How to Validate ICP-MS Through External Calibration Methods
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ICP-MS Technology Background and Validation Objectives
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has evolved significantly since its commercial introduction in the 1980s, establishing itself as a cornerstone analytical technique in various scientific and industrial applications. This technology combines the high-temperature ICP source with a mass spectrometer, enabling precise detection and quantification of elements at concentrations as low as parts per trillion (ppt). The evolution of ICP-MS has been marked by continuous improvements in sensitivity, precision, and interference management capabilities.
The historical development of ICP-MS reflects broader trends in analytical instrumentation, moving from single quadrupole systems to more sophisticated configurations including collision/reaction cells, high-resolution magnetic sector instruments, and triple quadrupole systems. Each advancement has addressed specific limitations, particularly in handling spectral interferences that previously constrained accurate analysis of complex matrices.
Current technological trends in ICP-MS focus on enhancing automation, reducing sample preparation requirements, and integrating with complementary techniques such as chromatography for speciation analysis. The miniaturization of components and development of more energy-efficient systems represent additional vectors of innovation, aligning with broader sustainability goals in laboratory operations.
External calibration methods for ICP-MS validation have similarly evolved, transitioning from simple linear calibration approaches to more robust methodologies that account for matrix effects and instrumental drift. These methods rely on establishing a relationship between instrument response and known concentrations of analytes in standard solutions, forming the foundation for accurate quantitative analysis.
The primary validation objectives for ICP-MS through external calibration include establishing analytical performance parameters such as linearity, range, accuracy, precision, detection limits, and quantification limits. Additionally, validation aims to demonstrate method robustness across different matrices and operating conditions, ensuring reliable performance in routine analytical applications.
Regulatory frameworks from organizations such as the FDA, EPA, and ICH have increasingly emphasized the importance of thorough validation protocols for analytical methods, including those based on ICP-MS. These requirements have driven the development of more comprehensive validation approaches that address method transferability and long-term stability.
The technical goals of this research include evaluating current best practices in external calibration for ICP-MS, identifying key validation parameters specific to different application domains, and developing optimized protocols that balance analytical rigor with practical laboratory constraints. Furthermore, we aim to explore emerging calibration strategies that leverage computational approaches for handling non-linear responses and complex matrix effects.
The historical development of ICP-MS reflects broader trends in analytical instrumentation, moving from single quadrupole systems to more sophisticated configurations including collision/reaction cells, high-resolution magnetic sector instruments, and triple quadrupole systems. Each advancement has addressed specific limitations, particularly in handling spectral interferences that previously constrained accurate analysis of complex matrices.
Current technological trends in ICP-MS focus on enhancing automation, reducing sample preparation requirements, and integrating with complementary techniques such as chromatography for speciation analysis. The miniaturization of components and development of more energy-efficient systems represent additional vectors of innovation, aligning with broader sustainability goals in laboratory operations.
External calibration methods for ICP-MS validation have similarly evolved, transitioning from simple linear calibration approaches to more robust methodologies that account for matrix effects and instrumental drift. These methods rely on establishing a relationship between instrument response and known concentrations of analytes in standard solutions, forming the foundation for accurate quantitative analysis.
The primary validation objectives for ICP-MS through external calibration include establishing analytical performance parameters such as linearity, range, accuracy, precision, detection limits, and quantification limits. Additionally, validation aims to demonstrate method robustness across different matrices and operating conditions, ensuring reliable performance in routine analytical applications.
Regulatory frameworks from organizations such as the FDA, EPA, and ICH have increasingly emphasized the importance of thorough validation protocols for analytical methods, including those based on ICP-MS. These requirements have driven the development of more comprehensive validation approaches that address method transferability and long-term stability.
The technical goals of this research include evaluating current best practices in external calibration for ICP-MS, identifying key validation parameters specific to different application domains, and developing optimized protocols that balance analytical rigor with practical laboratory constraints. Furthermore, we aim to explore emerging calibration strategies that leverage computational approaches for handling non-linear responses and complex matrix effects.
Market Demand for Accurate Analytical Methods
The analytical instrumentation market has witnessed substantial growth in recent years, driven by increasing demands for accurate and reliable analytical methods across various industries. The global market for analytical instruments was valued at approximately $85 billion in 2022, with a projected compound annual growth rate of 6.7% through 2028. Within this broader market, ICP-MS (Inductively Coupled Plasma Mass Spectrometry) technology represents a significant segment, valued at around $1.2 billion in 2022, with expectations to reach $1.8 billion by 2027.
The demand for accurate analytical methods, particularly ICP-MS with external calibration validation, is being fueled by several key market drivers. Stringent regulatory requirements in pharmaceutical, environmental, and food safety sectors have necessitated more precise analytical techniques. The FDA, EPA, and similar regulatory bodies worldwide have established increasingly rigorous standards for trace element analysis, creating substantial market pull for validated analytical methods.
The pharmaceutical and biotechnology industries represent major consumers of ICP-MS technology, accounting for approximately 28% of the market share. These sectors require highly accurate methods for detecting elemental impurities in drug products, as mandated by USP <232> and <233> guidelines and ICH Q3D regulations. The implementation of these standards has significantly increased demand for validated external calibration methods.
Environmental monitoring constitutes another substantial market segment, representing about 23% of ICP-MS applications. Government agencies and private environmental testing laboratories require reliable analytical methods to monitor trace elements in water, soil, and air samples. The growing public concern over environmental contamination has further accelerated this demand.
Food safety testing has emerged as a rapidly growing application area, with a market growth rate exceeding 8% annually. Consumers and regulatory bodies are increasingly concerned about heavy metal contamination in food products, driving the need for validated analytical methods that can accurately quantify trace elements at parts-per-billion levels.
The semiconductor and electronics manufacturing industry has also become a significant consumer of ICP-MS technology, requiring ultra-trace elemental analysis for quality control. This sector demands exceptionally accurate calibration methods to ensure the purity of materials used in high-performance electronic components.
Geographically, North America and Europe currently dominate the market for advanced analytical methods, collectively accounting for approximately 60% of global demand. However, the Asia-Pacific region is experiencing the fastest growth rate at nearly 9% annually, driven by expanding pharmaceutical manufacturing, environmental regulations, and food safety concerns in countries like China, India, and South Korea.
The demand for accurate analytical methods, particularly ICP-MS with external calibration validation, is being fueled by several key market drivers. Stringent regulatory requirements in pharmaceutical, environmental, and food safety sectors have necessitated more precise analytical techniques. The FDA, EPA, and similar regulatory bodies worldwide have established increasingly rigorous standards for trace element analysis, creating substantial market pull for validated analytical methods.
The pharmaceutical and biotechnology industries represent major consumers of ICP-MS technology, accounting for approximately 28% of the market share. These sectors require highly accurate methods for detecting elemental impurities in drug products, as mandated by USP <232> and <233> guidelines and ICH Q3D regulations. The implementation of these standards has significantly increased demand for validated external calibration methods.
Environmental monitoring constitutes another substantial market segment, representing about 23% of ICP-MS applications. Government agencies and private environmental testing laboratories require reliable analytical methods to monitor trace elements in water, soil, and air samples. The growing public concern over environmental contamination has further accelerated this demand.
Food safety testing has emerged as a rapidly growing application area, with a market growth rate exceeding 8% annually. Consumers and regulatory bodies are increasingly concerned about heavy metal contamination in food products, driving the need for validated analytical methods that can accurately quantify trace elements at parts-per-billion levels.
The semiconductor and electronics manufacturing industry has also become a significant consumer of ICP-MS technology, requiring ultra-trace elemental analysis for quality control. This sector demands exceptionally accurate calibration methods to ensure the purity of materials used in high-performance electronic components.
Geographically, North America and Europe currently dominate the market for advanced analytical methods, collectively accounting for approximately 60% of global demand. However, the Asia-Pacific region is experiencing the fastest growth rate at nearly 9% annually, driven by expanding pharmaceutical manufacturing, environmental regulations, and food safety concerns in countries like China, India, and South Korea.
Current State and Challenges in ICP-MS Validation
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has evolved into a cornerstone analytical technique for elemental analysis across various industries. Currently, the global landscape of ICP-MS validation exhibits significant variations in methodological approaches, regulatory compliance requirements, and technical capabilities. The technique has reached maturity in many developed markets, with established protocols for external calibration validation, while emerging economies are still developing standardized frameworks.
The primary challenge facing ICP-MS validation through external calibration methods is the persistent issue of matrix effects, which can significantly impact measurement accuracy. These effects occur when sample components alter the ionization efficiency of analytes compared to calibration standards, leading to systematic errors that are difficult to quantify and correct. Despite advances in collision/reaction cell technologies, matrix interferences remain problematic for complex sample types.
Instrument drift represents another significant challenge, necessitating frequent recalibration and implementation of internal standardization. The selection of appropriate internal standards that match the ionization behavior of target analytes across diverse sample matrices remains technically demanding. This challenge is particularly acute in high-throughput environments where analytical speed must be balanced with validation rigor.
Regulatory frameworks governing ICP-MS validation vary considerably across regions and industries, creating compliance challenges for global organizations. While pharmaceutical applications follow stringent ICH and pharmacopeia guidelines, environmental and food safety sectors often operate under different validation paradigms. This regulatory heterogeneity complicates the development of universally applicable external calibration validation protocols.
The availability and quality of certified reference materials (CRMs) present another significant limitation. For many specialized applications, matrix-matched CRMs are either unavailable or prohibitively expensive, forcing analysts to compromise on validation robustness. This gap is particularly evident for emerging contaminants and specialized industrial applications where reference materials lag behind analytical requirements.
Technical expertise requirements pose additional challenges, as effective ICP-MS validation demands multidisciplinary knowledge spanning analytical chemistry, statistics, and regulatory compliance. The shortage of qualified personnel capable of designing and implementing comprehensive validation protocols represents a bottleneck in many organizations, particularly in regions where ICP-MS technology has been more recently adopted.
Data processing and uncertainty estimation remain complex aspects of validation, with many laboratories struggling to implement metrologically sound approaches to measurement uncertainty. The statistical treatment of calibration data, detection limit determination, and uncertainty propagation often lack standardization, leading to inconsistent validation outcomes across different laboratories analyzing identical samples.
The primary challenge facing ICP-MS validation through external calibration methods is the persistent issue of matrix effects, which can significantly impact measurement accuracy. These effects occur when sample components alter the ionization efficiency of analytes compared to calibration standards, leading to systematic errors that are difficult to quantify and correct. Despite advances in collision/reaction cell technologies, matrix interferences remain problematic for complex sample types.
Instrument drift represents another significant challenge, necessitating frequent recalibration and implementation of internal standardization. The selection of appropriate internal standards that match the ionization behavior of target analytes across diverse sample matrices remains technically demanding. This challenge is particularly acute in high-throughput environments where analytical speed must be balanced with validation rigor.
Regulatory frameworks governing ICP-MS validation vary considerably across regions and industries, creating compliance challenges for global organizations. While pharmaceutical applications follow stringent ICH and pharmacopeia guidelines, environmental and food safety sectors often operate under different validation paradigms. This regulatory heterogeneity complicates the development of universally applicable external calibration validation protocols.
The availability and quality of certified reference materials (CRMs) present another significant limitation. For many specialized applications, matrix-matched CRMs are either unavailable or prohibitively expensive, forcing analysts to compromise on validation robustness. This gap is particularly evident for emerging contaminants and specialized industrial applications where reference materials lag behind analytical requirements.
Technical expertise requirements pose additional challenges, as effective ICP-MS validation demands multidisciplinary knowledge spanning analytical chemistry, statistics, and regulatory compliance. The shortage of qualified personnel capable of designing and implementing comprehensive validation protocols represents a bottleneck in many organizations, particularly in regions where ICP-MS technology has been more recently adopted.
Data processing and uncertainty estimation remain complex aspects of validation, with many laboratories struggling to implement metrologically sound approaches to measurement uncertainty. The statistical treatment of calibration data, detection limit determination, and uncertainty propagation often lack standardization, leading to inconsistent validation outcomes across different laboratories analyzing identical samples.
Existing External Calibration Protocols for ICP-MS
01 Validation methods for ICP-MS analysis
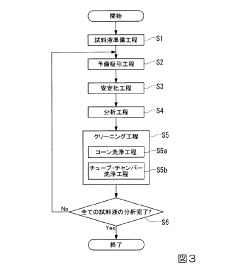
Various validation methods are employed to ensure the accuracy and reliability of ICP-MS analysis. These methods include calibration procedures, quality control measures, and performance verification protocols. Validation typically involves assessing parameters such as linearity, precision, accuracy, detection limits, and quantification limits. Proper validation ensures that the analytical results obtained from ICP-MS are reliable and reproducible for various applications in elemental analysis.- Validation methods for ICP-MS analysis: Various validation methods are employed to ensure the accuracy and reliability of ICP-MS analysis. These methods include calibration procedures, quality control measures, and validation protocols that verify the performance of the ICP-MS system. Validation typically involves assessing parameters such as linearity, precision, accuracy, detection limits, and robustness of the analytical method.
- Sample preparation techniques for ICP-MS validation: Proper sample preparation is crucial for accurate ICP-MS validation. This includes digestion methods, dilution protocols, and matrix modification techniques to minimize interferences. The preparation process must be validated to ensure consistent and reliable results across different sample types and concentrations, with special attention to potential contamination sources and recovery rates.
- Interference reduction and elimination strategies: ICP-MS validation requires effective strategies to reduce or eliminate spectral and non-spectral interferences that can affect measurement accuracy. These strategies include collision/reaction cell technologies, mathematical correction models, and optimized instrument parameters. Validation protocols must verify that these interference reduction methods work effectively across the range of expected sample matrices.
- Automated systems and equipment for ICP-MS validation: Automated systems and specialized equipment have been developed to streamline the ICP-MS validation process. These include automated sample introduction systems, integrated calibration devices, and software solutions for method validation. Such systems help improve reproducibility, reduce human error, and increase throughput during the validation process while maintaining data integrity.
- Application-specific validation protocols: Different applications of ICP-MS require specialized validation protocols tailored to specific industries or analytical needs. These include pharmaceutical testing, environmental monitoring, food safety analysis, and clinical diagnostics. Each application domain has unique requirements for validation parameters, acceptance criteria, and regulatory compliance that must be addressed in the validation process.
02 Sample preparation techniques for ICP-MS validation
Effective sample preparation is crucial for successful ICP-MS validation. This includes digestion methods, dilution protocols, and matrix modification techniques to minimize interferences. Proper sample preparation ensures that analytes are in appropriate forms for detection and quantification. Techniques may include acid digestion, microwave-assisted extraction, and filtration procedures to remove particulates that could interfere with analysis. These preparation methods significantly impact the quality and reliability of validation results.Expand Specific Solutions03 Interference reduction strategies in ICP-MS validation
Various strategies are implemented to reduce interferences during ICP-MS validation. These include collision/reaction cell technologies, mathematical correction models, and optimized instrument parameters. Interference reduction is essential for accurate quantification of elements, particularly in complex matrices. Techniques may involve using specific gases in collision cells, applying kinetic energy discrimination, or utilizing high-resolution mass analyzers to separate interfering species from analytes of interest.Expand Specific Solutions04 Automated systems for ICP-MS validation
Automated systems enhance the efficiency and reproducibility of ICP-MS validation procedures. These systems include automated sample introduction, data acquisition, and processing software. Automation reduces human error, increases throughput, and improves consistency in validation protocols. Features may include automated calibration, quality control checks, and performance monitoring to ensure consistent analytical performance over time. These systems are particularly valuable for routine validation in high-throughput laboratories.Expand Specific Solutions05 Application-specific validation protocols for ICP-MS
Different applications require specialized validation protocols for ICP-MS analysis. These protocols are tailored to specific industries such as pharmaceuticals, environmental monitoring, food safety, and clinical diagnostics. Application-specific validation considers unique matrix effects, regulatory requirements, and critical quality attributes relevant to each field. Protocols may include specific reference materials, acceptance criteria, and robustness testing designed to address the particular challenges of the intended application area.Expand Specific Solutions
Key Players in Analytical Instrumentation Industry
The ICP-MS external calibration market is in a mature growth phase, characterized by established methodologies and widespread adoption across pharmaceutical, environmental, and research sectors. The global market size for ICP-MS technologies exceeds $1 billion, with steady annual growth of 5-7%. Leading players demonstrate varying levels of technical maturity: Agilent Technologies, Roche Diagnostics, and SPEX CertiPrep show advanced capabilities with comprehensive validation protocols; while Fujifilm, DH Technologies, and Skyray Instrument offer specialized solutions with moderate maturity. Academic institutions like ETH Zurich and University of Tokyo contribute significant research advancements. The competitive landscape features both specialized analytical instrument manufacturers and diversified scientific equipment providers, with increasing focus on automated validation workflows and reference material standardization.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed a pharmaceutical-focused approach to ICP-MS validation through external calibration that emphasizes regulatory compliance for elemental impurity testing. Their methodology incorporates bracketed calibration designs where samples are surrounded by calibration standards to compensate for temporal drift. Roche's validation protocol includes extensive robustness testing of calibration stability under varying plasma conditions and sample matrices typical in pharmaceutical applications. Their approach emphasizes method transfer validation between different laboratories and instruments, with standardized external calibration procedures that can be implemented across global manufacturing sites. Roche has pioneered the use of multi-isotope calibration strategies for elements with multiple stable isotopes, allowing internal cross-validation of calibration accuracy. Their validation procedures include comprehensive uncertainty budgets that quantify all sources of calibration error, from standard preparation to instrumental measurement variability.
Strengths: Extensive experience with pharmaceutical regulatory requirements; robust validation protocols designed for method transfer between sites; comprehensive uncertainty quantification approaches. Weaknesses: Calibration methods highly specialized for pharmaceutical matrices may not translate well to environmental or geological samples; protocols may be more rigorous than necessary for non-regulated applications; significant resources required for full implementation.
DH Technologies Development Pte Ltd.
Technical Solution: DH Technologies (parent company of SCIEX) has developed advanced external calibration methodologies for ICP-MS that leverage their patented Dynamic Reaction Cell (DRC) technology. Their approach integrates collision/reaction cell optimization with external calibration to eliminate polyatomic interferences that can compromise calibration accuracy. The company's validation protocol includes automated calibration curve optimization that selects appropriate weighting factors based on concentration range and detector response characteristics. Their external calibration method incorporates automatic correction for detector dead time and analog-to-digital conversion factors, ensuring linearity across the full dynamic range. DH Technologies has pioneered calibration approaches that simultaneously validate both quantitative accuracy and isotope ratio precision, critical for applications requiring isotopic analysis. Their methodology includes specialized calibration procedures for challenging elements like mercury and selenium that exhibit memory effects or volatility issues that can compromise conventional calibration approaches.
Strengths: Integration of collision/reaction cell technology with calibration methodology; advanced detector calibration capabilities; specialized solutions for problematic elements. Weaknesses: Complex calibration procedures may require significant expertise; optimization of collision/reaction parameters adds additional validation requirements; proprietary approaches may limit transferability to other instrument platforms.
Critical Technical Aspects of ICP-MS Validation
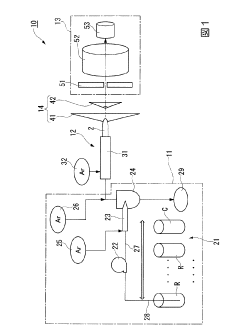
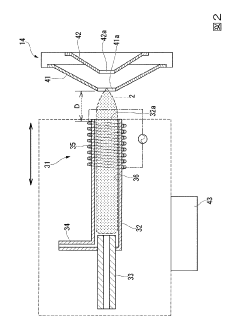
Inductively coupled plasma mass spectroscopy apparatus and measured data processing method in the inductively coupled plasma mass spectroscopy apparatus
PatentActiveUS8530829B2
Innovation
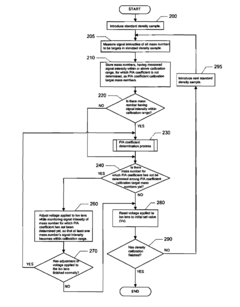
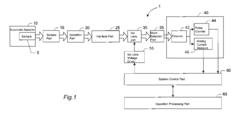
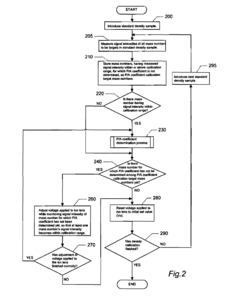
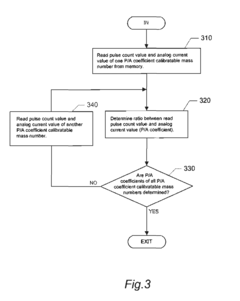
- An ICP-MS apparatus and method that uses standard density samples to generate a working curve and adjusts ion lens voltage to ensure signal intensities fall within a calibration range, combined with a P/A coefficient estimation method that correlates P/A coefficients from different measurements to accurately determine coefficients for mass numbers outside the initial calibration range.
Inductively coupled plasma mass spectrometry method
PatentInactiveJP2016008918A
Innovation
- An inductively coupled plasma mass spectrometry method that allows undiluted high-salt sample analysis, incorporating a cleaning step to remove deposits on the sampler using plasma exposure and a cleaning liquid, maintaining detection sensitivity and accuracy.
Regulatory Compliance Requirements for ICP-MS Methods
Regulatory compliance for ICP-MS methods is governed by multiple international and regional frameworks that establish stringent validation requirements. The FDA's Guidance for Industry on Analytical Procedures and Methods Validation mandates specific performance characteristics for ICP-MS, including accuracy, precision, specificity, detection limit, quantitation limit, linearity, range, and robustness. These parameters must be thoroughly documented when using external calibration methods.
The International Conference on Harmonisation (ICH) guidelines, particularly ICH Q2(R1), provide detailed requirements for analytical method validation applicable to ICP-MS. When implementing external calibration, laboratories must demonstrate compliance with these guidelines through comprehensive validation protocols that address all required parameters and establish acceptance criteria before method implementation.
USP <233> specifically addresses elemental impurities analysis using ICP-MS and outlines validation requirements for external calibration approaches. This includes system suitability tests, calibration curve requirements, and quality control measures that must be incorporated into routine analysis. The chapter stipulates minimum correlation coefficient values for calibration curves and requires demonstration of accuracy within specified recovery ranges.
For environmental applications, EPA Method 6020B establishes regulatory requirements for ICP-MS validation, including specific protocols for external calibration. This method mandates minimum calibration points, quality control samples, and internal standard requirements that laboratories must follow to maintain compliance.
The European Medicines Agency (EMA) guidelines provide additional regulatory frameworks applicable to pharmaceutical analysis using ICP-MS. These guidelines emphasize the importance of uncertainty measurements and traceability in external calibration methods, requiring laboratories to establish measurement uncertainty budgets for their analytical procedures.
ISO/IEC 17025 accreditation requirements add another layer of compliance considerations for laboratories performing ICP-MS analysis. This standard necessitates documented validation procedures, proficiency testing participation, and quality assurance measures for all analytical methods, including those utilizing external calibration approaches.
Compliance with these regulatory frameworks requires laboratories to maintain comprehensive documentation of all validation activities, including raw data, statistical analyses, and conclusions regarding method suitability. Regular revalidation and continuous monitoring through quality control samples are essential components of maintaining regulatory compliance for ICP-MS methods using external calibration.
The International Conference on Harmonisation (ICH) guidelines, particularly ICH Q2(R1), provide detailed requirements for analytical method validation applicable to ICP-MS. When implementing external calibration, laboratories must demonstrate compliance with these guidelines through comprehensive validation protocols that address all required parameters and establish acceptance criteria before method implementation.
USP <233> specifically addresses elemental impurities analysis using ICP-MS and outlines validation requirements for external calibration approaches. This includes system suitability tests, calibration curve requirements, and quality control measures that must be incorporated into routine analysis. The chapter stipulates minimum correlation coefficient values for calibration curves and requires demonstration of accuracy within specified recovery ranges.
For environmental applications, EPA Method 6020B establishes regulatory requirements for ICP-MS validation, including specific protocols for external calibration. This method mandates minimum calibration points, quality control samples, and internal standard requirements that laboratories must follow to maintain compliance.
The European Medicines Agency (EMA) guidelines provide additional regulatory frameworks applicable to pharmaceutical analysis using ICP-MS. These guidelines emphasize the importance of uncertainty measurements and traceability in external calibration methods, requiring laboratories to establish measurement uncertainty budgets for their analytical procedures.
ISO/IEC 17025 accreditation requirements add another layer of compliance considerations for laboratories performing ICP-MS analysis. This standard necessitates documented validation procedures, proficiency testing participation, and quality assurance measures for all analytical methods, including those utilizing external calibration approaches.
Compliance with these regulatory frameworks requires laboratories to maintain comprehensive documentation of all validation activities, including raw data, statistical analyses, and conclusions regarding method suitability. Regular revalidation and continuous monitoring through quality control samples are essential components of maintaining regulatory compliance for ICP-MS methods using external calibration.
Quality Assurance Strategies for Analytical Validation
Quality assurance is a critical component in the validation of ICP-MS through external calibration methods. Establishing robust quality assurance protocols ensures that analytical results are reliable, reproducible, and meet regulatory requirements. These strategies should encompass comprehensive documentation practices, including detailed standard operating procedures (SOPs) that outline each step of the validation process, from sample preparation to data analysis and reporting.
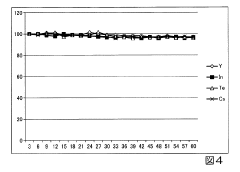
Regular performance verification is essential within quality assurance frameworks. This includes system suitability tests performed before analytical runs to confirm that the ICP-MS instrument is operating within specified parameters. Performance checks should evaluate sensitivity, mass calibration accuracy, oxide formation rates, and double-charged ion ratios to ensure optimal instrument conditions for accurate external calibration.
Proficiency testing participation represents another crucial quality assurance element. Laboratories should regularly participate in interlaboratory comparison programs specific to ICP-MS analysis, allowing for external assessment of analytical capabilities and identification of systematic errors that might not be apparent through internal quality control measures.
Control charting methodologies provide valuable tools for monitoring analytical performance over time. By tracking key performance indicators such as calibration slopes, correlation coefficients, and quality control sample recoveries, analysts can identify trends or shifts in analytical performance before they impact data quality. Statistical process control techniques can be applied to establish warning and action limits for these parameters.
Reference material utilization forms the backbone of effective quality assurance strategies. Certified reference materials (CRMs) that closely match sample matrices should be analyzed regularly to verify calibration accuracy and method performance. These materials provide traceability to recognized standards and help quantify method bias and recovery efficiency across different concentration ranges.
Quality assurance strategies must also incorporate comprehensive staff training programs. Analysts should receive thorough training on ICP-MS principles, external calibration techniques, and quality control procedures. Regular competency assessments ensure that personnel maintain the necessary skills for proper method execution and data interpretation.
Audit trails and electronic data management systems play an increasingly important role in modern quality assurance frameworks. These systems provide secure documentation of all analytical activities, including calibration standard preparation, instrument parameters, and data processing steps, ensuring data integrity throughout the validation process.
Regular performance verification is essential within quality assurance frameworks. This includes system suitability tests performed before analytical runs to confirm that the ICP-MS instrument is operating within specified parameters. Performance checks should evaluate sensitivity, mass calibration accuracy, oxide formation rates, and double-charged ion ratios to ensure optimal instrument conditions for accurate external calibration.
Proficiency testing participation represents another crucial quality assurance element. Laboratories should regularly participate in interlaboratory comparison programs specific to ICP-MS analysis, allowing for external assessment of analytical capabilities and identification of systematic errors that might not be apparent through internal quality control measures.
Control charting methodologies provide valuable tools for monitoring analytical performance over time. By tracking key performance indicators such as calibration slopes, correlation coefficients, and quality control sample recoveries, analysts can identify trends or shifts in analytical performance before they impact data quality. Statistical process control techniques can be applied to establish warning and action limits for these parameters.
Reference material utilization forms the backbone of effective quality assurance strategies. Certified reference materials (CRMs) that closely match sample matrices should be analyzed regularly to verify calibration accuracy and method performance. These materials provide traceability to recognized standards and help quantify method bias and recovery efficiency across different concentration ranges.
Quality assurance strategies must also incorporate comprehensive staff training programs. Analysts should receive thorough training on ICP-MS principles, external calibration techniques, and quality control procedures. Regular competency assessments ensure that personnel maintain the necessary skills for proper method execution and data interpretation.
Audit trails and electronic data management systems play an increasingly important role in modern quality assurance frameworks. These systems provide secure documentation of all analytical activities, including calibration standard preparation, instrument parameters, and data processing steps, ensuring data integrity throughout the validation process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!