Assessing ICP-MS vs IC for Ionic Element Quantification in Water
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ICP-MS and IC Technology Evolution and Objectives
The evolution of analytical techniques for water quality assessment has seen significant advancements over the past several decades, with Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Ion Chromatography (IC) emerging as two pivotal technologies. These methodologies have revolutionized our ability to detect and quantify ionic elements in water matrices with unprecedented precision and sensitivity.
ICP-MS technology originated in the early 1980s, combining the high-temperature ICP source with a mass spectrometer. This innovation marked a significant leap forward from earlier techniques such as atomic absorption spectroscopy (AAS) and inductively coupled plasma optical emission spectrometry (ICP-OES). The technology has progressively evolved from quadrupole-based systems to high-resolution magnetic sector instruments, and more recently to triple quadrupole systems capable of addressing polyatomic interferences.
Concurrently, IC technology has undergone its own transformation since its commercial introduction in the mid-1970s. Initially developed for the analysis of anions and cations in aqueous solutions, IC has expanded its capabilities through innovations in stationary phases, detection methods, and sample preparation techniques. The integration of conductivity detectors, followed by the development of suppressed conductivity detection, significantly enhanced the sensitivity and applicability of IC systems.
The technological trajectory of both methods has been driven by increasing demands for lower detection limits, higher throughput, and greater analytical versatility. Modern ICP-MS systems can now achieve detection limits in the parts-per-trillion range for many elements, while IC systems have become more specialized for specific ionic species with improved resolution and reduced analysis times.
The primary objective in comparing these technologies is to establish optimal methodological frameworks for different water analysis scenarios. This includes determining which technique provides superior performance for specific ionic elements, considering factors such as detection limits, linear dynamic range, susceptibility to interferences, sample throughput, and cost-effectiveness.
Additionally, this assessment aims to explore the complementary nature of these techniques, identifying scenarios where a combined analytical approach might yield more comprehensive water quality data. The integration of these technologies with automated sample preparation systems and data processing algorithms represents another frontier in the evolution of water analysis methodologies.
Future technological developments are expected to focus on miniaturization, portability, and real-time monitoring capabilities, potentially transforming these laboratory-based techniques into field-deployable solutions for environmental monitoring and emergency response scenarios. Understanding the evolutionary paths and current capabilities of ICP-MS and IC is essential for predicting their future applications and limitations in water quality assessment.
ICP-MS technology originated in the early 1980s, combining the high-temperature ICP source with a mass spectrometer. This innovation marked a significant leap forward from earlier techniques such as atomic absorption spectroscopy (AAS) and inductively coupled plasma optical emission spectrometry (ICP-OES). The technology has progressively evolved from quadrupole-based systems to high-resolution magnetic sector instruments, and more recently to triple quadrupole systems capable of addressing polyatomic interferences.
Concurrently, IC technology has undergone its own transformation since its commercial introduction in the mid-1970s. Initially developed for the analysis of anions and cations in aqueous solutions, IC has expanded its capabilities through innovations in stationary phases, detection methods, and sample preparation techniques. The integration of conductivity detectors, followed by the development of suppressed conductivity detection, significantly enhanced the sensitivity and applicability of IC systems.
The technological trajectory of both methods has been driven by increasing demands for lower detection limits, higher throughput, and greater analytical versatility. Modern ICP-MS systems can now achieve detection limits in the parts-per-trillion range for many elements, while IC systems have become more specialized for specific ionic species with improved resolution and reduced analysis times.
The primary objective in comparing these technologies is to establish optimal methodological frameworks for different water analysis scenarios. This includes determining which technique provides superior performance for specific ionic elements, considering factors such as detection limits, linear dynamic range, susceptibility to interferences, sample throughput, and cost-effectiveness.
Additionally, this assessment aims to explore the complementary nature of these techniques, identifying scenarios where a combined analytical approach might yield more comprehensive water quality data. The integration of these technologies with automated sample preparation systems and data processing algorithms represents another frontier in the evolution of water analysis methodologies.
Future technological developments are expected to focus on miniaturization, portability, and real-time monitoring capabilities, potentially transforming these laboratory-based techniques into field-deployable solutions for environmental monitoring and emergency response scenarios. Understanding the evolutionary paths and current capabilities of ICP-MS and IC is essential for predicting their future applications and limitations in water quality assessment.
Market Demand for Water Analysis Technologies
The global water analysis market has witnessed substantial growth in recent years, driven by increasing concerns about water quality and safety across various sectors. The market for water analysis technologies was valued at approximately $3.4 billion in 2022 and is projected to reach $5.1 billion by 2027, growing at a CAGR of 8.5%. This growth is primarily fueled by stringent regulatory frameworks, heightened environmental awareness, and the escalating need for clean water resources worldwide.
Industrial sectors, particularly pharmaceuticals, semiconductors, and food and beverage, represent significant demand drivers for precise ionic element quantification technologies. The semiconductor industry, with its requirement for ultrapure water in manufacturing processes, demands detection limits in the parts-per-trillion range, creating a specialized market segment for high-sensitivity analytical instruments like ICP-MS.
Environmental monitoring constitutes another substantial market segment, with government agencies and environmental consultancies requiring reliable methods to detect trace elements in natural water bodies, groundwater, and drinking water supplies. The global environmental testing market specifically for water analysis is expanding at approximately 7.2% annually, reflecting increased regulatory pressure and public concern over water contamination issues.
Healthcare applications represent an emerging market opportunity, with growing recognition of the importance of water quality in medical settings and the potential health impacts of trace elements. Hospitals, clinical laboratories, and research institutions increasingly require sophisticated analytical capabilities for water quality assurance.
Regional analysis reveals that North America currently holds the largest market share (approximately 35%) for advanced water analysis technologies, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the fastest growth rate, driven by rapid industrialization, urbanization, and strengthening environmental regulations in countries like China and India.
Customer segmentation within the water analysis market shows distinct needs across different sectors. While research institutions and regulatory bodies often prioritize analytical accuracy and detection limits, industrial users typically value throughput, ease of use, and total cost of ownership. This divergence in requirements creates opportunities for specialized instrumentation tailored to specific market segments.
The competitive landscape for water analysis technologies is characterized by ongoing innovation, with customers increasingly demanding integrated solutions that combine hardware, software, and services. Subscription-based models and remote monitoring capabilities are gaining traction, reflecting broader trends toward digitalization in analytical instrumentation.
Industrial sectors, particularly pharmaceuticals, semiconductors, and food and beverage, represent significant demand drivers for precise ionic element quantification technologies. The semiconductor industry, with its requirement for ultrapure water in manufacturing processes, demands detection limits in the parts-per-trillion range, creating a specialized market segment for high-sensitivity analytical instruments like ICP-MS.
Environmental monitoring constitutes another substantial market segment, with government agencies and environmental consultancies requiring reliable methods to detect trace elements in natural water bodies, groundwater, and drinking water supplies. The global environmental testing market specifically for water analysis is expanding at approximately 7.2% annually, reflecting increased regulatory pressure and public concern over water contamination issues.
Healthcare applications represent an emerging market opportunity, with growing recognition of the importance of water quality in medical settings and the potential health impacts of trace elements. Hospitals, clinical laboratories, and research institutions increasingly require sophisticated analytical capabilities for water quality assurance.
Regional analysis reveals that North America currently holds the largest market share (approximately 35%) for advanced water analysis technologies, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the fastest growth rate, driven by rapid industrialization, urbanization, and strengthening environmental regulations in countries like China and India.
Customer segmentation within the water analysis market shows distinct needs across different sectors. While research institutions and regulatory bodies often prioritize analytical accuracy and detection limits, industrial users typically value throughput, ease of use, and total cost of ownership. This divergence in requirements creates opportunities for specialized instrumentation tailored to specific market segments.
The competitive landscape for water analysis technologies is characterized by ongoing innovation, with customers increasingly demanding integrated solutions that combine hardware, software, and services. Subscription-based models and remote monitoring capabilities are gaining traction, reflecting broader trends toward digitalization in analytical instrumentation.
Current Capabilities and Limitations of ICP-MS and IC
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Ion Chromatography (IC) represent two distinct analytical approaches for quantifying ionic elements in water samples, each with specific capabilities and inherent limitations that determine their suitability for different analytical scenarios.
ICP-MS demonstrates exceptional sensitivity, routinely achieving detection limits in the parts-per-trillion (ppt) range for most elements. This makes it particularly valuable for ultra-trace analysis in environmental monitoring and regulatory compliance testing. The technique offers multi-element capability, allowing simultaneous determination of over 70 elements in a single analytical run with minimal sample volume requirements. Modern ICP-MS systems feature rapid analysis times, typically processing samples in 2-3 minutes, enabling high-throughput workflows in commercial laboratories.
However, ICP-MS faces significant challenges with certain sample types. High dissolved solid content (>0.2%) can cause signal suppression and instrument contamination, necessitating sample dilution that may compromise detection limits. The technique struggles with polyatomic interferences, particularly for elements like iron, chromium, and arsenic, where molecular species formed in the plasma can overlap with analyte signals. While collision/reaction cell technology has mitigated many interference issues, complete elimination remains challenging for complex matrices.
Ion Chromatography excels in speciation analysis, distinguishing between different oxidation states of elements (e.g., As(III) vs. As(V)), which is critical for toxicity assessments. The technique handles high-salt matrices effectively without significant dilution requirements and offers excellent precision for anion analysis, typically achieving relative standard deviations below 2%. IC systems generally require less capital investment and maintenance than ICP-MS, making them more accessible to smaller laboratories.
IC's limitations include substantially higher detection limits (typically in the ppb range) compared to ICP-MS, restricting its application for ultra-trace analysis. The technique also demonstrates limited multi-element capability, typically analyzing only 5-10 ions per run, and requires longer analysis times (10-30 minutes per sample) for adequate separation. Additionally, IC primarily targets anions and cannot detect many metallic elements that ICP-MS routinely measures.
Recent technological developments have improved both techniques. Advanced ICP-MS systems now incorporate triple quadrupole technology to further reduce interferences, while high-pressure IC systems have enhanced separation efficiency and reduced analysis times. The emergence of hyphenated techniques, particularly IC-ICP-MS, combines the separation power of IC with the sensitivity of ICP-MS, enabling both speciation and ultra-trace detection in a single analytical workflow.
ICP-MS demonstrates exceptional sensitivity, routinely achieving detection limits in the parts-per-trillion (ppt) range for most elements. This makes it particularly valuable for ultra-trace analysis in environmental monitoring and regulatory compliance testing. The technique offers multi-element capability, allowing simultaneous determination of over 70 elements in a single analytical run with minimal sample volume requirements. Modern ICP-MS systems feature rapid analysis times, typically processing samples in 2-3 minutes, enabling high-throughput workflows in commercial laboratories.
However, ICP-MS faces significant challenges with certain sample types. High dissolved solid content (>0.2%) can cause signal suppression and instrument contamination, necessitating sample dilution that may compromise detection limits. The technique struggles with polyatomic interferences, particularly for elements like iron, chromium, and arsenic, where molecular species formed in the plasma can overlap with analyte signals. While collision/reaction cell technology has mitigated many interference issues, complete elimination remains challenging for complex matrices.
Ion Chromatography excels in speciation analysis, distinguishing between different oxidation states of elements (e.g., As(III) vs. As(V)), which is critical for toxicity assessments. The technique handles high-salt matrices effectively without significant dilution requirements and offers excellent precision for anion analysis, typically achieving relative standard deviations below 2%. IC systems generally require less capital investment and maintenance than ICP-MS, making them more accessible to smaller laboratories.
IC's limitations include substantially higher detection limits (typically in the ppb range) compared to ICP-MS, restricting its application for ultra-trace analysis. The technique also demonstrates limited multi-element capability, typically analyzing only 5-10 ions per run, and requires longer analysis times (10-30 minutes per sample) for adequate separation. Additionally, IC primarily targets anions and cannot detect many metallic elements that ICP-MS routinely measures.
Recent technological developments have improved both techniques. Advanced ICP-MS systems now incorporate triple quadrupole technology to further reduce interferences, while high-pressure IC systems have enhanced separation efficiency and reduced analysis times. The emergence of hyphenated techniques, particularly IC-ICP-MS, combines the separation power of IC with the sensitivity of ICP-MS, enabling both speciation and ultra-trace detection in a single analytical workflow.
Comparative Analysis of ICP-MS and IC Methodologies
01 ICP-MS techniques for quantitative elemental analysis
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) provides high accuracy quantification of elements in various samples. The technique offers superior detection limits and multi-element analysis capabilities. Advanced ICP-MS methods incorporate internal standardization and calibration techniques to improve quantification accuracy, particularly for trace elements and isotope ratio measurements.- ICP-MS technique for quantitative elemental analysis: Inductively Coupled Plasma Mass Spectrometry (ICP-MS) is used for highly accurate quantitative analysis of elements in various samples. This technique offers exceptional sensitivity, allowing for detection of trace elements at parts per billion (ppb) or parts per trillion (ppt) levels. The accuracy of ICP-MS is enhanced through proper sample preparation, calibration with certified reference materials, and optimization of instrument parameters to minimize interferences. This analytical method is particularly valuable for multi-element analysis with high precision.
- Ion Chromatography (IC) for ionic species quantification: Ion Chromatography (IC) provides accurate quantification of ionic species in solution. This technique separates ions based on their interactions with ion-exchange resins, allowing for precise determination of anions and cations. The quantification accuracy of IC depends on proper calibration, sample preparation techniques, and the use of appropriate eluents. IC offers high selectivity and sensitivity for ionic compounds, making it suitable for environmental, pharmaceutical, and industrial applications where accurate ionic concentration measurements are critical.
- Combined ICP-MS and IC techniques for comprehensive analysis: The combination of ICP-MS and IC techniques provides comprehensive analytical capabilities for complex samples. This hyphenated approach allows for separation of species by IC followed by sensitive detection using ICP-MS, enhancing both selectivity and sensitivity. The combined methodology is particularly valuable for speciation analysis, where determining the chemical form of elements is as important as their total concentration. This approach improves quantification accuracy by reducing matrix interferences and enabling simultaneous analysis of multiple analytes with different chemical properties.
- Calibration and validation methods for accuracy improvement: Achieving high quantification accuracy in ICP-MS and IC analyses requires robust calibration and validation methods. These include the use of internal standards, standard addition techniques, and certified reference materials to compensate for matrix effects and instrument drift. Method validation protocols involve determining limits of detection and quantification, assessing linearity, precision, and accuracy through recovery studies. Statistical approaches for uncertainty estimation and quality control procedures are essential for ensuring reliable analytical results and maintaining instrument performance over time.
- Sample preparation techniques for enhanced quantification accuracy: Proper sample preparation is crucial for achieving high quantification accuracy in both ICP-MS and IC analyses. Techniques include acid digestion, microwave-assisted extraction, dilution protocols, and filtration methods to minimize matrix interferences and contamination. Preconcentration methods can be employed to improve detection limits for trace analysis. The selection of appropriate sample preparation techniques depends on sample type, target analytes, and required detection limits. Standardized sample handling procedures help ensure reproducibility and comparability of analytical results across different laboratories and instruments.
02 Ion Chromatography (IC) methods for accurate ion quantification
Ion Chromatography techniques enable precise quantification of ionic species in solution. These methods utilize specialized columns and detectors to separate and measure anions and cations with high accuracy. Recent advancements in IC technology have improved detection limits and quantification accuracy through enhanced separation efficiency and detector sensitivity.Expand Specific Solutions03 Combined ICP-MS and IC techniques for comprehensive analysis
The integration of ICP-MS and IC analytical techniques provides complementary data for more accurate and comprehensive sample characterization. This hyphenated approach allows for speciation analysis and improved quantification of complex matrices by leveraging the strengths of both techniques. The combined methodology enhances accuracy through validation of results across different analytical platforms.Expand Specific Solutions04 Calibration and standardization methods for quantification accuracy
Advanced calibration strategies significantly improve quantification accuracy in both ICP-MS and IC analyses. These include matrix-matched calibration, standard addition methods, and the use of certified reference materials. Proper calibration accounts for matrix effects and instrumental drift, ensuring reliable quantitative results across different sample types and concentration ranges.Expand Specific Solutions05 Sample preparation techniques affecting quantification accuracy
Sample preparation methodologies directly impact the accuracy of ICP-MS and IC quantification. Optimized digestion, extraction, and preconcentration techniques minimize contamination and analyte loss. Proper sample handling procedures, including filtration and preservation methods, are essential for maintaining sample integrity and achieving accurate quantitative results.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The market for ionic element quantification in water analysis is in a mature growth phase, with ICP-MS and IC technologies representing complementary approaches with distinct advantages. The global analytical instrumentation market for water analysis exceeds $3 billion, growing at 5-7% annually. Technologically, ICP-MS offers superior detection limits and multi-element capabilities, with Agilent Technologies, Thermo Fisher Scientific, and Shimadzu leading innovation through advanced spectrometry platforms. Meanwhile, IC technology, championed by Thermo Fisher and Shimadzu, provides excellent specificity for ionic species with lower operational costs. Emerging players like Elemental Scientific are disrupting the space with automated sample preparation solutions, while established companies like DuPont and SAES Getters contribute specialized materials and components enhancing instrument performance.
Agilent Technologies, Inc.
Technical Solution: Agilent has developed advanced ICP-MS systems specifically optimized for water analysis, including their 7900 and 8900 ICP-MS platforms with helium collision cell technology that effectively removes polyatomic interferences common in water matrices. Their ICP-MS MassHunter software incorporates dedicated workflows for environmental water testing with pre-configured methods for EPA compliance. Agilent's latest systems feature Ultra High Matrix Introduction (UHMI) technology allowing direct analysis of samples with up to 25% dissolved solids without dilution, significantly improving throughput for challenging water samples. Their integrated sample introduction systems with aerosol dilution capabilities enable automated handling of variable matrix samples, while their Triple Quadrupole ICP-MS technology provides MS/MS capabilities for eliminating complex interferences in difficult water matrices.
Strengths: Superior interference management through helium collision cell and MS/MS capabilities; high sample throughput with UHMI technology; comprehensive software solutions for regulatory compliance. Weaknesses: Higher initial capital investment compared to IC systems; requires specialized training and expertise; higher operating costs due to argon gas consumption and maintenance requirements.
Shimadzu Corp.
Technical Solution: Shimadzu has developed a dual-analysis approach combining their ICPMS-2030 system with their HIC-ESP ion chromatography platform for comprehensive water analysis. Their ICPMS-2030 features patented mini-torch technology that reduces argon consumption by up to 50% compared to conventional systems, making it more economical for routine water testing. For ionic element quantification, Shimadzu's Development Assistant software includes automatic method development tools with built-in EPA and ISO method parameters specifically for water analysis. Their unique Eco mode automatically reduces gas and power consumption during standby periods, while their proprietary collision cell technology (CCT) effectively removes polyatomic interferences common in environmental water samples. Shimadzu also offers integrated IC-ICPMS hyphenated systems with specialized software for speciation analysis of ionic elements in water.
Strengths: Lower operating costs through mini-torch technology and Eco mode; comprehensive solution offering both ICP-MS and IC technologies; specialized software for environmental applications. Weaknesses: Less sensitive than some competing high-end ICP-MS systems; more complex setup required for hyphenated techniques; limited third-party integration options compared to market leaders.
Key Technical Innovations in Elemental Analysis
ICP-ms method for multi-element determination by direct sample injection of high salt water
PatentActiveNL2033445A
Innovation
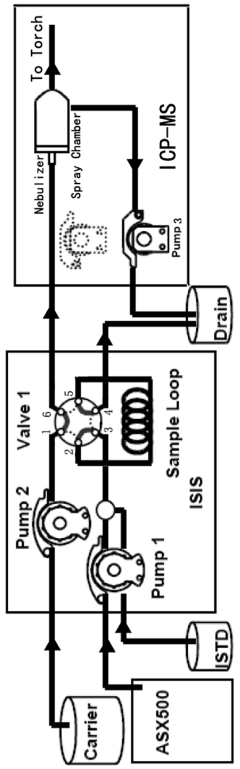
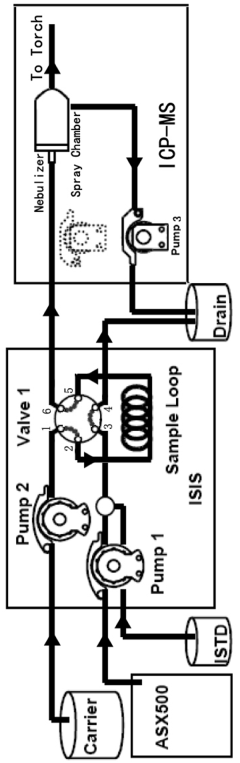
- A Discrete Flow Injection Sampling (DFIS) method using a six-way valve and peristaltic pumps to directly inject seawater samples into an ICP-MS system, optimizing parameters like sample loop diameter, carrier acidity, and pumping speed to minimize salt deposition and ensure stable sample delivery, allowing simultaneous analysis of multiple elements.
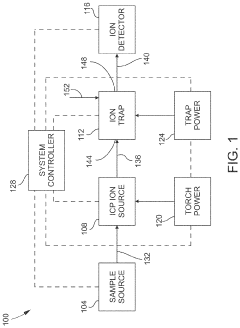
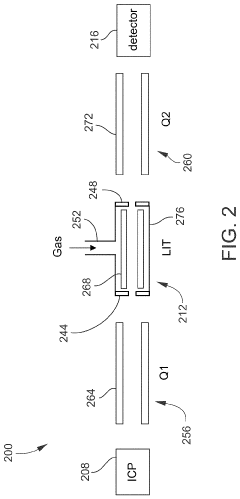
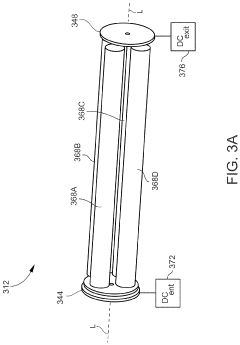
Inductively coupled plasma mass spectrometry (ICP-MS) with ion trapping
PatentActiveUS11443933B1
Innovation
- Incorporating an ion trap, such as a linear ion trap, into the ICP-MS system to confine and mass-selectively eject ions, allowing for the simultaneous analysis of multiple elements from transient signals by preventing ion exit and entry during a confinement period and transmitting selected ions to a detector for measurement.
Environmental Regulatory Compliance Considerations
Environmental regulatory frameworks worldwide have established strict guidelines for water quality monitoring, making compliance a critical consideration when selecting analytical methods. The choice between ICP-MS (Inductively Coupled Plasma Mass Spectrometry) and IC (Ion Chromatography) for ionic element quantification must align with these regulatory requirements to ensure valid reporting and avoid potential legal consequences.
The United States Environmental Protection Agency (EPA) has approved both ICP-MS (Method 200.8) and IC (Method 300.0/300.1) for different water quality parameters. ICP-MS is specifically recognized for trace metal analysis with detection limits that satisfy the stringent requirements of the Safe Drinking Water Act and Clean Water Act. Meanwhile, IC is the preferred method for anion analysis including chloride, fluoride, and sulfate determinations.
European regulations, particularly the Water Framework Directive and Drinking Water Directive, similarly recognize both techniques but emphasize method validation and uncertainty measurements. European regulatory bodies often require lower detection limits for certain contaminants compared to their US counterparts, potentially favoring ICP-MS in some applications due to its superior sensitivity.
Quality assurance protocols mandated by regulatory agencies necessitate regular calibration, certified reference materials, and participation in proficiency testing programs. ICP-MS laboratories typically face more rigorous quality control requirements due to the technique's complexity and susceptibility to interferences, requiring additional compliance documentation and validation procedures.
Record-keeping requirements differ significantly between methods, with ICP-MS generally demanding more comprehensive documentation of instrument parameters, interference corrections, and quality control measures. Organizations must consider the additional administrative burden when selecting between these analytical approaches.
Method-specific certification requirements present another compliance consideration. Laboratory personnel operating ICP-MS equipment typically require specialized training and certification, while IC operation may have less stringent certification requirements in some jurisdictions, potentially affecting staffing decisions and operational costs.
Emerging regulations concerning previously unmonitored contaminants, such as per- and polyfluoroalkyl substances (PFAS), are increasingly shaping analytical method selection. The adaptability of analytical platforms to accommodate new regulatory targets without significant reconfiguration may influence long-term compliance strategies and technology investment decisions.
The United States Environmental Protection Agency (EPA) has approved both ICP-MS (Method 200.8) and IC (Method 300.0/300.1) for different water quality parameters. ICP-MS is specifically recognized for trace metal analysis with detection limits that satisfy the stringent requirements of the Safe Drinking Water Act and Clean Water Act. Meanwhile, IC is the preferred method for anion analysis including chloride, fluoride, and sulfate determinations.
European regulations, particularly the Water Framework Directive and Drinking Water Directive, similarly recognize both techniques but emphasize method validation and uncertainty measurements. European regulatory bodies often require lower detection limits for certain contaminants compared to their US counterparts, potentially favoring ICP-MS in some applications due to its superior sensitivity.
Quality assurance protocols mandated by regulatory agencies necessitate regular calibration, certified reference materials, and participation in proficiency testing programs. ICP-MS laboratories typically face more rigorous quality control requirements due to the technique's complexity and susceptibility to interferences, requiring additional compliance documentation and validation procedures.
Record-keeping requirements differ significantly between methods, with ICP-MS generally demanding more comprehensive documentation of instrument parameters, interference corrections, and quality control measures. Organizations must consider the additional administrative burden when selecting between these analytical approaches.
Method-specific certification requirements present another compliance consideration. Laboratory personnel operating ICP-MS equipment typically require specialized training and certification, while IC operation may have less stringent certification requirements in some jurisdictions, potentially affecting staffing decisions and operational costs.
Emerging regulations concerning previously unmonitored contaminants, such as per- and polyfluoroalkyl substances (PFAS), are increasingly shaping analytical method selection. The adaptability of analytical platforms to accommodate new regulatory targets without significant reconfiguration may influence long-term compliance strategies and technology investment decisions.
Sample Preparation Techniques and Challenges
Sample preparation represents a critical phase in both ICP-MS (Inductively Coupled Plasma Mass Spectrometry) and IC (Ion Chromatography) analytical workflows for water analysis. The quality of results obtained from these sophisticated instruments depends significantly on proper sample handling and preparation techniques.
For ICP-MS analysis, water samples typically require filtration through 0.45 μm membrane filters to remove particulate matter that could potentially clog the nebulizer or interfere with measurements. Acidification with high-purity nitric acid to pH <2 is essential to stabilize metal ions and prevent adsorption to container walls. This preservation step must occur within 24 hours of collection to maintain sample integrity.
IC sample preparation presents different challenges, particularly regarding sample stability. Unlike ICP-MS samples, IC samples should generally not be acidified as this can interfere with ion separation mechanisms. Instead, samples should be kept refrigerated at 4°C and analyzed within 48 hours to prevent microbial activity that might alter ionic composition.
Cross-contamination represents a significant challenge for both techniques. For trace element analysis by ICP-MS, even minor contamination can lead to substantial analytical errors. This necessitates the use of ultra-clean laboratory environments, acid-washed containers, and powder-free gloves during sample handling. IC analysis faces similar concerns, with particular attention needed to avoid contamination from common ions present in laboratory environments.
Matrix effects pose another substantial challenge, especially for complex water samples. High dissolved solid content can suppress ionization in ICP-MS, leading to signal reduction. Sample dilution may be necessary, though this reduces sensitivity for trace elements. For IC analysis, high ionic strength samples may require dilution to prevent column overloading and peak distortion.
Pre-concentration techniques become essential when target analytes exist below direct detection limits. For ICP-MS, solid-phase extraction using chelating resins can effectively concentrate trace metals while removing matrix interferences. IC may employ pre-concentration cartridges for specific ionic species, though these add complexity to the analytical workflow.
Quality control measures must be integrated throughout the sample preparation process. These include preparation blanks to monitor contamination, certified reference materials to verify accuracy, and duplicate samples to assess precision. The implementation of strict protocols for sample collection, preservation, and preparation is fundamental to obtaining reliable quantitative results from both analytical platforms.
For ICP-MS analysis, water samples typically require filtration through 0.45 μm membrane filters to remove particulate matter that could potentially clog the nebulizer or interfere with measurements. Acidification with high-purity nitric acid to pH <2 is essential to stabilize metal ions and prevent adsorption to container walls. This preservation step must occur within 24 hours of collection to maintain sample integrity.
IC sample preparation presents different challenges, particularly regarding sample stability. Unlike ICP-MS samples, IC samples should generally not be acidified as this can interfere with ion separation mechanisms. Instead, samples should be kept refrigerated at 4°C and analyzed within 48 hours to prevent microbial activity that might alter ionic composition.
Cross-contamination represents a significant challenge for both techniques. For trace element analysis by ICP-MS, even minor contamination can lead to substantial analytical errors. This necessitates the use of ultra-clean laboratory environments, acid-washed containers, and powder-free gloves during sample handling. IC analysis faces similar concerns, with particular attention needed to avoid contamination from common ions present in laboratory environments.
Matrix effects pose another substantial challenge, especially for complex water samples. High dissolved solid content can suppress ionization in ICP-MS, leading to signal reduction. Sample dilution may be necessary, though this reduces sensitivity for trace elements. For IC analysis, high ionic strength samples may require dilution to prevent column overloading and peak distortion.
Pre-concentration techniques become essential when target analytes exist below direct detection limits. For ICP-MS, solid-phase extraction using chelating resins can effectively concentrate trace metals while removing matrix interferences. IC may employ pre-concentration cartridges for specific ionic species, though these add complexity to the analytical workflow.
Quality control measures must be integrated throughout the sample preparation process. These include preparation blanks to monitor contamination, certified reference materials to verify accuracy, and duplicate samples to assess precision. The implementation of strict protocols for sample collection, preservation, and preparation is fundamental to obtaining reliable quantitative results from both analytical platforms.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!