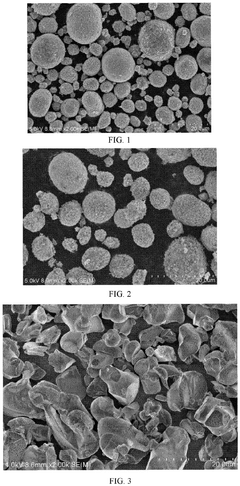
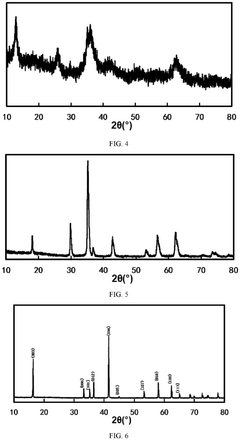
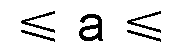How Sodium-Ion Battery Cathode Materials Influence Electrochemical Stability
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium-Ion Battery Cathode Evolution and Research Objectives
Sodium-ion batteries (SIBs) have emerged as a promising alternative to lithium-ion batteries due to the abundance and low cost of sodium resources. The development of sodium-ion battery technology can be traced back to the 1970s and 1980s, when researchers first began exploring sodium intercalation chemistry. However, significant progress in this field has only been achieved in the past decade, driven by concerns about lithium resource limitations and the increasing demand for large-scale energy storage solutions.
The evolution of cathode materials for sodium-ion batteries has followed several distinct phases. Initially, research focused on layered oxide materials similar to those used in lithium-ion batteries. These early materials suffered from structural instability during sodium insertion and extraction, leading to rapid capacity fading and poor cycle life. The second generation of cathode materials explored polyanionic compounds, which offered improved structural stability but often at the cost of energy density.
Recent advancements have centered on developing novel cathode architectures that can accommodate the larger ionic radius of sodium (1.02 Å) compared to lithium (0.76 Å). This size difference creates unique challenges for designing stable host structures that can withstand repeated sodium insertion and extraction without significant structural degradation. Researchers have explored various strategies including vacancy-rich structures, three-dimensional frameworks, and composite materials to address these challenges.
The technological trajectory shows a clear trend toward multi-component systems that combine the advantages of different material classes. For example, P2-type layered oxides modified with dopants to enhance structural stability, or Prussian blue analogs with controlled defect chemistry to improve sodium diffusion kinetics. These hybrid approaches represent the current frontier in cathode material development.
The primary research objectives in this field now focus on understanding the fundamental mechanisms that govern electrochemical stability. This includes investigating the correlation between crystal structure evolution and voltage profiles, elucidating the role of transition metal dissolution in capacity fading, and developing in-situ characterization techniques to monitor structural changes during cycling.
Another critical research goal is to establish clear design principles for cathode materials that can achieve both high energy density and long cycle life. This requires a comprehensive understanding of how different cathode compositions influence the formation and stability of the solid-electrolyte interphase (SEI), which plays a crucial role in determining battery performance and longevity.
Looking forward, research aims to develop cathode materials that can enable sodium-ion batteries with energy densities approaching those of commercial lithium-ion systems (>200 Wh/kg) while maintaining stable performance over thousands of cycles. Achieving this ambitious target will require innovative approaches to materials design and a deeper understanding of the complex interplay between cathode composition, structure, and electrochemical stability.
The evolution of cathode materials for sodium-ion batteries has followed several distinct phases. Initially, research focused on layered oxide materials similar to those used in lithium-ion batteries. These early materials suffered from structural instability during sodium insertion and extraction, leading to rapid capacity fading and poor cycle life. The second generation of cathode materials explored polyanionic compounds, which offered improved structural stability but often at the cost of energy density.
Recent advancements have centered on developing novel cathode architectures that can accommodate the larger ionic radius of sodium (1.02 Å) compared to lithium (0.76 Å). This size difference creates unique challenges for designing stable host structures that can withstand repeated sodium insertion and extraction without significant structural degradation. Researchers have explored various strategies including vacancy-rich structures, three-dimensional frameworks, and composite materials to address these challenges.
The technological trajectory shows a clear trend toward multi-component systems that combine the advantages of different material classes. For example, P2-type layered oxides modified with dopants to enhance structural stability, or Prussian blue analogs with controlled defect chemistry to improve sodium diffusion kinetics. These hybrid approaches represent the current frontier in cathode material development.
The primary research objectives in this field now focus on understanding the fundamental mechanisms that govern electrochemical stability. This includes investigating the correlation between crystal structure evolution and voltage profiles, elucidating the role of transition metal dissolution in capacity fading, and developing in-situ characterization techniques to monitor structural changes during cycling.
Another critical research goal is to establish clear design principles for cathode materials that can achieve both high energy density and long cycle life. This requires a comprehensive understanding of how different cathode compositions influence the formation and stability of the solid-electrolyte interphase (SEI), which plays a crucial role in determining battery performance and longevity.
Looking forward, research aims to develop cathode materials that can enable sodium-ion batteries with energy densities approaching those of commercial lithium-ion systems (>200 Wh/kg) while maintaining stable performance over thousands of cycles. Achieving this ambitious target will require innovative approaches to materials design and a deeper understanding of the complex interplay between cathode composition, structure, and electrochemical stability.
Market Analysis for Sodium-Ion Battery Technologies
The global sodium-ion battery market is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions. Current market valuations indicate a compound annual growth rate exceeding 15% between 2023 and 2030, with projections suggesting the market could reach $1.2 billion by 2025. This growth trajectory is primarily fueled by the inherent advantages of sodium-ion technology, particularly its cost-effectiveness compared to lithium-ion alternatives.
Cathode materials represent approximately 30% of a sodium-ion battery's total cost, making them a critical component for market competitiveness. The influence of cathode materials on electrochemical stability directly impacts battery performance metrics that consumers and industries prioritize, including cycle life, safety, and operational temperature range. Market research indicates that improvements in cathode stability could potentially reduce battery degradation by 25-40%, significantly enhancing product appeal.
Regional market analysis reveals China leading sodium-ion battery development and production, controlling nearly 40% of the global market share. European markets follow with growing investments in research and manufacturing facilities, while North American adoption remains in early stages but shows accelerating interest from both automotive and grid storage sectors.
Consumer electronics currently represents the largest application segment for sodium-ion batteries, accounting for approximately 35% of market demand. However, grid-scale energy storage is projected to become the fastest-growing segment, with an anticipated growth rate of 20% annually through 2028, driven by renewable energy integration requirements and the favorable stability characteristics of sodium-ion systems.
Market segmentation by cathode material type shows layered oxide materials currently dominating with 45% market share, followed by polyanionic compounds at 30% and Prussian blue analogs at 20%. The remaining market consists of emerging cathode technologies. Notably, cathode materials demonstrating superior electrochemical stability command premium pricing, with manufacturers willing to pay 15-25% more for materials that can deliver demonstrable improvements in cycle life.
Industry surveys indicate that 78% of potential commercial adopters cite electrochemical stability as a "very important" or "critical" factor in their purchasing decisions for energy storage technologies. This market preference is creating strong commercial incentives for research focused specifically on enhancing cathode stability rather than pursuing marginal improvements in energy density.
Cathode materials represent approximately 30% of a sodium-ion battery's total cost, making them a critical component for market competitiveness. The influence of cathode materials on electrochemical stability directly impacts battery performance metrics that consumers and industries prioritize, including cycle life, safety, and operational temperature range. Market research indicates that improvements in cathode stability could potentially reduce battery degradation by 25-40%, significantly enhancing product appeal.
Regional market analysis reveals China leading sodium-ion battery development and production, controlling nearly 40% of the global market share. European markets follow with growing investments in research and manufacturing facilities, while North American adoption remains in early stages but shows accelerating interest from both automotive and grid storage sectors.
Consumer electronics currently represents the largest application segment for sodium-ion batteries, accounting for approximately 35% of market demand. However, grid-scale energy storage is projected to become the fastest-growing segment, with an anticipated growth rate of 20% annually through 2028, driven by renewable energy integration requirements and the favorable stability characteristics of sodium-ion systems.
Market segmentation by cathode material type shows layered oxide materials currently dominating with 45% market share, followed by polyanionic compounds at 30% and Prussian blue analogs at 20%. The remaining market consists of emerging cathode technologies. Notably, cathode materials demonstrating superior electrochemical stability command premium pricing, with manufacturers willing to pay 15-25% more for materials that can deliver demonstrable improvements in cycle life.
Industry surveys indicate that 78% of potential commercial adopters cite electrochemical stability as a "very important" or "critical" factor in their purchasing decisions for energy storage technologies. This market preference is creating strong commercial incentives for research focused specifically on enhancing cathode stability rather than pursuing marginal improvements in energy density.
Current Challenges in Cathode Material Electrochemical Stability
Despite significant advancements in sodium-ion battery technology, cathode materials continue to face substantial electrochemical stability challenges that hinder widespread commercial adoption. The primary issue stems from the larger ionic radius of Na+ (1.02 Å) compared to Li+ (0.76 Å), which causes greater structural stress during charge-discharge cycles. This fundamental difference leads to accelerated capacity fading and reduced cycle life in many sodium-ion cathode materials.
Layered oxide cathodes (NaxMO2, where M represents transition metals) exhibit particularly severe phase transitions during sodium extraction/insertion processes. These structural changes often result in irreversible transformations that permanently damage the cathode's crystalline framework. For instance, P2-type Na0.67MnO2 undergoes multiple phase transitions during cycling, leading to capacity loss exceeding 30% after just 50 cycles in many experimental studies.
Prussian blue analogs (PBAs), while promising for their open framework structure, suffer from significant challenges related to water coordination and vacancies in their crystal structure. These defects create instability during cycling, particularly at higher voltages above 4.0V vs. Na/Na+, where electrolyte decomposition further exacerbates capacity fade. Recent research indicates that water molecules trapped in the PBA framework can trigger side reactions that progressively degrade electrochemical performance.
Polyanionic compounds such as NaFePO4 and Na3V2(PO4)3 face stability issues related to their complex sodium diffusion pathways. The migration energy barriers for sodium ions in these materials are typically 20-40% higher than their lithium counterparts, resulting in kinetic limitations that manifest as voltage hysteresis and reduced rate capability. This energy barrier difference becomes particularly problematic at high cycling rates, where incomplete sodium reinsertion leads to cumulative capacity loss.
Interface stability represents another critical challenge, as cathode materials often form unstable solid-electrolyte interphase (SEI) layers when in contact with conventional electrolytes. These interfaces evolve continuously during cycling, consuming active sodium and increasing cell impedance. Recent studies using advanced characterization techniques have revealed that the SEI composition in sodium systems differs significantly from lithium counterparts, with more inorganic components that offer less effective passivation.
Transition metal dissolution from cathode materials into the electrolyte presents a particularly vexing challenge for long-term stability. Manganese and vanadium-containing cathodes are especially susceptible to this phenomenon, which not only depletes active material but also leads to contamination of the anode surface. Experimental evidence suggests dissolution rates in sodium systems can exceed those in lithium analogs by factors of 2-5 under identical conditions.
Layered oxide cathodes (NaxMO2, where M represents transition metals) exhibit particularly severe phase transitions during sodium extraction/insertion processes. These structural changes often result in irreversible transformations that permanently damage the cathode's crystalline framework. For instance, P2-type Na0.67MnO2 undergoes multiple phase transitions during cycling, leading to capacity loss exceeding 30% after just 50 cycles in many experimental studies.
Prussian blue analogs (PBAs), while promising for their open framework structure, suffer from significant challenges related to water coordination and vacancies in their crystal structure. These defects create instability during cycling, particularly at higher voltages above 4.0V vs. Na/Na+, where electrolyte decomposition further exacerbates capacity fade. Recent research indicates that water molecules trapped in the PBA framework can trigger side reactions that progressively degrade electrochemical performance.
Polyanionic compounds such as NaFePO4 and Na3V2(PO4)3 face stability issues related to their complex sodium diffusion pathways. The migration energy barriers for sodium ions in these materials are typically 20-40% higher than their lithium counterparts, resulting in kinetic limitations that manifest as voltage hysteresis and reduced rate capability. This energy barrier difference becomes particularly problematic at high cycling rates, where incomplete sodium reinsertion leads to cumulative capacity loss.
Interface stability represents another critical challenge, as cathode materials often form unstable solid-electrolyte interphase (SEI) layers when in contact with conventional electrolytes. These interfaces evolve continuously during cycling, consuming active sodium and increasing cell impedance. Recent studies using advanced characterization techniques have revealed that the SEI composition in sodium systems differs significantly from lithium counterparts, with more inorganic components that offer less effective passivation.
Transition metal dissolution from cathode materials into the electrolyte presents a particularly vexing challenge for long-term stability. Manganese and vanadium-containing cathodes are especially susceptible to this phenomenon, which not only depletes active material but also leads to contamination of the anode surface. Experimental evidence suggests dissolution rates in sodium systems can exceed those in lithium analogs by factors of 2-5 under identical conditions.
Contemporary Cathode Design Strategies for Enhanced Stability
01 Layered transition metal oxide cathodes
Layered transition metal oxides, particularly sodium-containing compounds like NaxMO2 (where M represents transition metals such as Fe, Mn, Co, Ni), offer promising electrochemical stability for sodium-ion battery cathodes. These materials feature a layered structure that facilitates sodium ion intercalation and extraction while maintaining structural integrity during cycling. Various dopants and compositional modifications can enhance their voltage stability window and cycling performance.- Layered transition metal oxide cathodes for sodium-ion batteries: Layered transition metal oxides, particularly those containing sodium, such as NaxMO2 (where M represents transition metals like Fe, Mn, Co, Ni), offer promising electrochemical stability for sodium-ion battery cathodes. These materials provide high specific capacity and good cycling performance due to their stable crystal structure that facilitates sodium ion intercalation and extraction. The incorporation of multiple transition metals can further enhance the structural stability during charge-discharge cycles.
- Polyanionic compounds as stable cathode materials: Polyanionic compounds, including phosphates, fluorophosphates, and sulfates, demonstrate excellent electrochemical stability as sodium-ion battery cathodes. These materials feature strong covalent bonds between oxygen and non-metal elements (P, S), which stabilize the crystal structure during sodium insertion/extraction processes. The robust three-dimensional frameworks of these compounds resist structural collapse during cycling, leading to enhanced cycle life and safety performance.
- Carbon-based composite cathode materials: Carbon-based composite cathode materials, incorporating conductive carbon with active materials, significantly improve the electrochemical stability of sodium-ion batteries. The carbon component enhances electronic conductivity, buffers volume changes during cycling, and prevents agglomeration of active particles. These composites often demonstrate reduced polarization, improved rate capability, and extended cycle life compared to non-composite counterparts.
- Surface coating and modification techniques: Surface coating and modification techniques are effective approaches to enhance the electrochemical stability of sodium-ion battery cathode materials. Applying protective layers of metal oxides, fluorides, or conductive polymers on cathode particles can suppress undesirable side reactions with the electrolyte, prevent dissolution of active components, and maintain structural integrity during cycling. These modifications effectively mitigate capacity fading and extend battery lifespan.
- Prussian blue analogs for stable sodium storage: Prussian blue analogs (PBAs) represent a class of open-framework materials with excellent electrochemical stability for sodium-ion battery cathodes. Their cubic structure with large interstitial sites facilitates rapid sodium ion diffusion with minimal structural strain. PBAs demonstrate low volume change during cycling, good thermal stability, and consistent performance over extended charge-discharge cycles, making them promising candidates for long-life sodium-ion batteries.
02 Polyanionic framework compounds
Polyanionic framework compounds, including phosphates (NaMPO4), pyrophosphates (Na2MP2O7), and fluorophosphates (NaMPO4F), demonstrate excellent electrochemical stability due to their robust three-dimensional frameworks. The strong covalent bonds between oxygen and phosphorus create stable structures that resist degradation during repeated sodium insertion/extraction cycles. These materials typically offer higher operating voltages and better thermal stability compared to oxide-based cathodes.Expand Specific Solutions03 Prussian blue analogs and hexacyanoferrates
Prussian blue analogs and hexacyanoferrates with the general formula NaxM[Fe(CN)6] (where M represents transition metals) exhibit exceptional electrochemical stability due to their open framework structure with large interstitial spaces. These materials allow for rapid and reversible sodium-ion insertion/extraction with minimal structural changes, resulting in excellent cycling stability. Their low-cost synthesis and environmental friendliness make them particularly attractive for large-scale sodium-ion battery applications.Expand Specific Solutions04 Surface coating and modification techniques
Surface coating and modification techniques significantly enhance the electrochemical stability of sodium-ion battery cathode materials. Applying thin layers of metal oxides, carbon, or conductive polymers on cathode particles creates protective barriers against electrolyte attack and suppresses unwanted side reactions. These coatings improve interfacial stability, reduce dissolution of active materials, and enhance electronic/ionic conductivity, resulting in improved cycling performance and extended battery lifespan.Expand Specific Solutions05 Electrolyte compatibility and interface engineering
Electrolyte compatibility and interface engineering are crucial for ensuring electrochemical stability of sodium-ion battery cathodes. Optimizing electrolyte compositions with appropriate additives helps form stable solid electrolyte interphase (SEI) layers that prevent continuous electrolyte decomposition. Interface engineering approaches, such as artificial SEI formation and functional electrolyte additives, effectively mitigate parasitic reactions between cathode materials and electrolytes, enhancing voltage stability and reducing capacity fading during long-term cycling.Expand Specific Solutions
Leading Research Institutions and Industrial Manufacturers
The sodium-ion battery cathode materials market is in an early growth phase, characterized by increasing R&D investments and emerging commercial applications. The competitive landscape features established lithium-ion battery manufacturers expanding into sodium-ion technology, including Shenzhen Zhenhua New Material Co., which achieved ten-ton sales by 2022, and Ningde Amperex Technology Ltd. Academic-industrial collaborations are prominent, with research institutions like Nankai University, CNRS, and Argonne National Laboratory advancing fundamental understanding of electrochemical stability mechanisms. Technical challenges remain in optimizing cathode materials' stability, with companies like GEM Co., Ronbay New Energy, and Easpring Material Technology developing proprietary solutions for multi-element doping, crystal structure control, and surface modification to enhance performance and durability in commercial applications.
Nankai University
Technical Solution: Nankai University has developed innovative Prussian blue analogue (PBA) cathode materials for sodium-ion batteries with enhanced structural stability. Their approach focuses on controlling vacancies and water content in the PBA framework to improve electrochemical performance. Researchers at Nankai have pioneered a controlled crystallization method that produces highly ordered rhombohedral Na2-xFe[Fe(CN)6]1-y□y structures with minimal lattice defects and optimized sodium transport channels. Their technology incorporates strategic substitution of transition metals in the PBA framework to enhance structural integrity during repeated sodium insertion/extraction. Nankai's research has demonstrated that controlling the coordination environment of iron centers significantly impacts the redox potential and reversibility of the cathode material. They have also developed advanced carbon composite architectures where PBA nanoparticles are embedded in conductive carbon networks, effectively addressing the poor electronic conductivity of PBA materials while maintaining high ionic diffusivity. Their cathode materials have demonstrated capacity retention exceeding 90% after 1000 cycles at 1C rate.
Strengths: Cutting-edge research in Prussian blue analogue materials; innovative approaches to addressing fundamental stability issues; strong academic research capabilities. Weaknesses: Limited industrial partnerships for commercialization; challenges in scaling laboratory synthesis methods to industrial production.
Beijing Easpring Material Technology Co., Ltd.
Technical Solution: Easpring has developed advanced layered oxide cathode materials for sodium-ion batteries with optimized composition and surface modifications. Their approach focuses on industrially viable synthesis methods for Na0.67Ni0.33Mn0.67O2 cathode materials with controlled particle morphology and size distribution. Easpring employs a proprietary co-precipitation process followed by carefully controlled calcination to produce cathode materials with uniform composition and minimal impurities. Their technology incorporates aluminum and titanium dopants at specific concentrations to stabilize the crystal structure during sodium extraction/insertion, effectively suppressing detrimental phase transitions. Easpring has developed specialized surface coating technologies using phosphates and fluorides to create protective layers that minimize electrolyte decomposition at high voltages. Their cathode materials feature hierarchical particle architectures with primary particles of 100-200nm assembled into secondary particles of 2-5μm, optimizing both ionic diffusion kinetics and volumetric energy density. Easpring's materials demonstrate capacity retention exceeding 80% after 500 cycles at practical current densities.
Strengths: Established commercial production capabilities; experience transitioning from lithium-ion to sodium-ion materials; strong supply chain integration in China. Weaknesses: Relatively new to sodium-ion technology; facing competition from larger battery material manufacturers; challenges in achieving consistent quality at scale.
Critical Patents and Scientific Breakthroughs in Cathode Materials
Sodium ion battery cathode material and preparation method thereof and sodium ion battery
PatentActiveEP4516744A1
Innovation
- A sodium ion battery cathode material with improved air and structural stability is developed, characterized by specific XRD peak arrangements and ratios, an O3-type monocrystal structure, and a controlled composition and particle size distribution, achieved through a precise pre-sintering and calcination process.
Sodium ion cathode active materials for batteries
PatentWO2025132336A1
Innovation
- A sodium-ion cathode active material with a chemical composition of Na, M, and O, where M consists of Fe, Mn, and X (with X being at least one element selected from B, Si, K, Co, Ga, Rb, Rh, Cs, Re, Tl, and Pb), is developed. This composition is optimized to improve structural and air stability, reducing transition metal dissolution.
Sustainability Impact of Cathode Material Selection
The selection of cathode materials in sodium-ion batteries carries significant implications for environmental sustainability throughout the entire battery lifecycle. Unlike lithium-ion batteries that rely heavily on cobalt and nickel—elements associated with resource scarcity and problematic mining practices—sodium-ion battery cathodes can utilize more abundant and environmentally benign materials such as iron, manganese, and titanium.
Cathode materials based on earth-abundant elements demonstrate substantially lower environmental footprints. Life cycle assessments indicate that iron and manganese-based cathodes can reduce greenhouse gas emissions by 30-40% compared to conventional lithium cobalt oxide cathodes. Furthermore, the extraction processes for these elements typically consume less water and energy, while generating fewer toxic byproducts.
The manufacturing processes for sodium-ion cathodes generally require lower temperatures than their lithium counterparts, particularly for layered oxide materials. This translates to reduced energy consumption during production and consequently lower carbon emissions. For instance, P2-type layered oxides can be synthesized at temperatures approximately 100-200°C lower than NMC cathodes, representing a significant energy saving at industrial scale.
Recycling potential represents another crucial sustainability advantage of certain sodium cathode materials. Prussian blue analogs and polyanionic compounds demonstrate superior structural stability during recycling processes, allowing for more efficient material recovery. Current research indicates recovery rates exceeding 90% for sodium and transition metals from these cathode types, compared to 60-70% for conventional lithium-ion cathodes.
The reduced dependency on critical raw materials enhances supply chain resilience and reduces geopolitical vulnerabilities. This aspect is particularly important as battery production scales up to meet growing energy storage demands. Economic analyses suggest that cathode materials based on sodium, iron, and manganese could reduce material costs by 40-60% while simultaneously decreasing supply chain risks.
Water usage and land impact assessments reveal that sodium-based cathode production typically requires 25-35% less water than lithium-ion equivalents. Additionally, the mining footprint for iron and manganese is substantially smaller than that required for nickel and cobalt extraction, resulting in reduced ecosystem disruption and biodiversity impact.
Cathode materials based on earth-abundant elements demonstrate substantially lower environmental footprints. Life cycle assessments indicate that iron and manganese-based cathodes can reduce greenhouse gas emissions by 30-40% compared to conventional lithium cobalt oxide cathodes. Furthermore, the extraction processes for these elements typically consume less water and energy, while generating fewer toxic byproducts.
The manufacturing processes for sodium-ion cathodes generally require lower temperatures than their lithium counterparts, particularly for layered oxide materials. This translates to reduced energy consumption during production and consequently lower carbon emissions. For instance, P2-type layered oxides can be synthesized at temperatures approximately 100-200°C lower than NMC cathodes, representing a significant energy saving at industrial scale.
Recycling potential represents another crucial sustainability advantage of certain sodium cathode materials. Prussian blue analogs and polyanionic compounds demonstrate superior structural stability during recycling processes, allowing for more efficient material recovery. Current research indicates recovery rates exceeding 90% for sodium and transition metals from these cathode types, compared to 60-70% for conventional lithium-ion cathodes.
The reduced dependency on critical raw materials enhances supply chain resilience and reduces geopolitical vulnerabilities. This aspect is particularly important as battery production scales up to meet growing energy storage demands. Economic analyses suggest that cathode materials based on sodium, iron, and manganese could reduce material costs by 40-60% while simultaneously decreasing supply chain risks.
Water usage and land impact assessments reveal that sodium-based cathode production typically requires 25-35% less water than lithium-ion equivalents. Additionally, the mining footprint for iron and manganese is substantially smaller than that required for nickel and cobalt extraction, resulting in reduced ecosystem disruption and biodiversity impact.
Comparative Analysis with Lithium-Ion Battery Technologies
When comparing sodium-ion battery (SIB) cathode materials with lithium-ion battery (LIB) technologies, several key differences emerge that significantly impact electrochemical stability. Lithium-ion batteries have dominated the energy storage market for decades due to their high energy density, long cycle life, and established manufacturing infrastructure. However, sodium-ion batteries offer compelling advantages in terms of resource abundance and cost-effectiveness, with sodium being approximately 1,000 times more abundant in the Earth's crust than lithium.
The cathode materials in both technologies play a crucial role in determining electrochemical stability. LIBs typically employ layered oxide materials such as LiCoO₂, LiNiMnCoO₂ (NMC), or LiFePO₄ (LFP), which have been extensively optimized over decades. These materials generally demonstrate excellent structural stability during cycling, with volume changes typically limited to 2-7%. In contrast, SIB cathode materials often experience more significant volume changes during sodium insertion/extraction, sometimes exceeding 10%, which can lead to accelerated capacity fading.
Voltage stability represents another critical difference. LIB cathodes generally operate at higher voltages (3.7-4.2V vs. Li/Li⁺) compared to their sodium counterparts (typically 2.7-3.8V vs. Na/Na⁺). This voltage difference directly impacts energy density, with commercial LIBs achieving 150-260 Wh/kg while current SIBs typically reach only 90-150 Wh/kg. However, the lower operating voltage of SIBs can potentially offer advantages in terms of electrolyte stability and reduced side reactions.
Regarding cycling performance, state-of-the-art LIBs routinely achieve 1,000-2,000 cycles with 80% capacity retention, whereas most SIB cathode materials currently demonstrate 500-1,000 cycles under optimal conditions. This performance gap stems largely from the larger ionic radius of Na⁺ (1.02Å) compared to Li⁺ (0.76Å), which causes greater structural strain during intercalation processes.
The electrolyte-cathode interface stability also differs significantly between the two technologies. LIBs benefit from the formation of a stable solid electrolyte interphase (SEI) that protects against continuous electrolyte decomposition. In SIBs, the SEI formation is often less stable and more dynamic, leading to ongoing electrolyte consumption and impedance growth during cycling. Recent research has focused on electrolyte additives and surface coatings specifically designed for sodium systems to address these interface stability challenges.
Temperature performance represents another divergence point, with many SIB cathode materials showing improved low-temperature performance compared to LIBs, potentially offering advantages for cold-climate applications. However, SIBs typically demonstrate accelerated capacity fading at elevated temperatures due to increased solubility of cathode components in conventional electrolytes.
The cathode materials in both technologies play a crucial role in determining electrochemical stability. LIBs typically employ layered oxide materials such as LiCoO₂, LiNiMnCoO₂ (NMC), or LiFePO₄ (LFP), which have been extensively optimized over decades. These materials generally demonstrate excellent structural stability during cycling, with volume changes typically limited to 2-7%. In contrast, SIB cathode materials often experience more significant volume changes during sodium insertion/extraction, sometimes exceeding 10%, which can lead to accelerated capacity fading.
Voltage stability represents another critical difference. LIB cathodes generally operate at higher voltages (3.7-4.2V vs. Li/Li⁺) compared to their sodium counterparts (typically 2.7-3.8V vs. Na/Na⁺). This voltage difference directly impacts energy density, with commercial LIBs achieving 150-260 Wh/kg while current SIBs typically reach only 90-150 Wh/kg. However, the lower operating voltage of SIBs can potentially offer advantages in terms of electrolyte stability and reduced side reactions.
Regarding cycling performance, state-of-the-art LIBs routinely achieve 1,000-2,000 cycles with 80% capacity retention, whereas most SIB cathode materials currently demonstrate 500-1,000 cycles under optimal conditions. This performance gap stems largely from the larger ionic radius of Na⁺ (1.02Å) compared to Li⁺ (0.76Å), which causes greater structural strain during intercalation processes.
The electrolyte-cathode interface stability also differs significantly between the two technologies. LIBs benefit from the formation of a stable solid electrolyte interphase (SEI) that protects against continuous electrolyte decomposition. In SIBs, the SEI formation is often less stable and more dynamic, leading to ongoing electrolyte consumption and impedance growth during cycling. Recent research has focused on electrolyte additives and surface coatings specifically designed for sodium systems to address these interface stability challenges.
Temperature performance represents another divergence point, with many SIB cathode materials showing improved low-temperature performance compared to LIBs, potentially offering advantages for cold-climate applications. However, SIBs typically demonstrate accelerated capacity fading at elevated temperatures due to increased solubility of cathode components in conventional electrolytes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!