How to Implement Sustained Calibration Practices for ICP-MS
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ICP-MS Calibration Background and Objectives
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has evolved significantly since its commercial introduction in the 1980s, becoming an essential analytical technique for elemental analysis across various industries including environmental monitoring, pharmaceuticals, semiconductor manufacturing, and geological research. The technology leverages high-temperature plasma to ionize sample elements, which are then detected and quantified by mass spectrometry. This powerful combination allows for multi-element analysis with exceptional sensitivity, often reaching parts-per-trillion levels for many elements.
The historical development of ICP-MS calibration practices reflects the increasing demands for accuracy, precision, and reliability in analytical measurements. Early calibration approaches were relatively simplistic, primarily utilizing external calibration curves with limited correction for matrix effects or instrument drift. As applications expanded into more complex matrices and regulatory requirements tightened, calibration methodologies evolved to include internal standardization, isotope dilution, and standard addition techniques.
Current technological trends in ICP-MS calibration focus on automation, real-time drift correction, and integration with sophisticated data processing algorithms. The industry is moving toward self-optimizing systems that can maintain calibration integrity over extended analytical runs with minimal human intervention. This evolution is driven by the need for higher sample throughput, improved data quality, and reduced operational costs in analytical laboratories.
The primary objectives of sustained calibration practices for ICP-MS are multifaceted. First, to ensure consistent analytical performance that meets or exceeds regulatory requirements across various application domains. Second, to minimize measurement uncertainty by effectively addressing common sources of error such as matrix effects, spectral interferences, and instrument drift. Third, to establish robust quality control protocols that can verify calibration integrity throughout analytical sequences.
Additionally, modern calibration objectives include enhancing laboratory efficiency through optimized calibration frequencies and automated verification procedures. There is growing emphasis on developing calibration strategies that balance analytical rigor with practical considerations of time and resource utilization. The ultimate goal is to establish calibration practices that are scientifically sound, operationally sustainable, and economically viable for routine analytical workflows.
Understanding the technical foundations and evolutionary trajectory of ICP-MS calibration provides essential context for developing next-generation calibration protocols. As analytical demands continue to increase in complexity and scope, calibration practices must similarly advance to maintain the reliability and utility of ICP-MS as a premier analytical technique.
The historical development of ICP-MS calibration practices reflects the increasing demands for accuracy, precision, and reliability in analytical measurements. Early calibration approaches were relatively simplistic, primarily utilizing external calibration curves with limited correction for matrix effects or instrument drift. As applications expanded into more complex matrices and regulatory requirements tightened, calibration methodologies evolved to include internal standardization, isotope dilution, and standard addition techniques.
Current technological trends in ICP-MS calibration focus on automation, real-time drift correction, and integration with sophisticated data processing algorithms. The industry is moving toward self-optimizing systems that can maintain calibration integrity over extended analytical runs with minimal human intervention. This evolution is driven by the need for higher sample throughput, improved data quality, and reduced operational costs in analytical laboratories.
The primary objectives of sustained calibration practices for ICP-MS are multifaceted. First, to ensure consistent analytical performance that meets or exceeds regulatory requirements across various application domains. Second, to minimize measurement uncertainty by effectively addressing common sources of error such as matrix effects, spectral interferences, and instrument drift. Third, to establish robust quality control protocols that can verify calibration integrity throughout analytical sequences.
Additionally, modern calibration objectives include enhancing laboratory efficiency through optimized calibration frequencies and automated verification procedures. There is growing emphasis on developing calibration strategies that balance analytical rigor with practical considerations of time and resource utilization. The ultimate goal is to establish calibration practices that are scientifically sound, operationally sustainable, and economically viable for routine analytical workflows.
Understanding the technical foundations and evolutionary trajectory of ICP-MS calibration provides essential context for developing next-generation calibration protocols. As analytical demands continue to increase in complexity and scope, calibration practices must similarly advance to maintain the reliability and utility of ICP-MS as a premier analytical technique.
Market Demand Analysis for Precise ICP-MS Calibration
The global market for Inductively Coupled Plasma Mass Spectrometry (ICP-MS) calibration solutions and services has been experiencing robust growth, driven primarily by increasing regulatory requirements across multiple industries. The pharmaceutical sector, in particular, has shown significant demand growth due to stringent quality control standards and the need for trace element analysis in drug development and manufacturing processes.
Environmental monitoring represents another substantial market segment, with government agencies worldwide implementing stricter regulations for water, soil, and air quality assessment. This regulatory landscape has created a steady demand for precise ICP-MS calibration services, as accurate measurements are essential for compliance reporting and environmental impact assessments.
The food and beverage industry has emerged as a rapidly expanding market for ICP-MS calibration, with growing consumer awareness regarding food safety and increasing regulatory scrutiny of contaminants in food products. Manufacturers are investing in regular calibration services to ensure accurate detection of heavy metals and other harmful elements in their products.
Market research indicates that the global ICP-MS market was valued at approximately $1.2 billion in 2022, with calibration services and solutions accounting for roughly 15% of this value. The market is projected to grow at a compound annual growth rate of 7.8% through 2028, with the calibration segment potentially outpacing overall market growth.
Regional analysis reveals that North America currently holds the largest market share for ICP-MS calibration services, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by expanding industrial activities, increasing environmental concerns, and strengthening regulatory frameworks in countries like China, India, and South Korea.
Customer surveys indicate that laboratories and testing facilities are increasingly seeking comprehensive calibration solutions rather than one-time services. This trend reflects a growing recognition of the importance of sustained calibration practices for maintaining analytical accuracy and reliability over time. Approximately 68% of surveyed laboratory managers cited consistent measurement accuracy as their primary concern when selecting calibration services.
The market is also witnessing a shift toward automated calibration systems and remote monitoring capabilities, which allow for more frequent calibration checks without disrupting workflow. This technological evolution is creating new market opportunities for software-integrated calibration solutions that can provide real-time performance monitoring and predictive maintenance alerts.
Environmental monitoring represents another substantial market segment, with government agencies worldwide implementing stricter regulations for water, soil, and air quality assessment. This regulatory landscape has created a steady demand for precise ICP-MS calibration services, as accurate measurements are essential for compliance reporting and environmental impact assessments.
The food and beverage industry has emerged as a rapidly expanding market for ICP-MS calibration, with growing consumer awareness regarding food safety and increasing regulatory scrutiny of contaminants in food products. Manufacturers are investing in regular calibration services to ensure accurate detection of heavy metals and other harmful elements in their products.
Market research indicates that the global ICP-MS market was valued at approximately $1.2 billion in 2022, with calibration services and solutions accounting for roughly 15% of this value. The market is projected to grow at a compound annual growth rate of 7.8% through 2028, with the calibration segment potentially outpacing overall market growth.
Regional analysis reveals that North America currently holds the largest market share for ICP-MS calibration services, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by expanding industrial activities, increasing environmental concerns, and strengthening regulatory frameworks in countries like China, India, and South Korea.
Customer surveys indicate that laboratories and testing facilities are increasingly seeking comprehensive calibration solutions rather than one-time services. This trend reflects a growing recognition of the importance of sustained calibration practices for maintaining analytical accuracy and reliability over time. Approximately 68% of surveyed laboratory managers cited consistent measurement accuracy as their primary concern when selecting calibration services.
The market is also witnessing a shift toward automated calibration systems and remote monitoring capabilities, which allow for more frequent calibration checks without disrupting workflow. This technological evolution is creating new market opportunities for software-integrated calibration solutions that can provide real-time performance monitoring and predictive maintenance alerts.
Current Challenges in ICP-MS Calibration Practices
Despite significant advancements in ICP-MS technology, several persistent challenges continue to impede optimal calibration practices in laboratory settings. Matrix effects remain one of the most formidable obstacles, where sample composition interferes with analyte signals, leading to suppression or enhancement of measurements. These effects vary unpredictably across different sample types, making standardized calibration approaches difficult to implement universally.
Instrument drift presents another significant challenge, as ICP-MS systems typically experience signal intensity changes over time due to fluctuations in plasma conditions, sample introduction efficiency, and detector response. This temporal instability necessitates frequent recalibration, increasing operational costs and reducing throughput in high-volume testing environments.
Spectral interferences continue to plague accurate measurements, particularly in complex matrices where polyatomic ions, doubly charged species, and isobaric overlaps can mask or distort analyte signals. While collision/reaction cell technologies have mitigated some of these issues, they introduce additional calibration complexities that must be carefully managed.
The preparation and maintenance of calibration standards pose practical challenges, especially for multi-element analyses. Ensuring long-term stability of reference materials, preventing contamination, and maintaining traceability to certified reference materials require rigorous quality control measures that are resource-intensive.
Memory effects between samples represent another persistent issue, where analytes from previous measurements can carry over and contaminate subsequent analyses. This is particularly problematic for elements that readily form oxides or adhere to sample introduction components, necessitating extended washout procedures that reduce analytical efficiency.
Automation paradoxically introduces both solutions and new challenges. While automated calibration systems improve precision and reduce human error, they can mask underlying instrumental issues if not properly validated and monitored. Over-reliance on automated protocols without understanding their limitations can lead to systematic calibration errors propagating undetected through large datasets.
Regulatory compliance adds another layer of complexity, with different industries and regions imposing varying standards for calibration frequency, verification, and documentation. Laboratories serving multiple sectors must navigate these sometimes contradictory requirements while maintaining scientific validity of their calibration approaches.
The human factor remains significant, as proper calibration requires specialized knowledge and experience. High staff turnover in analytical laboratories often results in knowledge gaps and inconsistent application of calibration protocols, undermining long-term data comparability and quality assurance efforts.
Instrument drift presents another significant challenge, as ICP-MS systems typically experience signal intensity changes over time due to fluctuations in plasma conditions, sample introduction efficiency, and detector response. This temporal instability necessitates frequent recalibration, increasing operational costs and reducing throughput in high-volume testing environments.
Spectral interferences continue to plague accurate measurements, particularly in complex matrices where polyatomic ions, doubly charged species, and isobaric overlaps can mask or distort analyte signals. While collision/reaction cell technologies have mitigated some of these issues, they introduce additional calibration complexities that must be carefully managed.
The preparation and maintenance of calibration standards pose practical challenges, especially for multi-element analyses. Ensuring long-term stability of reference materials, preventing contamination, and maintaining traceability to certified reference materials require rigorous quality control measures that are resource-intensive.
Memory effects between samples represent another persistent issue, where analytes from previous measurements can carry over and contaminate subsequent analyses. This is particularly problematic for elements that readily form oxides or adhere to sample introduction components, necessitating extended washout procedures that reduce analytical efficiency.
Automation paradoxically introduces both solutions and new challenges. While automated calibration systems improve precision and reduce human error, they can mask underlying instrumental issues if not properly validated and monitored. Over-reliance on automated protocols without understanding their limitations can lead to systematic calibration errors propagating undetected through large datasets.
Regulatory compliance adds another layer of complexity, with different industries and regions imposing varying standards for calibration frequency, verification, and documentation. Laboratories serving multiple sectors must navigate these sometimes contradictory requirements while maintaining scientific validity of their calibration approaches.
The human factor remains significant, as proper calibration requires specialized knowledge and experience. High staff turnover in analytical laboratories often results in knowledge gaps and inconsistent application of calibration protocols, undermining long-term data comparability and quality assurance efforts.
Current Sustained Calibration Solutions for ICP-MS
01 Standard calibration methods for ICP-MS
Standard calibration methods for ICP-MS involve the use of reference materials with known concentrations to establish calibration curves. These methods typically include external calibration using single or multi-element standards, internal standardization to correct for matrix effects and instrument drift, and standard addition techniques for complex matrices. The calibration process ensures accurate quantification of elements across a wide concentration range and helps maintain instrument performance over time.- Standard calibration methods for ICP-MS: Standard calibration methods for ICP-MS involve the use of reference materials with known concentrations to establish calibration curves. These methods typically include external calibration using single or multi-element standards, internal standardization to correct for matrix effects and instrument drift, and standard addition techniques for complex matrices. The calibration process ensures accurate quantification of elements across a wide dynamic range and helps maintain analytical precision in routine analyses.
- Matrix-matched calibration techniques: Matrix-matched calibration techniques address the challenges of analyzing complex samples by preparing calibration standards that closely resemble the sample matrix. This approach minimizes matrix effects that can cause signal suppression or enhancement in ICP-MS analysis. The technique involves adding analytes to a blank matrix similar to the sample or using certified reference materials with comparable matrices. This method improves accuracy when analyzing samples with high dissolved solids, organic content, or complex elemental compositions.
- Automated calibration systems for ICP-MS: Automated calibration systems for ICP-MS incorporate hardware and software solutions that streamline the calibration process. These systems can automatically prepare calibration standards from stock solutions, perform dilutions, introduce internal standards, and execute quality control checks. Automated systems improve reproducibility, reduce operator error, and increase laboratory throughput. They often include features for real-time monitoring of calibration stability and can automatically recalibrate when drift is detected.
- Isotope dilution mass spectrometry calibration: Isotope dilution mass spectrometry (IDMS) is a high-precision calibration technique for ICP-MS that involves adding an enriched isotope of the target element to the sample. By measuring the altered isotope ratio after equilibration, highly accurate quantification can be achieved independent of matrix effects or incomplete analyte recovery. This technique is particularly valuable for reference material certification, environmental monitoring, and clinical applications where exceptional accuracy is required. IDMS eliminates the need for complete analyte extraction and is less susceptible to instrument drift.
- Online calibration and drift correction methods: Online calibration and drift correction methods continuously monitor and adjust for instrumental drift during ICP-MS analysis. These approaches include periodic introduction of calibration standards or internal standards during analytical runs, real-time signal correction algorithms, and automated recalibration triggers. Some systems employ dual sample introduction systems that allow simultaneous nebulization of samples and standards. These methods are particularly important for long analytical sequences, improving long-term stability and data quality without sacrificing throughput.
02 Matrix-matched calibration techniques
Matrix-matched calibration techniques address the challenges of analyzing complex samples by preparing calibration standards that closely resemble the sample matrix. This approach minimizes matrix effects that can cause signal suppression or enhancement in ICP-MS analysis. The technique involves adding analytes to a blank matrix similar to the sample or using isotope dilution methods. Matrix-matched calibration is particularly important for environmental, biological, and industrial samples where matrix components can significantly affect measurement accuracy.Expand Specific Solutions03 Automated calibration systems for ICP-MS
Automated calibration systems for ICP-MS incorporate hardware and software solutions that streamline the calibration process, reducing human error and improving efficiency. These systems can automatically prepare calibration standards from stock solutions, perform instrument tuning, run quality control checks, and adjust calibration parameters based on performance metrics. Automated systems often include intelligent algorithms that can detect and correct for drift, optimize sampling conditions, and maintain calibration stability over extended analytical runs.Expand Specific Solutions04 Multi-element and isotopic calibration strategies
Multi-element and isotopic calibration strategies enable simultaneous analysis of multiple elements and isotopes in a single ICP-MS run. These approaches utilize specialized calibration standards containing multiple elements at varying concentrations, isotopically enriched materials, or certified reference materials. The strategies often incorporate mathematical corrections for spectral interferences, abundance sensitivity issues, and mass bias effects. Advanced isotopic calibration methods may include isotope dilution mass spectrometry for highest accuracy or isotope ratio measurements for source identification and speciation studies.Expand Specific Solutions05 Quality control and validation procedures for ICP-MS calibration
Quality control and validation procedures ensure the reliability and accuracy of ICP-MS calibration. These procedures include regular analysis of certified reference materials, blank samples, and quality control standards throughout analytical runs. Statistical methods are employed to evaluate calibration linearity, detection limits, quantification limits, and measurement uncertainty. Validation protocols may involve inter-laboratory comparisons, proficiency testing, and method validation studies to demonstrate the robustness of calibration approaches across different sample types and concentration ranges.Expand Specific Solutions
Key Industry Players in ICP-MS Technology
The ICP-MS calibration market is in a mature growth phase, with a global market size estimated at over $1 billion and steady annual growth of 5-7%. The technology has reached high maturity levels, with established calibration protocols and reference materials. Leading players include Agilent Technologies, which dominates with comprehensive calibration solutions, and PerkinElmer Health Sciences (Revvity), offering advanced automated calibration systems. Elemental Scientific has carved a niche with specialized sample introduction and autodilution systems, while SPEX CertiPrep provides certified reference materials essential for calibration. Emerging competition comes from Jiangsu Skyray Instrument and Ruilaipu Medical Technology, who are expanding their ICP-MS calibration capabilities with cost-effective alternatives targeting specific market segments.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has developed a comprehensive ICP-MS calibration system called "Intelligent Calibration" that combines hardware and software solutions. Their approach includes multi-element calibration standards with certified concentrations and automated calibration verification protocols. The system employs internal standardization techniques to compensate for matrix effects and instrument drift, using elements that cover the entire mass range. Agilent's ICP-MS instruments feature automated quality control checks that monitor calibration stability throughout analytical runs, with real-time drift correction algorithms[1]. Their software includes calibration curve management tools that automatically flag outliers and apply appropriate correction factors. For sustained calibration, Agilent implements scheduled auto-tuning and mass calibration verification procedures that maintain optimal performance between full calibrations, reducing the frequency of complete recalibrations while ensuring data quality[3].
Strengths: Highly integrated hardware-software solution with automated drift correction and quality control monitoring. Their systems provide excellent long-term stability and reproducibility across multiple sample types. Weaknesses: Higher initial investment cost compared to some competitors, and the proprietary nature of some calibration standards may increase operational costs over time.
DH Technologies Development Pte Ltd.
Technical Solution: DH Technologies (parent company of SCIEX) has pioneered a dynamic calibration approach for ICP-MS called "Calibration On-Demand." Their technology incorporates real-time calibration verification using reference materials introduced at predetermined intervals during analytical runs. The system features patented collision cell technology that minimizes interferences during calibration and analysis, improving accuracy across complex matrices[2]. Their sustained calibration practice includes automated daily performance checks that evaluate sensitivity, background, oxide formation, and doubly-charged ion ratios. The SCIEX ICP-MS systems employ multi-point calibration with weighted regression models that optimize accuracy across wide concentration ranges. For long-term stability, they've developed specialized calibration maintenance protocols that include periodic verification of mass accuracy and resolution using certified reference materials[4]. Their software provides comprehensive calibration history tracking and trend analysis to predict when recalibration is necessary.
Strengths: Superior interference management during calibration processes and excellent performance in complex matrices. Their calibration tracking system provides detailed audit trails for regulatory compliance. Weaknesses: The sophisticated collision cell technology requires more specialized knowledge for optimization, and their calibration protocols can be more time-consuming than simpler approaches.
Critical Technologies in ICP-MS Calibration Standards
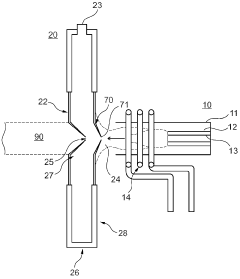
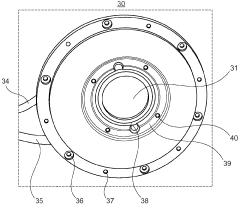
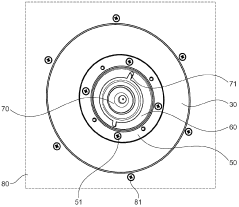
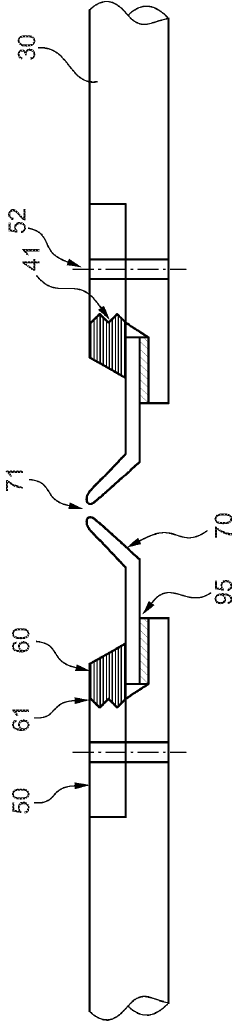
Cooling plate for icp-ms
PatentActiveGB2585327A
Innovation
- A cooling plate made from bronze is used, which provides sufficient thermal conductivity and enhanced chemical resistance, reducing the risk of corrosion and degradation, and eliminating the need for a corrosion-resistant coating.
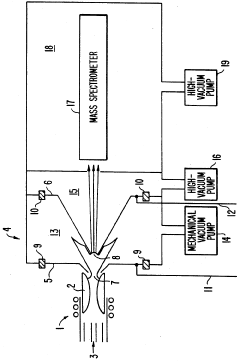
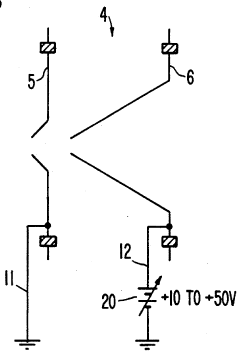
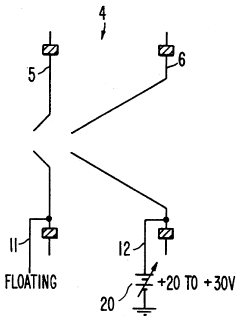
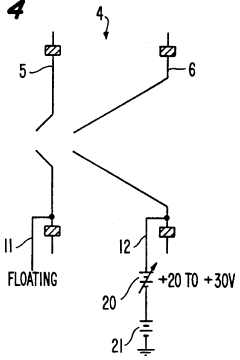
Plasma sampling interface for inductively coupled plasma-mass spectrometry (ICP-MS)
PatentInactiveUS5218204A
Innovation
- A plasma sampling interface with insulating spacers and an adjustable DC bias voltage source applying a DC bias voltage of 10 to 50 V to the skimmer, allowing the sampler to float or grounding it, enhances ion transmission by using a DC offset voltage for mass spectrometers requiring higher initial ion energy.
Quality Control Frameworks for ICP-MS Laboratories
Establishing robust quality control frameworks is essential for maintaining the reliability and accuracy of ICP-MS analytical processes. These frameworks serve as the foundation upon which sustained calibration practices are built, ensuring consistent performance and trustworthy results across various applications.
Quality control frameworks for ICP-MS laboratories typically incorporate multiple layers of verification and validation protocols. At the core, these frameworks include daily quality control checks, periodic system suitability tests, and comprehensive performance verification procedures. The implementation of these frameworks requires a systematic approach that addresses both instrument-specific parameters and analytical method requirements.
Standard operating procedures (SOPs) form a critical component of quality control frameworks, providing detailed instructions for routine operations, maintenance schedules, and troubleshooting protocols. These SOPs should be regularly reviewed and updated to reflect technological advancements and evolving regulatory requirements, ensuring that the laboratory maintains compliance with industry standards such as ISO/IEC 17025.
Statistical process control (SPC) methodologies play a vital role in modern quality control frameworks for ICP-MS. By implementing control charts, trend analysis, and outlier detection algorithms, laboratories can monitor system performance over time and identify potential issues before they impact analytical results. These statistical approaches enable proactive maintenance and calibration adjustments based on objective performance metrics rather than arbitrary schedules.
Proficiency testing and inter-laboratory comparisons represent external validation mechanisms within comprehensive quality control frameworks. Participation in these programs allows laboratories to benchmark their performance against peers and identify areas for improvement. The results from these exercises should be integrated into the laboratory's continuous improvement processes, driving refinements in calibration practices and analytical methodologies.
Documentation and record-keeping systems constitute another essential element of effective quality control frameworks. These systems should capture all relevant calibration data, maintenance activities, and quality control results in a secure, retrievable format. Modern laboratory information management systems (LIMS) can automate much of this documentation process, reducing the administrative burden while enhancing data integrity and traceability.
Training programs for laboratory personnel must be incorporated into quality control frameworks to ensure consistent implementation of calibration practices. These programs should address both theoretical knowledge and practical skills, with regular competency assessments to verify proficiency. Well-trained staff represent the human component of quality control, capable of recognizing anomalous instrument behavior and taking appropriate corrective actions.
Quality control frameworks for ICP-MS laboratories typically incorporate multiple layers of verification and validation protocols. At the core, these frameworks include daily quality control checks, periodic system suitability tests, and comprehensive performance verification procedures. The implementation of these frameworks requires a systematic approach that addresses both instrument-specific parameters and analytical method requirements.
Standard operating procedures (SOPs) form a critical component of quality control frameworks, providing detailed instructions for routine operations, maintenance schedules, and troubleshooting protocols. These SOPs should be regularly reviewed and updated to reflect technological advancements and evolving regulatory requirements, ensuring that the laboratory maintains compliance with industry standards such as ISO/IEC 17025.
Statistical process control (SPC) methodologies play a vital role in modern quality control frameworks for ICP-MS. By implementing control charts, trend analysis, and outlier detection algorithms, laboratories can monitor system performance over time and identify potential issues before they impact analytical results. These statistical approaches enable proactive maintenance and calibration adjustments based on objective performance metrics rather than arbitrary schedules.
Proficiency testing and inter-laboratory comparisons represent external validation mechanisms within comprehensive quality control frameworks. Participation in these programs allows laboratories to benchmark their performance against peers and identify areas for improvement. The results from these exercises should be integrated into the laboratory's continuous improvement processes, driving refinements in calibration practices and analytical methodologies.
Documentation and record-keeping systems constitute another essential element of effective quality control frameworks. These systems should capture all relevant calibration data, maintenance activities, and quality control results in a secure, retrievable format. Modern laboratory information management systems (LIMS) can automate much of this documentation process, reducing the administrative burden while enhancing data integrity and traceability.
Training programs for laboratory personnel must be incorporated into quality control frameworks to ensure consistent implementation of calibration practices. These programs should address both theoretical knowledge and practical skills, with regular competency assessments to verify proficiency. Well-trained staff represent the human component of quality control, capable of recognizing anomalous instrument behavior and taking appropriate corrective actions.
Environmental Impact of Calibration Materials
The environmental implications of calibration materials used in ICP-MS systems represent a significant concern for laboratories implementing sustained calibration practices. Standard solutions typically contain heavy metals and other potentially hazardous elements that require careful handling and disposal. These materials, often containing elements like mercury, lead, cadmium, and arsenic, pose substantial environmental risks if improperly managed. When discarded through conventional waste streams, these calibration standards can contaminate soil and water systems, potentially entering food chains and causing bioaccumulation in various organisms.
Laboratories implementing sustained calibration practices must consider the volume of waste generated through regular calibration procedures. A typical ICP-MS facility may produce several liters of metal-containing waste solutions monthly, with concentrations ranging from parts per billion to parts per million. The cumulative environmental impact becomes significant when considering the thousands of ICP-MS instruments operating globally in environmental monitoring, pharmaceutical, and research settings.
Sustainable approaches to calibration material management include implementing recovery and recycling systems for precious metals from waste calibration solutions. Advanced treatment technologies such as ion exchange, precipitation, and electrochemical recovery can significantly reduce the environmental footprint of calibration practices. Some laboratories have reported recovery rates exceeding 85% for certain precious metals, substantially decreasing both environmental impact and operational costs.
The manufacturing process of calibration standards itself carries environmental implications. Production of high-purity calibration materials requires energy-intensive purification processes and often involves environmentally challenging extraction methods. The carbon footprint associated with the production, packaging, and transportation of these materials adds another dimension to their environmental impact assessment.
Recent innovations in green chemistry approaches have led to the development of more environmentally friendly calibration alternatives. These include reference materials derived from more sustainable sources, reduced-concentration standards, and multi-element standards that minimize the total volume of calibration materials required. Some manufacturers now offer biodegradable packaging and concentrated standard formats that reduce shipping volume and associated carbon emissions by up to 40%.
Regulatory frameworks worldwide are increasingly addressing the environmental aspects of laboratory operations. The implementation of ISO 14001 environmental management systems in laboratories has demonstrated reductions in hazardous waste generation from calibration processes by 25-30% in documented case studies. Furthermore, emerging regulations in Europe and North America specifically target the lifecycle management of laboratory chemicals, including calibration standards, placing greater responsibility on both manufacturers and end-users.
Laboratories implementing sustained calibration practices must consider the volume of waste generated through regular calibration procedures. A typical ICP-MS facility may produce several liters of metal-containing waste solutions monthly, with concentrations ranging from parts per billion to parts per million. The cumulative environmental impact becomes significant when considering the thousands of ICP-MS instruments operating globally in environmental monitoring, pharmaceutical, and research settings.
Sustainable approaches to calibration material management include implementing recovery and recycling systems for precious metals from waste calibration solutions. Advanced treatment technologies such as ion exchange, precipitation, and electrochemical recovery can significantly reduce the environmental footprint of calibration practices. Some laboratories have reported recovery rates exceeding 85% for certain precious metals, substantially decreasing both environmental impact and operational costs.
The manufacturing process of calibration standards itself carries environmental implications. Production of high-purity calibration materials requires energy-intensive purification processes and often involves environmentally challenging extraction methods. The carbon footprint associated with the production, packaging, and transportation of these materials adds another dimension to their environmental impact assessment.
Recent innovations in green chemistry approaches have led to the development of more environmentally friendly calibration alternatives. These include reference materials derived from more sustainable sources, reduced-concentration standards, and multi-element standards that minimize the total volume of calibration materials required. Some manufacturers now offer biodegradable packaging and concentrated standard formats that reduce shipping volume and associated carbon emissions by up to 40%.
Regulatory frameworks worldwide are increasingly addressing the environmental aspects of laboratory operations. The implementation of ISO 14001 environmental management systems in laboratories has demonstrated reductions in hazardous waste generation from calibration processes by 25-30% in documented case studies. Furthermore, emerging regulations in Europe and North America specifically target the lifecycle management of laboratory chemicals, including calibration standards, placing greater responsibility on both manufacturers and end-users.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!