Analyzing Organic Samples Using ICP-MS: Methods and Challenges
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ICP-MS for Organic Analysis: Background and Objectives
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has evolved significantly since its commercial introduction in the 1980s, transforming from a specialized analytical technique to an essential tool across multiple scientific disciplines. The technology's development trajectory has been characterized by continuous improvements in sensitivity, precision, and application versatility, particularly in the challenging domain of organic sample analysis.
The historical progression of ICP-MS technology reveals a gradual shift from primarily inorganic applications toward increasingly sophisticated organic sample analysis capabilities. Early systems faced substantial limitations when processing organic matrices due to carbon deposition, plasma instability, and spectral interferences. These challenges prompted technological innovations including collision/reaction cells, improved sample introduction systems, and enhanced data processing algorithms specifically designed to address organic matrix complexities.
Current technological trends in ICP-MS for organic analysis focus on miniaturization, automation, and integration with complementary techniques such as chromatography and spectrometry. The convergence of these technologies has expanded the analytical capabilities beyond traditional elemental analysis to include speciation studies, metabolomics, and proteomics applications involving complex organic matrices.
The primary objective of this technical research is to comprehensively evaluate current methodologies for analyzing organic samples using ICP-MS, with particular emphasis on sample preparation techniques, matrix effect mitigation strategies, and instrumental parameter optimization. We aim to identify the most effective approaches for overcoming persistent challenges in organic sample analysis, including carbon buildup, polyatomic interferences, and matrix-induced signal suppression.
Additionally, this investigation seeks to assess emerging technologies that promise to enhance organic sample analysis capabilities, such as triple-quadrupole systems, time-of-flight mass analyzers, and novel plasma interface designs. By examining these technological developments, we intend to forecast future directions in ICP-MS instrumentation specifically tailored for organic applications.
The ultimate goal is to establish a technical foundation for developing standardized protocols that maximize analytical performance when processing diverse organic matrices, ranging from biological fluids and tissues to environmental samples and food products. This research will provide valuable insights for laboratories seeking to expand their analytical capabilities into challenging organic sample types while maintaining the high sensitivity and precision that characterize modern ICP-MS analysis.
The historical progression of ICP-MS technology reveals a gradual shift from primarily inorganic applications toward increasingly sophisticated organic sample analysis capabilities. Early systems faced substantial limitations when processing organic matrices due to carbon deposition, plasma instability, and spectral interferences. These challenges prompted technological innovations including collision/reaction cells, improved sample introduction systems, and enhanced data processing algorithms specifically designed to address organic matrix complexities.
Current technological trends in ICP-MS for organic analysis focus on miniaturization, automation, and integration with complementary techniques such as chromatography and spectrometry. The convergence of these technologies has expanded the analytical capabilities beyond traditional elemental analysis to include speciation studies, metabolomics, and proteomics applications involving complex organic matrices.
The primary objective of this technical research is to comprehensively evaluate current methodologies for analyzing organic samples using ICP-MS, with particular emphasis on sample preparation techniques, matrix effect mitigation strategies, and instrumental parameter optimization. We aim to identify the most effective approaches for overcoming persistent challenges in organic sample analysis, including carbon buildup, polyatomic interferences, and matrix-induced signal suppression.
Additionally, this investigation seeks to assess emerging technologies that promise to enhance organic sample analysis capabilities, such as triple-quadrupole systems, time-of-flight mass analyzers, and novel plasma interface designs. By examining these technological developments, we intend to forecast future directions in ICP-MS instrumentation specifically tailored for organic applications.
The ultimate goal is to establish a technical foundation for developing standardized protocols that maximize analytical performance when processing diverse organic matrices, ranging from biological fluids and tissues to environmental samples and food products. This research will provide valuable insights for laboratories seeking to expand their analytical capabilities into challenging organic sample types while maintaining the high sensitivity and precision that characterize modern ICP-MS analysis.
Market Demand for Organic Sample Analysis
The global market for organic sample analysis using ICP-MS has experienced significant growth over the past decade, driven primarily by increasing regulatory requirements across multiple industries. Environmental monitoring agencies, pharmaceutical companies, food safety organizations, and research institutions represent the core demand sectors, collectively contributing to a market valuation exceeding $2.5 billion in 2023, with projected annual growth rates of 6-8% through 2028.
Environmental testing represents the largest market segment, accounting for approximately 35% of the total demand. This is largely attributed to stricter global regulations on water quality, soil contamination, and air pollution monitoring. The Clean Water Act amendments and similar international regulations have mandated more comprehensive testing protocols, specifically requiring the detection of trace organic contaminants at increasingly lower concentration levels.
The pharmaceutical and biotechnology sectors constitute the fastest-growing segment, with demand increasing at nearly 9% annually. This growth is driven by the need for ultra-sensitive detection of impurities in drug formulations and biological samples. The FDA's implementation of more stringent quality control requirements has significantly expanded the application of ICP-MS in pharmaceutical manufacturing and quality assurance processes.
Food safety testing represents another substantial market segment, particularly following several high-profile contamination incidents that have heightened consumer awareness and regulatory scrutiny. The demand for organic sample analysis in this sector has grown by approximately 7.5% annually, with particular emphasis on detecting pesticide residues, heavy metals in organic matrices, and food adulterants.
Geographically, North America and Europe currently dominate the market, collectively accounting for over 60% of global demand. However, the Asia-Pacific region, particularly China and India, is experiencing the most rapid growth, driven by expanding industrial bases, increasing environmental concerns, and strengthening regulatory frameworks. Market analysts project that the Asia-Pacific region will represent nearly 30% of the global market by 2027.
Customer requirements are increasingly focused on higher sensitivity, improved matrix tolerance, and more efficient sample preparation methods. End users consistently report challenges with complex organic matrices interfering with accurate analysis, creating significant demand for advanced sample preparation technologies and specialized ICP-MS instruments designed specifically for organic sample analysis.
The service segment of this market is also expanding rapidly, with contract testing laboratories reporting annual growth rates exceeding 10%. This trend reflects the increasing outsourcing of complex analytical testing by companies seeking to reduce capital expenditures while maintaining access to cutting-edge analytical capabilities.
Environmental testing represents the largest market segment, accounting for approximately 35% of the total demand. This is largely attributed to stricter global regulations on water quality, soil contamination, and air pollution monitoring. The Clean Water Act amendments and similar international regulations have mandated more comprehensive testing protocols, specifically requiring the detection of trace organic contaminants at increasingly lower concentration levels.
The pharmaceutical and biotechnology sectors constitute the fastest-growing segment, with demand increasing at nearly 9% annually. This growth is driven by the need for ultra-sensitive detection of impurities in drug formulations and biological samples. The FDA's implementation of more stringent quality control requirements has significantly expanded the application of ICP-MS in pharmaceutical manufacturing and quality assurance processes.
Food safety testing represents another substantial market segment, particularly following several high-profile contamination incidents that have heightened consumer awareness and regulatory scrutiny. The demand for organic sample analysis in this sector has grown by approximately 7.5% annually, with particular emphasis on detecting pesticide residues, heavy metals in organic matrices, and food adulterants.
Geographically, North America and Europe currently dominate the market, collectively accounting for over 60% of global demand. However, the Asia-Pacific region, particularly China and India, is experiencing the most rapid growth, driven by expanding industrial bases, increasing environmental concerns, and strengthening regulatory frameworks. Market analysts project that the Asia-Pacific region will represent nearly 30% of the global market by 2027.
Customer requirements are increasingly focused on higher sensitivity, improved matrix tolerance, and more efficient sample preparation methods. End users consistently report challenges with complex organic matrices interfering with accurate analysis, creating significant demand for advanced sample preparation technologies and specialized ICP-MS instruments designed specifically for organic sample analysis.
The service segment of this market is also expanding rapidly, with contract testing laboratories reporting annual growth rates exceeding 10%. This trend reflects the increasing outsourcing of complex analytical testing by companies seeking to reduce capital expenditures while maintaining access to cutting-edge analytical capabilities.
Technical Challenges in Organic ICP-MS Applications
The application of ICP-MS (Inductively Coupled Plasma Mass Spectrometry) to organic samples presents numerous technical challenges that significantly impact analytical performance and reliability. One of the primary obstacles is sample introduction, as organic matrices often exhibit high viscosity, volatility, and surface tension properties that differ substantially from aqueous solutions. These physical characteristics can lead to inconsistent nebulization, affecting the stability of the plasma and ultimately compromising measurement precision.
Matrix effects represent another substantial challenge, with organic carbon causing significant spectral interferences. When introduced into the plasma, carbon-rich samples can form polyatomic species (e.g., 12C40Ar+, 12C35Cl+) that overlap with analyte signals, particularly for elements like Fe, As, and V. Additionally, carbon deposition on sampler and skimmer cones progressively degrades instrument sensitivity and stability during analytical runs, necessitating frequent maintenance interventions.
Plasma stability issues are particularly problematic when analyzing organic samples. The high carbon content can cool the plasma, altering ionization conditions and causing signal suppression or enhancement for different elements. This phenomenon, known as carbon-induced matrix effects, creates significant challenges for accurate quantification, especially when calibration standards do not precisely match sample matrices.
Memory effects present additional complications, as many organic compounds can adsorb onto sample introduction components, causing cross-contamination between successive measurements. This is particularly problematic for volatile organic compounds and certain organometallic species, requiring extensive washout procedures that reduce analytical throughput and efficiency.
Sample preparation techniques for organic ICP-MS analysis also face significant limitations. Traditional acid digestion methods may be ineffective for certain organic matrices, while alternative approaches like microwave-assisted digestion or combustion methods can introduce additional contamination or analyte loss. The incomplete decomposition of organic matter often leads to residual carbon in the final solution, perpetuating matrix effect issues.
Calibration strategies present further challenges, as matrix-matched calibration standards are difficult to prepare for complex organic samples. Internal standardization, while helpful, cannot fully compensate for the dynamic matrix effects that occur during analysis. The lack of certified reference materials for many organic sample types compounds this problem, making method validation particularly challenging.
Instrument configuration optimization remains complex, requiring careful balance between sensitivity, stability, and interference reduction. Technologies like collision/reaction cells can mitigate some spectral interferences but may introduce new analytical complexities and reduce overall sensitivity for certain elements of interest.
Matrix effects represent another substantial challenge, with organic carbon causing significant spectral interferences. When introduced into the plasma, carbon-rich samples can form polyatomic species (e.g., 12C40Ar+, 12C35Cl+) that overlap with analyte signals, particularly for elements like Fe, As, and V. Additionally, carbon deposition on sampler and skimmer cones progressively degrades instrument sensitivity and stability during analytical runs, necessitating frequent maintenance interventions.
Plasma stability issues are particularly problematic when analyzing organic samples. The high carbon content can cool the plasma, altering ionization conditions and causing signal suppression or enhancement for different elements. This phenomenon, known as carbon-induced matrix effects, creates significant challenges for accurate quantification, especially when calibration standards do not precisely match sample matrices.
Memory effects present additional complications, as many organic compounds can adsorb onto sample introduction components, causing cross-contamination between successive measurements. This is particularly problematic for volatile organic compounds and certain organometallic species, requiring extensive washout procedures that reduce analytical throughput and efficiency.
Sample preparation techniques for organic ICP-MS analysis also face significant limitations. Traditional acid digestion methods may be ineffective for certain organic matrices, while alternative approaches like microwave-assisted digestion or combustion methods can introduce additional contamination or analyte loss. The incomplete decomposition of organic matter often leads to residual carbon in the final solution, perpetuating matrix effect issues.
Calibration strategies present further challenges, as matrix-matched calibration standards are difficult to prepare for complex organic samples. Internal standardization, while helpful, cannot fully compensate for the dynamic matrix effects that occur during analysis. The lack of certified reference materials for many organic sample types compounds this problem, making method validation particularly challenging.
Instrument configuration optimization remains complex, requiring careful balance between sensitivity, stability, and interference reduction. Technologies like collision/reaction cells can mitigate some spectral interferences but may introduce new analytical complexities and reduce overall sensitivity for certain elements of interest.
Current Methodologies for Organic Sample Preparation
01 Sample preparation techniques for ICP-MS analysis
Various sample preparation methods are crucial for accurate ICP-MS analysis. These include digestion procedures for solid samples, dilution protocols for liquid samples, and extraction techniques for complex matrices. Proper sample preparation helps minimize matrix effects, reduce interferences, and improve detection limits. Advanced techniques such as microwave-assisted digestion and automated sample preparation systems can enhance efficiency and reproducibility of the analysis.- Sample preparation techniques for ICP-MS analysis: Various sample preparation methods are crucial for accurate ICP-MS analysis. These include digestion procedures for solid samples, dilution protocols for liquid samples, and extraction techniques for complex matrices. Proper sample preparation helps minimize matrix effects, reduce interferences, and improve detection limits. Advanced preparation techniques may involve microwave-assisted digestion, ultrasonic extraction, or automated sample handling systems to enhance efficiency and reproducibility.
- Interference reduction and elimination strategies: ICP-MS analysis faces challenges from spectral and non-spectral interferences that can affect measurement accuracy. Various strategies have been developed to address these issues, including collision/reaction cell technology, mathematical correction models, and high-resolution mass spectrometry. These approaches help separate analyte ions from interfering species, particularly important when analyzing complex environmental or biological samples containing multiple elements with overlapping mass-to-charge ratios.
- Calibration and quantification methods: Accurate quantification in ICP-MS requires robust calibration strategies. These include external calibration with matrix-matched standards, standard addition methods, and isotope dilution techniques. Internal standardization is commonly employed to compensate for matrix effects and instrument drift. Quality control procedures involving certified reference materials and regular performance checks are essential for maintaining analytical reliability and ensuring accurate trace element determination across different sample types.
- Specialized ICP-MS instrumentation and modifications: Advancements in ICP-MS instrumentation have led to specialized systems for particular analytical challenges. These include high-resolution ICP-MS for resolving spectral interferences, triple quadrupole systems for improved selectivity, and laser ablation systems for direct solid sample analysis. Modifications to sample introduction systems, plasma generation, and ion optics have improved sensitivity, stability, and detection limits. Automated systems with integrated sample preparation capabilities enhance throughput and reproducibility.
- Applications and analytical challenges in specific fields: ICP-MS is applied across diverse fields including environmental monitoring, food safety, pharmaceutical analysis, and geological studies. Each application presents unique analytical challenges related to sample complexity, required detection limits, and regulatory compliance. For instance, environmental samples may contain high salt content interfering with analysis, while biological samples often require specialized digestion procedures. Emerging applications include single-particle analysis for nanoparticle characterization and speciation analysis for determining different chemical forms of elements.
02 Interference reduction and elimination strategies
ICP-MS analysis faces challenges from spectral and non-spectral interferences that can affect measurement accuracy. Various strategies have been developed to address these issues, including collision/reaction cell technology, mathematical correction models, and high-resolution mass spectrometry. These approaches help to eliminate polyatomic interferences, reduce matrix effects, and improve the accuracy of trace element analysis, particularly for complex samples containing high levels of matrix elements.Expand Specific Solutions03 Specialized ICP-MS instrumentation and configurations
Advanced ICP-MS instrumentation designs address specific analytical challenges. These include triple quadrupole systems for enhanced interference removal, time-of-flight analyzers for rapid multi-element analysis, and hyphenated techniques that couple ICP-MS with separation methods like chromatography. Specialized sample introduction systems, such as laser ablation and desolvation nebulizers, expand the range of sample types that can be analyzed and improve sensitivity for challenging elements.Expand Specific Solutions04 Calibration and quantification methods
Accurate quantification in ICP-MS requires robust calibration strategies. These include external calibration with matrix-matched standards, standard addition methods, isotope dilution techniques, and internal standardization. Each approach offers advantages for specific analytical scenarios, with isotope dilution providing the highest accuracy for many applications. Quality control procedures, including the use of certified reference materials and regular instrument performance checks, are essential for maintaining analytical reliability.Expand Specific Solutions05 Applications and method development for challenging samples
ICP-MS methods have been developed for challenging sample types including biological tissues, environmental matrices, semiconductor materials, and nanoparticles. These specialized methods address unique challenges such as high dissolved solids, organic content, or ultra-trace detection requirements. Single particle ICP-MS enables characterization of nanoparticles, while speciation analysis using hyphenated techniques allows determination of different chemical forms of elements, providing crucial information for toxicological and environmental studies.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The ICP-MS organic sample analysis market is in a growth phase, characterized by increasing adoption across environmental, pharmaceutical, and food safety sectors. The global market size for ICP-MS technology is expanding steadily, driven by stringent regulatory requirements and growing demand for trace element analysis. Technologically, the field shows varying maturity levels, with companies like Thermo Fisher Scientific and PerkinElmer (Revvity) leading innovation with advanced instrumentation and sample preparation techniques. SPECTRO Analytical, Shimadzu, and Agilent are also significant competitors offering specialized solutions. Academic institutions including ETH Zurich and CNRS are advancing fundamental research, while industry-academia collaborations are addressing key challenges in organic matrix interference, sample preparation, and calibration methodologies.
Thermo Fisher Scientific (Bremen) GmbH
Technical Solution: Thermo Fisher Scientific has developed advanced ICP-MS systems specifically optimized for organic sample analysis, featuring collision/reaction cell technology that effectively removes polyatomic interferences common in organic matrices. Their iCAP RQ and iCAP TQ ICP-MS systems incorporate specialized sample introduction systems including the FAST valve system and specialized nebulizers designed to handle volatile organic solvents. The company has pioneered the integration of chromatography systems (LC, GC, IC) with ICP-MS for speciation analysis of organometallic compounds, allowing researchers to distinguish between different chemical forms of elements in organic samples. Their instruments feature oxygen addition technology that converts carbon to CO2, reducing carbon deposition on cones and improving long-term stability during organic sample analysis. Thermo Fisher also provides specialized software with pre-configured methods for organic sample preparation, analysis, and quality control protocols specifically designed for pharmaceutical, environmental, and food safety applications.
Strengths: Industry-leading sensitivity and interference removal capabilities; comprehensive integration with chromatography systems; robust hardware designed specifically for organic matrices. Weaknesses: Higher acquisition and operational costs compared to competitors; complex systems require specialized training; some applications may require extensive method development despite pre-configured templates.
SPECTRO Analytical Instruments GmbH
Technical Solution: SPECTRO Analytical Instruments has developed proprietary technology for organic sample analysis via ICP-MS through their SPECTRO MS platform, which utilizes a unique double-focusing sector field mass spectrometer design. Their approach incorporates specialized sample introduction systems including temperature-controlled spray chambers and organic-resistant torch designs that minimize carbon deposition and plasma instability when analyzing organic matrices. SPECTRO's technology employs a patented ion optic system that maintains high sensitivity even with carbon-rich samples, while their MatrixGuard interface effectively reduces maintenance requirements when working with organic solvents. The company has developed specific protocols for oxygen addition to the plasma, which converts carbon to CO2 and prevents carbon buildup on interface cones. Their SPECTRO MS systems feature rapid scanning capabilities that allow for effective transient signal analysis when coupled with chromatographic separation techniques, making them particularly suitable for speciation analysis in complex organic samples such as petroleum products, biofuels, and pharmaceutical compounds.
Strengths: Robust hardware design specifically engineered for challenging organic matrices; excellent long-term stability with carbon-rich samples; comprehensive application support for various organic sample types. Weaknesses: More limited global service network compared to larger competitors; higher initial investment for some specialized configurations; steeper learning curve for new users transitioning from other platforms.
Key Innovations in Interference Reduction Techniques
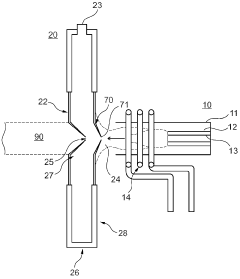
Ion source for inductively coupled plasma mass spectrometry
PatentWO2020187856A1
Innovation
- An ICP source with a vertically oriented plasma torch and injector tube allows sample introduction along a downwards-pointing vertical direction, reducing dependence on carrier gas flow and enabling 100% transport efficiency by utilizing gravity, and includes a metallic cooling plate and electromagnetic coupling element for efficient plasma generation.
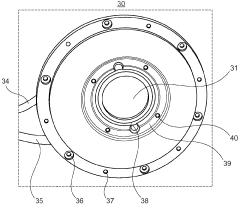
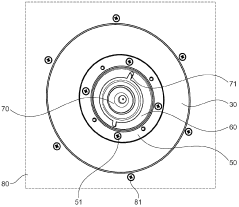
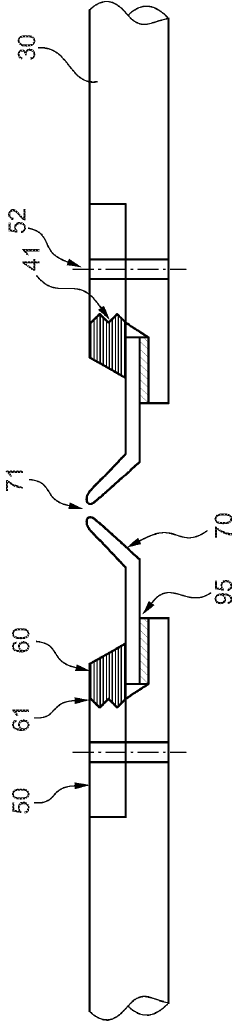
Cooling plate for icp-ms
PatentActiveGB2585327A
Innovation
- A cooling plate made from bronze is used, which provides sufficient thermal conductivity and enhanced chemical resistance, reducing the risk of corrosion and degradation, and eliminating the need for a corrosion-resistant coating.
Environmental and Safety Considerations
The environmental and safety considerations associated with ICP-MS analysis of organic samples require careful attention due to the potential hazards involved in sample preparation, analysis, and waste disposal. Laboratory personnel must adhere to strict protocols when handling concentrated acids, organic solvents, and other reagents commonly used in sample digestion and preparation. These chemicals present significant risks including corrosion, toxicity, and flammability. Proper personal protective equipment (PPE) including chemical-resistant gloves, lab coats, and safety goggles is essential, while all sample preparation should be conducted in properly ventilated fume hoods to minimize exposure to harmful vapors.
The operation of ICP-MS instruments introduces additional safety concerns. The high-temperature plasma (typically 6,000-10,000K) presents thermal hazards, while the radio frequency (RF) generators used to maintain the plasma emit electromagnetic radiation that requires appropriate shielding. Argon gas cylinders used to generate the plasma must be properly secured and handled according to compressed gas safety protocols. Regular maintenance and inspection of gas lines and connections is critical to prevent leaks that could lead to asphyxiation or explosion risks.
Waste management represents a significant environmental consideration in ICP-MS analysis of organic samples. The analytical process generates various waste streams containing heavy metals, acids, and organic compounds that require proper segregation, treatment, and disposal in accordance with local regulations. Many facilities implement waste reduction strategies such as acid recycling systems and microwave digestion techniques that minimize reagent consumption. Laboratories should maintain detailed waste disposal logs and ensure compliance with environmental protection standards.
Energy consumption is another important environmental factor, as ICP-MS instruments require substantial power for operation, particularly for maintaining the high-temperature plasma. Implementation of energy-efficient protocols, such as optimizing instrument warm-up times and scheduling batch analyses, can significantly reduce the carbon footprint of analytical operations. Some facilities are exploring renewable energy sources to power laboratory equipment as part of broader sustainability initiatives.
Water usage in sample preparation and instrument operation also presents environmental concerns. Advanced laboratories are implementing water recycling systems and adopting microsampling techniques that reduce both water consumption and waste generation. Additionally, the selection of environmentally friendly reagents and the development of "green chemistry" approaches for sample preparation are emerging trends that aim to minimize the environmental impact of ICP-MS analysis while maintaining analytical performance and reliability.
The operation of ICP-MS instruments introduces additional safety concerns. The high-temperature plasma (typically 6,000-10,000K) presents thermal hazards, while the radio frequency (RF) generators used to maintain the plasma emit electromagnetic radiation that requires appropriate shielding. Argon gas cylinders used to generate the plasma must be properly secured and handled according to compressed gas safety protocols. Regular maintenance and inspection of gas lines and connections is critical to prevent leaks that could lead to asphyxiation or explosion risks.
Waste management represents a significant environmental consideration in ICP-MS analysis of organic samples. The analytical process generates various waste streams containing heavy metals, acids, and organic compounds that require proper segregation, treatment, and disposal in accordance with local regulations. Many facilities implement waste reduction strategies such as acid recycling systems and microwave digestion techniques that minimize reagent consumption. Laboratories should maintain detailed waste disposal logs and ensure compliance with environmental protection standards.
Energy consumption is another important environmental factor, as ICP-MS instruments require substantial power for operation, particularly for maintaining the high-temperature plasma. Implementation of energy-efficient protocols, such as optimizing instrument warm-up times and scheduling batch analyses, can significantly reduce the carbon footprint of analytical operations. Some facilities are exploring renewable energy sources to power laboratory equipment as part of broader sustainability initiatives.
Water usage in sample preparation and instrument operation also presents environmental concerns. Advanced laboratories are implementing water recycling systems and adopting microsampling techniques that reduce both water consumption and waste generation. Additionally, the selection of environmentally friendly reagents and the development of "green chemistry" approaches for sample preparation are emerging trends that aim to minimize the environmental impact of ICP-MS analysis while maintaining analytical performance and reliability.
Validation and Quality Control Protocols
Quality control and validation protocols are essential components in ICP-MS analysis of organic samples to ensure reliable, accurate, and reproducible results. These protocols typically begin with instrument qualification procedures that verify the proper functioning of all system components. Daily performance checks must be conducted using certified reference materials to monitor sensitivity, mass calibration accuracy, and oxide formation rates. These checks establish baseline performance metrics against which subsequent analytical runs can be compared.
Method validation for organic sample analysis via ICP-MS requires comprehensive assessment of several critical parameters. Limit of detection (LOD) and limit of quantification (LOQ) determinations are particularly important when analyzing trace elements in complex organic matrices. These limits should be established through multiple blank measurements and calculation of standard deviations, following international guidelines such as those from ICH or FDA.
Accuracy validation involves analyzing certified reference materials with known concentrations of target analytes in matrices similar to the samples being tested. Recovery rates between 80-120% are generally considered acceptable, though specific applications may require tighter tolerances. Precision assessment through replicate analyses helps quantify method variability, with relative standard deviation targets typically set at <5% for major elements and <10% for trace elements in organic matrices.
Matrix effect evaluation is particularly crucial for organic samples due to their complex composition. Standard addition methods and isotope dilution techniques can be employed to quantify and compensate for matrix-induced signal suppression or enhancement. Internal standardization using elements with similar mass and ionization potential to the analytes of interest helps correct for instrument drift and matrix effects during analytical runs.
Robust quality control measures must include regular analysis of quality control samples at defined intervals throughout analytical batches. These should include method blanks to assess contamination, laboratory control samples to verify method performance, and duplicate samples to confirm precision. Control charts tracking key performance indicators over time enable early detection of systematic errors or instrument deterioration.
Interlaboratory comparison studies provide external validation of method performance and help identify laboratory-specific biases. Participation in proficiency testing programs specific to organic sample analysis via ICP-MS is highly recommended to maintain analytical excellence and ensure comparability of results across different facilities and analytical platforms.
Method validation for organic sample analysis via ICP-MS requires comprehensive assessment of several critical parameters. Limit of detection (LOD) and limit of quantification (LOQ) determinations are particularly important when analyzing trace elements in complex organic matrices. These limits should be established through multiple blank measurements and calculation of standard deviations, following international guidelines such as those from ICH or FDA.
Accuracy validation involves analyzing certified reference materials with known concentrations of target analytes in matrices similar to the samples being tested. Recovery rates between 80-120% are generally considered acceptable, though specific applications may require tighter tolerances. Precision assessment through replicate analyses helps quantify method variability, with relative standard deviation targets typically set at <5% for major elements and <10% for trace elements in organic matrices.
Matrix effect evaluation is particularly crucial for organic samples due to their complex composition. Standard addition methods and isotope dilution techniques can be employed to quantify and compensate for matrix-induced signal suppression or enhancement. Internal standardization using elements with similar mass and ionization potential to the analytes of interest helps correct for instrument drift and matrix effects during analytical runs.
Robust quality control measures must include regular analysis of quality control samples at defined intervals throughout analytical batches. These should include method blanks to assess contamination, laboratory control samples to verify method performance, and duplicate samples to confirm precision. Control charts tracking key performance indicators over time enable early detection of systematic errors or instrument deterioration.
Interlaboratory comparison studies provide external validation of method performance and help identify laboratory-specific biases. Participation in proficiency testing programs specific to organic sample analysis via ICP-MS is highly recommended to maintain analytical excellence and ensure comparability of results across different facilities and analytical platforms.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!