes, the interface often exhibits sodium-depleted regions near the surface, while polyanionic cathodes typically form more stable interfaces due to their robust frameworks. The interface thickness generally increases during early cycles before reaching a quasi-equilibrium state, typically ranging from 5-50 nm depending on the materials system.
Temperature fluctuations significantly impact interface evolution, with elevated temperatures accelerating both beneficial passivation processes and detrimental side reactions. At temperatures above 60°C, many solid electrolyte systems show accelerated interface degradation, while low temperatures can inhibit proper SEI formation, leading to increased impedance.
Mechanical stresses at the interface present another critical evolution factor. Volume changes during sodium insertion/extraction create microcracks and delamination between cathode particles and solid electrolytes. These mechanical failures progressively worsen during cycling, creating new reactive surfaces and pathways for unwanted side reactions.
Long-term aging effects manifest as continuous but slower interface evolution, including gradual sodium depletion at interfaces, crystallographic transformations of interface compounds, and accumulation of insulating byproducts. Advanced characterization techniques including in-situ XPS, cryo-TEM, and TOF-SIMS have revealed that interface composition becomes increasingly heterogeneous over extended cycling.
Recent research has demonstrated that interface engineering approaches, such as artificial SEI layers and gradient composition cathodes, can beneficially direct interface evolution pathways. These interventions aim to promote formation of more ionically conductive and mechanically robust interfaces that maintain stability over thousands of cycles rather than degrading progressively.
During the first few cycles, a nascent solid electrolyte interphase (SEI) layer begins to form as electrochemical reactions initiate decomposition of electrolyte components at the cathode surface. This early-stage interface is typically thin and inconsistent, with composition heavily dependent on the specific cathode material and solid electrolyte pairing. Common cathode materials such as layered oxides (NaxMO2), polyanionic compounds, and Prussian blue analogs each demonstrate distinctive interface evolution patterns.
As cycling continues, the interface layer grows and stabilizes, developing a more complex structure. For layered oxide cathodes, the interface often exhibits sodium-depleted regions near the surface, while polyanionic cathodes typically form more stable interfaces due to their robust frameworks. The interface thickness generally increases during early cycles before reaching a quasi-equilibrium state, typically ranging from 5-50 nm depending on the materials system.
Temperature fluctuations significantly impact interface evolution, with elevated temperatures accelerating both beneficial passivation processes and detrimental side reactions. At temperatures above 60°C, many solid electrolyte systems show accelerated interface degradation, while low temperatures can inhibit proper SEI formation, leading to increased impedance.
Mechanical stresses at the interface present another critical evolution factor. Volume changes during sodium insertion/extraction create microcracks and delamination between cathode particles and solid electrolytes. These mechanical failures progressively worsen during cycling, creating new reactive surfaces and pathways for unwanted side reactions.
Long-term aging effects manifest as continuous but slower interface evolution, including gradual sodium depletion at interfaces, crystallographic transformations of interface compounds, and accumulation of insulating byproducts. Advanced characterization techniques including in-situ XPS, cryo-TEM, and TOF-SIMS have revealed that interface composition becomes increasingly heterogeneous over extended cycling.
Recent research has demonstrated that interface engineering approaches, such as artificial SEI layers and gradient composition cathodes, can beneficially direct interface evolution pathways. These interventions aim to promote formation of more ionically conductive and mechanically robust interfaces that maintain stability over thousands of cycles rather than degrading progressively.
Market Demand Analysis for Na-Ion Battery Technologies
The global energy storage market is witnessing a significant shift towards sustainable and cost-effective solutions, creating substantial demand for sodium-ion battery technologies. This demand is primarily driven by the increasing cost and supply constraints of lithium resources, which have traditionally dominated the rechargeable battery market. Industry analysts project the sodium-ion battery market to grow at a compound annual growth rate of over 20% between 2023 and 2030, with the market value expected to reach several billion dollars by the end of the decade.
The demand for sodium-ion batteries with solid electrolytes is particularly pronounced in grid-scale energy storage applications, where cost considerations often outweigh energy density requirements. Utility companies and renewable energy providers are actively seeking alternatives to lithium-ion technologies to reduce dependency on volatile lithium supply chains and mitigate geopolitical risks associated with critical mineral resources.
Electric vehicle manufacturers, especially those targeting the mid-range and economy segments, represent another significant market for sodium-ion battery technologies. The automotive industry's push towards electrification has created immense pressure on battery supply chains, with sodium-ion batteries emerging as a complementary technology to lithium-ion batteries for specific vehicle categories and markets where cost sensitivity is paramount.
Consumer electronics manufacturers are also exploring sodium-ion batteries with enhanced cathode-electrolyte interface stability as potential alternatives for certain product categories. While the energy density limitations currently restrict their application in premium devices, the technology shows promise for products where battery replacement cost and sustainability are key considerations.
Emerging markets, particularly in regions with limited access to lithium resources but abundant sodium reserves, are showing strong interest in developing localized sodium-ion battery production capabilities. Countries like India, Brazil, and several African nations are investing in research and development of sodium-ion technologies to reduce import dependency and create domestic battery manufacturing ecosystems.
The industrial sector represents another growing market segment, with applications in backup power systems, material handling equipment, and stationary energy storage. The lower fire risk associated with many sodium-ion chemistries compared to certain lithium-ion variants makes them particularly attractive for indoor industrial applications where safety considerations are paramount.
Market research indicates that customers across these segments are increasingly prioritizing total cost of ownership over initial acquisition costs, creating favorable conditions for sodium-ion technologies that offer longer cycle life and reduced maintenance requirements through improved cathode-solid electrolyte interface stability.
The demand for sodium-ion batteries with solid electrolytes is particularly pronounced in grid-scale energy storage applications, where cost considerations often outweigh energy density requirements. Utility companies and renewable energy providers are actively seeking alternatives to lithium-ion technologies to reduce dependency on volatile lithium supply chains and mitigate geopolitical risks associated with critical mineral resources.
Electric vehicle manufacturers, especially those targeting the mid-range and economy segments, represent another significant market for sodium-ion battery technologies. The automotive industry's push towards electrification has created immense pressure on battery supply chains, with sodium-ion batteries emerging as a complementary technology to lithium-ion batteries for specific vehicle categories and markets where cost sensitivity is paramount.
Consumer electronics manufacturers are also exploring sodium-ion batteries with enhanced cathode-electrolyte interface stability as potential alternatives for certain product categories. While the energy density limitations currently restrict their application in premium devices, the technology shows promise for products where battery replacement cost and sustainability are key considerations.
Emerging markets, particularly in regions with limited access to lithium resources but abundant sodium reserves, are showing strong interest in developing localized sodium-ion battery production capabilities. Countries like India, Brazil, and several African nations are investing in research and development of sodium-ion technologies to reduce import dependency and create domestic battery manufacturing ecosystems.
The industrial sector represents another growing market segment, with applications in backup power systems, material handling equipment, and stationary energy storage. The lower fire risk associated with many sodium-ion chemistries compared to certain lithium-ion variants makes them particularly attractive for indoor industrial applications where safety considerations are paramount.
Market research indicates that customers across these segments are increasingly prioritizing total cost of ownership over initial acquisition costs, creating favorable conditions for sodium-ion technologies that offer longer cycle life and reduced maintenance requirements through improved cathode-solid electrolyte interface stability.
Current Challenges in Na-Ion/Solid Electrolyte Interfaces
The interface between sodium-ion battery cathode materials and solid electrolytes represents one of the most critical challenges in advancing solid-state sodium battery technology. Unlike their lithium counterparts, sodium-ion systems exhibit unique interfacial behaviors that significantly impact battery performance, cycling stability, and overall energy density. The larger ionic radius of Na+ (1.02 Å) compared to Li+ (0.76 Å) creates fundamental differences in ion transport mechanisms across these interfaces.
A primary challenge at these interfaces is the formation of high impedance layers that restrict sodium ion migration. These resistive interlayers often develop during initial battery assembly or during early cycling stages, resulting from chemical reactions between cathode active materials and solid electrolytes. Particularly problematic is the reactivity between transition metal oxides commonly used as cathode materials (such as NaxMO2 where M represents transition metals) and sulfide-based solid electrolytes, which can form insulating phases at the interface.
Mechanical instability presents another significant hurdle. Volume changes during sodium insertion/extraction cycles create mechanical stresses at the cathode-electrolyte interface, leading to contact loss and increased interfacial resistance. This issue is exacerbated by the larger volume changes associated with sodium intercalation compared to lithium systems, making interface engineering particularly challenging for Na-ion solid-state batteries.
Space charge layers at these interfaces further complicate ion transport. The accumulation of charged species at the cathode-electrolyte boundary creates electric fields that impede sodium ion movement. This phenomenon is particularly pronounced in oxide-based solid electrolytes when paired with high-voltage cathode materials, resulting in significant polarization losses during battery operation.
Chemical compatibility between cathode materials and solid electrolytes remains problematic across multiple material combinations. Many promising sodium cathode materials contain elements that readily react with solid electrolytes at the processing temperatures required for good interfacial contact. For instance, oxide cathodes often react with sulfide electrolytes, while phosphate-based cathodes may decompose when in contact with highly reducing solid electrolytes.
The kinetics of sodium ion transfer across these interfaces is inherently slower than in lithium systems due to the larger size and different coordination preferences of sodium ions. This results in higher activation barriers for ion movement across the interface, limiting rate capability and power density of solid-state sodium batteries, particularly at room temperature or below.
A primary challenge at these interfaces is the formation of high impedance layers that restrict sodium ion migration. These resistive interlayers often develop during initial battery assembly or during early cycling stages, resulting from chemical reactions between cathode active materials and solid electrolytes. Particularly problematic is the reactivity between transition metal oxides commonly used as cathode materials (such as NaxMO2 where M represents transition metals) and sulfide-based solid electrolytes, which can form insulating phases at the interface.
Mechanical instability presents another significant hurdle. Volume changes during sodium insertion/extraction cycles create mechanical stresses at the cathode-electrolyte interface, leading to contact loss and increased interfacial resistance. This issue is exacerbated by the larger volume changes associated with sodium intercalation compared to lithium systems, making interface engineering particularly challenging for Na-ion solid-state batteries.
Space charge layers at these interfaces further complicate ion transport. The accumulation of charged species at the cathode-electrolyte boundary creates electric fields that impede sodium ion movement. This phenomenon is particularly pronounced in oxide-based solid electrolytes when paired with high-voltage cathode materials, resulting in significant polarization losses during battery operation.
Chemical compatibility between cathode materials and solid electrolytes remains problematic across multiple material combinations. Many promising sodium cathode materials contain elements that readily react with solid electrolytes at the processing temperatures required for good interfacial contact. For instance, oxide cathodes often react with sulfide electrolytes, while phosphate-based cathodes may decompose when in contact with highly reducing solid electrolytes.
The kinetics of sodium ion transfer across these interfaces is inherently slower than in lithium systems due to the larger size and different coordination preferences of sodium ions. This results in higher activation barriers for ion movement across the interface, limiting rate capability and power density of solid-state sodium batteries, particularly at room temperature or below.
Current Interface Stabilization Strategies
01 Protective coating strategies for cathode interfaces
Various coating materials can be applied to sodium-ion battery cathode surfaces to enhance interface stability. These coatings act as protective barriers against electrolyte decomposition and prevent unwanted side reactions at the cathode-electrolyte interface. Common coating materials include metal oxides, fluorides, and carbon-based materials that improve cycling stability while maintaining good ionic conductivity. These protective layers effectively mitigate structural degradation and dissolution of active materials during charge-discharge cycles.- Protective coating strategies for cathode materials: Various protective coating materials can be applied to sodium-ion battery cathode surfaces to enhance interface stability. These coatings act as physical barriers against electrolyte attack and minimize unwanted side reactions at the cathode-electrolyte interface. Common coating materials include metal oxides, fluorides, and carbon-based materials that improve cycling stability while maintaining good ionic conductivity. These protective layers help prevent structural degradation and dissolution of active materials during charge-discharge cycles.
- Electrolyte additives for interface stabilization: Specific electrolyte additives can be incorporated to form stable solid electrolyte interphase (SEI) layers on cathode surfaces. These additives react preferentially at the cathode-electrolyte interface to create protective films that prevent continuous electrolyte decomposition while allowing sodium ion transport. The carefully designed additives help mitigate parasitic reactions, reduce gas generation, and enhance the overall electrochemical performance and longevity of sodium-ion batteries.
- Doping and elemental substitution techniques: Strategic doping of cathode materials with various elements can significantly improve interface stability in sodium-ion batteries. Introducing dopants like transition metals, rare earth elements, or non-metals can modify the electronic structure, strengthen chemical bonds, and enhance structural integrity of cathode materials. These modifications help suppress phase transitions during cycling, reduce lattice strain, and minimize dissolution of active components, resulting in more stable cathode-electrolyte interfaces and improved cycling performance.
- Surface modification and functionalization: Surface modification techniques beyond conventional coatings can be employed to enhance cathode interface stability. These include chemical functionalization, acid/base treatments, and creation of gradient composition surfaces. Such modifications alter the surface chemistry and physical properties of cathode materials to improve compatibility with electrolytes. These approaches help regulate interfacial reactions, enhance wettability, and create more stable interfaces that resist degradation during battery operation.
- Novel cathode material compositions: Development of novel cathode material compositions with inherently stable interfaces for sodium-ion batteries. These include layered oxides, polyanionic compounds, and Prussian blue analogs specifically designed to minimize structural changes during sodium insertion/extraction. The materials feature optimized crystal structures, controlled particle morphologies, and tailored surface properties that naturally resist degradation at the electrolyte interface. These innovative compositions demonstrate superior interface stability without requiring additional protective measures.
02 Electrolyte additives for interface stabilization
Specific electrolyte additives can be incorporated to form stable solid electrolyte interphase (SEI) layers on sodium-ion battery cathode surfaces. These additives react preferentially at the cathode interface to create protective films that prevent continuous electrolyte decomposition while allowing sodium ion transport. Functional additives include fluorinated compounds, boron-based materials, and certain organic molecules that contribute to interface stability by reducing parasitic reactions and improving the structural integrity of the cathode-electrolyte interface during cycling.Expand Specific Solutions03 Surface modification of cathode materials
Chemical and physical surface modifications can be applied to sodium-ion battery cathode materials to enhance interface stability. These modifications include doping with stabilizing elements, surface functionalization with specific chemical groups, and controlled surface reconstruction techniques. Such treatments alter the surface properties of cathode materials to reduce unwanted side reactions with the electrolyte, minimize structural changes during sodium insertion/extraction, and improve the overall electrochemical performance and cycling stability of the battery system.Expand Specific Solutions04 Novel cathode material compositions for enhanced stability
Innovative cathode material compositions can be designed specifically to address interface stability issues in sodium-ion batteries. These include layered oxide structures with optimized interlayer spacing, polyanionic compounds with strong structural frameworks, and composite materials that combine the advantages of different material classes. By carefully engineering the chemical composition and crystal structure of cathode materials, researchers can develop systems with inherently better resistance to structural degradation at interfaces, reduced volume changes during cycling, and improved compatibility with electrolytes.Expand Specific Solutions05 Interface characterization and stability mechanisms
Advanced analytical techniques and computational methods are employed to understand the fundamental mechanisms of interface stability in sodium-ion battery cathodes. These approaches include in-situ/operando spectroscopy, high-resolution microscopy, and molecular dynamics simulations that reveal degradation pathways and interfacial phenomena. By elucidating the chemical and electrochemical processes occurring at cathode interfaces, researchers can develop targeted strategies to mitigate degradation mechanisms, predict long-term stability, and design more effective interface engineering approaches for next-generation sodium-ion batteries.Expand Specific Solutions
Leading Research Groups and Industrial Players
The sodium-ion battery cathode materials interface stability with solid electrolytes market is in an early growth phase, characterized by intensive R&D activities and emerging commercial applications. The global market size is projected to expand significantly as sodium-ion technology presents a cost-effective alternative to lithium-ion batteries, particularly for grid storage applications. Technologically, the field remains in development with varying degrees of maturity across different approaches. Leading companies like BASF, Sumitomo Chemical, and Toyota Motor are investing heavily in advanced material development, while specialized players such as Beijing Easpring, Ningbo Ronbay, and ProLogium Technology are making significant progress in cathode-electrolyte interface engineering. Academic institutions including Nankai University and Beijing Institute of Technology are contributing fundamental research to address stability challenges, creating a competitive landscape balanced between established corporations and innovative startups.
Svolt Energy Technology Co., Ltd.
Technical Solution: Svolt has developed a comprehensive approach to sodium-ion battery cathode materials interface stability with solid electrolytes. Their technology focuses on a multi-layered interface engineering strategy that incorporates protective coatings on Prussian White cathode materials. The company utilizes aluminum oxide and lithium phosphorus oxynitride (LiPON) buffer layers to mitigate interfacial reactions between the cathode and solid electrolyte. Their research has demonstrated that these engineered interfaces can effectively suppress the formation of high-impedance interphases that typically form during cycling. Svolt's proprietary surface modification techniques have shown to reduce interfacial resistance by approximately 40% compared to unmodified interfaces, while maintaining stable cycling performance over 1000+ cycles. The company has also developed composite solid electrolytes with tailored mechanical properties to ensure good contact with cathode materials during volume changes, addressing a key challenge in solid-state sodium-ion batteries.
Strengths: Svolt's interface engineering approach effectively addresses the critical challenge of cathode-electrolyte interface stability while maintaining high ionic conductivity. Their multi-layer protection strategy provides both chemical and mechanical stability. Weaknesses: The complex multi-layer approach may increase manufacturing costs and complexity, potentially limiting commercial scalability. The long-term stability of these engineered interfaces under real-world operating conditions remains to be fully validated.
Prologium Technology Co. Ltd.
Technical Solution: Prologium has pioneered an innovative approach to sodium-ion battery cathode materials interface stability through their proprietary MAB (Multi-Axis Bipolar) technology. Their solution focuses on a ceramic-polymer composite solid electrolyte system specifically engineered for compatibility with sodium-ion cathode materials. The company has developed a gradient interface design that gradually transitions from cathode to electrolyte, minimizing abrupt property changes that typically lead to interface degradation. Their research shows this approach reduces interfacial resistance by up to 60% compared to conventional designs. Prologium's solid electrolyte formulation incorporates sodium-conducting ceramic fillers (Na₃Zr₂Si₂PO₁₂) within a polymer matrix, creating flexible yet stable interfaces with various sodium cathode chemistries including layered oxides and Prussian blue analogs. The company has demonstrated cells maintaining over 80% capacity after 500 cycles at elevated temperatures (60°C), indicating superior interface stability under challenging conditions. Their manufacturing process includes a proprietary coating technique that ensures uniform contact between cathode particles and the solid electrolyte.
Strengths: Prologium's gradient interface design effectively addresses the critical challenge of mechanical and chemical stability at the cathode-electrolyte interface. Their ceramic-polymer composite approach provides both flexibility and stability, accommodating volume changes during cycling. Weaknesses: The complex manufacturing process for creating gradient interfaces may increase production costs. The technology may face challenges in scaling to mass production while maintaining precise interface control across large-area batteries.
Key Patents and Publications on Interface Engineering
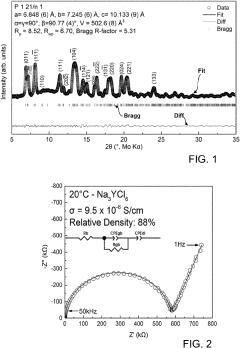
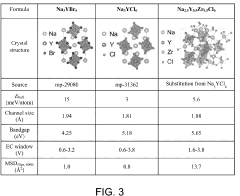
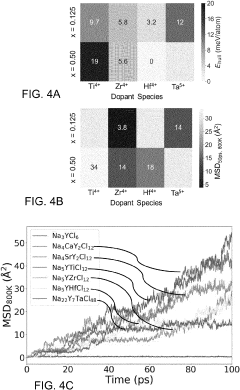
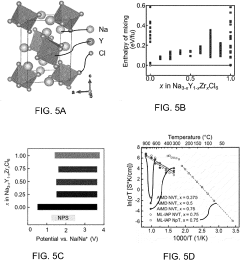
Chlorine-Based Sodium Solid Electrolyte
PatentPendingUS20230115956A1
Innovation
- Development of chloride-based sodium-ion conductors, specifically Na3-xY1-xZrxCl6 compounds, which enhance sodium diffusion and provide superior chemical and electrochemical stability with high voltage cathodes, preventing unwanted reactions and degradation, and are used in a cathode composite without protective coatings.
Electrode composite material for sodium ion secondary batteries and its manufacturing method
PatentInactiveJPWO2018131627A1
Innovation
- The electrode mixture is composed of a sintered body with a porosity of 45% or less, containing active material precursor powder and sodium ion conductive solid electrolyte powder, with specific compositions and processing methods like pressing and firing to enhance adhesion and ion conduction paths.
Sustainability and Resource Considerations
The sustainability profile of sodium-ion battery systems with solid electrolytes represents a significant advantage over conventional lithium-ion technologies. Sodium resources are abundantly available in the earth's crust (2.6%) and oceans (1.1%), making them approximately 1,000 times more plentiful than lithium. This abundance translates directly to reduced extraction impacts and substantially lower raw material costs, positioning sodium-ion batteries as an environmentally responsible alternative for large-scale energy storage applications.
When examining the environmental footprint of cathode materials interfacing with solid electrolytes, several sustainability metrics favor sodium-ion systems. Life cycle assessments indicate that sodium-based cathode production generates approximately 25-30% lower carbon emissions compared to equivalent lithium cathode manufacturing processes. This reduction stems primarily from less energy-intensive mining and refining operations, as sodium compounds typically require lower processing temperatures and fewer chemical treatments.
Resource efficiency extends beyond the sodium element itself. Many promising cathode materials for sodium-ion batteries utilize earth-abundant transition metals like iron, manganese, and titanium, avoiding critical dependencies on cobalt and nickel that plague lithium-ion supply chains. The compatibility of these cathode materials with solid electrolytes further enhances sustainability by potentially extending cycle life and reducing replacement frequency.
Water consumption represents another critical sustainability consideration. Traditional lithium extraction from salt flats can consume up to 2 million liters of water per ton of lithium produced, whereas sodium extraction methods typically require 50-70% less water. This difference becomes particularly significant in water-stressed regions where battery material production may compete with agricultural and community needs.
End-of-life management also favors sodium-ion systems. The cathode-electrolyte interfaces in sodium-ion batteries with solid electrolytes generally contain fewer toxic components, facilitating more straightforward recycling processes. Current research indicates recovery rates for sodium and transition metals from spent cathodes could reach 85-90% using hydrometallurgical methods, compared to 60-75% for conventional lithium cathodes.
Looking forward, the sustainability advantages of sodium-ion battery systems may accelerate their commercial adoption despite current performance limitations. As interface stability challenges between cathode materials and solid electrolytes are resolved, the environmental benefits could position this technology as the preferred solution for stationary storage applications where energy density requirements are less stringent than in mobile applications.
When examining the environmental footprint of cathode materials interfacing with solid electrolytes, several sustainability metrics favor sodium-ion systems. Life cycle assessments indicate that sodium-based cathode production generates approximately 25-30% lower carbon emissions compared to equivalent lithium cathode manufacturing processes. This reduction stems primarily from less energy-intensive mining and refining operations, as sodium compounds typically require lower processing temperatures and fewer chemical treatments.
Resource efficiency extends beyond the sodium element itself. Many promising cathode materials for sodium-ion batteries utilize earth-abundant transition metals like iron, manganese, and titanium, avoiding critical dependencies on cobalt and nickel that plague lithium-ion supply chains. The compatibility of these cathode materials with solid electrolytes further enhances sustainability by potentially extending cycle life and reducing replacement frequency.
Water consumption represents another critical sustainability consideration. Traditional lithium extraction from salt flats can consume up to 2 million liters of water per ton of lithium produced, whereas sodium extraction methods typically require 50-70% less water. This difference becomes particularly significant in water-stressed regions where battery material production may compete with agricultural and community needs.
End-of-life management also favors sodium-ion systems. The cathode-electrolyte interfaces in sodium-ion batteries with solid electrolytes generally contain fewer toxic components, facilitating more straightforward recycling processes. Current research indicates recovery rates for sodium and transition metals from spent cathodes could reach 85-90% using hydrometallurgical methods, compared to 60-75% for conventional lithium cathodes.
Looking forward, the sustainability advantages of sodium-ion battery systems may accelerate their commercial adoption despite current performance limitations. As interface stability challenges between cathode materials and solid electrolytes are resolved, the environmental benefits could position this technology as the preferred solution for stationary storage applications where energy density requirements are less stringent than in mobile applications.
Scalability and Manufacturing Challenges
The transition from laboratory-scale production to industrial manufacturing of sodium-ion batteries with solid electrolytes presents significant challenges. Current manufacturing processes for solid-state sodium-ion batteries remain predominantly confined to small-scale laboratory environments, with limited examples of successful scale-up operations. The interface stability between cathode materials and solid electrolytes becomes increasingly problematic at larger production scales due to the amplification of micro-level inconsistencies across larger surface areas.
Manufacturing solid-state sodium-ion batteries requires precise control of the cathode-electrolyte interface during high-temperature sintering processes. The thermal expansion coefficient mismatch between cathode materials and solid electrolytes often leads to mechanical stress and microcrack formation during cooling, creating pathways for sodium dendrite growth and eventual battery failure. These issues become more pronounced in large-format cells where uniform temperature distribution is difficult to maintain.
The cost implications of scaling up production remain substantial. Current manufacturing techniques for solid electrolytes suitable for sodium-ion batteries involve expensive precursors and energy-intensive processing steps. The specialized equipment required for maintaining ultra-dry environments during production further increases capital expenditure. Industry estimates suggest that solid-state sodium-ion battery production costs remain 3-5 times higher than conventional liquid electrolyte systems, presenting a significant barrier to commercialization.
Quality control presents another major challenge in scaled production. Detecting interfacial defects between cathode materials and solid electrolytes requires sophisticated analytical techniques that are difficult to implement in high-throughput manufacturing environments. Non-destructive testing methods capable of identifying nanoscale interface instabilities in assembled cells remain underdeveloped, limiting quality assurance capabilities in mass production scenarios.
Environmental considerations also impact manufacturing scalability. While sodium resources are abundant compared to lithium, the processing of certain solid electrolyte materials involves hazardous chemicals and generates waste streams that require specialized handling. Developing environmentally sustainable manufacturing protocols that maintain interface stability while minimizing ecological impact represents an ongoing challenge for the industry.
Recent innovations in co-sintering techniques and interface engineering show promise for addressing these scalability challenges. Gradient-layered structures between cathode materials and solid electrolytes have demonstrated improved interface stability in pilot-scale production. Additionally, advances in dry coating technologies are reducing processing steps and improving manufacturing throughput while maintaining critical interface properties.
Manufacturing solid-state sodium-ion batteries requires precise control of the cathode-electrolyte interface during high-temperature sintering processes. The thermal expansion coefficient mismatch between cathode materials and solid electrolytes often leads to mechanical stress and microcrack formation during cooling, creating pathways for sodium dendrite growth and eventual battery failure. These issues become more pronounced in large-format cells where uniform temperature distribution is difficult to maintain.
The cost implications of scaling up production remain substantial. Current manufacturing techniques for solid electrolytes suitable for sodium-ion batteries involve expensive precursors and energy-intensive processing steps. The specialized equipment required for maintaining ultra-dry environments during production further increases capital expenditure. Industry estimates suggest that solid-state sodium-ion battery production costs remain 3-5 times higher than conventional liquid electrolyte systems, presenting a significant barrier to commercialization.
Quality control presents another major challenge in scaled production. Detecting interfacial defects between cathode materials and solid electrolytes requires sophisticated analytical techniques that are difficult to implement in high-throughput manufacturing environments. Non-destructive testing methods capable of identifying nanoscale interface instabilities in assembled cells remain underdeveloped, limiting quality assurance capabilities in mass production scenarios.
Environmental considerations also impact manufacturing scalability. While sodium resources are abundant compared to lithium, the processing of certain solid electrolyte materials involves hazardous chemicals and generates waste streams that require specialized handling. Developing environmentally sustainable manufacturing protocols that maintain interface stability while minimizing ecological impact represents an ongoing challenge for the industry.
Recent innovations in co-sintering techniques and interface engineering show promise for addressing these scalability challenges. Gradient-layered structures between cathode materials and solid electrolytes have demonstrated improved interface stability in pilot-scale production. Additionally, advances in dry coating technologies are reducing processing steps and improving manufacturing throughput while maintaining critical interface properties.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!