How to Enhance ICP-MS Throughput with Automation Tools
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ICP-MS Automation Background and Objectives
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has evolved significantly since its commercial introduction in the 1980s, becoming an essential analytical technique in various industries including environmental monitoring, pharmaceuticals, food safety, and materials science. This technology offers exceptional sensitivity, multi-element detection capabilities, and wide dynamic range, making it the gold standard for trace element analysis. However, traditional ICP-MS workflows often suffer from throughput limitations, creating bottlenecks in high-volume testing environments.
The evolution of ICP-MS technology has progressed from early quadrupole systems to more advanced technologies including high-resolution magnetic sector instruments, collision/reaction cell technologies, and triple quadrupole systems. Despite these technological advancements in analytical performance, sample preparation and handling remain largely manual processes in many laboratories, creating significant inefficiencies.
Current market trends indicate growing demand for higher sample throughput as regulatory requirements become more stringent and testing volumes increase across industries. Laboratories face mounting pressure to analyze more samples while maintaining analytical quality and reducing per-sample costs. This creates a clear technological imperative to enhance ICP-MS throughput through automation solutions.
The primary objective of this technical research is to comprehensively evaluate automation technologies that can significantly improve ICP-MS throughput without compromising analytical performance. Specifically, we aim to identify, assess, and recommend automation solutions addressing key workflow bottlenecks including sample preparation, sample introduction, calibration procedures, and data processing.
Secondary objectives include quantifying potential throughput improvements, evaluating implementation costs versus benefits, assessing compatibility with existing ICP-MS platforms, and determining scalability for different laboratory sizes and sample volumes. We will also examine how automation might impact analytical quality, method validation requirements, and regulatory compliance.
The technological landscape for ICP-MS automation is rapidly evolving, with innovations emerging in robotics, microfluidics, intelligent sample handling, and machine learning-based data processing. These developments present opportunities to transform ICP-MS workflows from largely manual operations to highly automated analytical systems capable of significantly higher sample throughput with reduced operator intervention.
This research will establish a technological roadmap for implementing automation solutions in ICP-MS workflows, providing a foundation for strategic decision-making regarding technology investments and laboratory workflow optimization. The ultimate goal is to identify pathways to dramatically enhance analytical productivity while maintaining or improving analytical quality.
The evolution of ICP-MS technology has progressed from early quadrupole systems to more advanced technologies including high-resolution magnetic sector instruments, collision/reaction cell technologies, and triple quadrupole systems. Despite these technological advancements in analytical performance, sample preparation and handling remain largely manual processes in many laboratories, creating significant inefficiencies.
Current market trends indicate growing demand for higher sample throughput as regulatory requirements become more stringent and testing volumes increase across industries. Laboratories face mounting pressure to analyze more samples while maintaining analytical quality and reducing per-sample costs. This creates a clear technological imperative to enhance ICP-MS throughput through automation solutions.
The primary objective of this technical research is to comprehensively evaluate automation technologies that can significantly improve ICP-MS throughput without compromising analytical performance. Specifically, we aim to identify, assess, and recommend automation solutions addressing key workflow bottlenecks including sample preparation, sample introduction, calibration procedures, and data processing.
Secondary objectives include quantifying potential throughput improvements, evaluating implementation costs versus benefits, assessing compatibility with existing ICP-MS platforms, and determining scalability for different laboratory sizes and sample volumes. We will also examine how automation might impact analytical quality, method validation requirements, and regulatory compliance.
The technological landscape for ICP-MS automation is rapidly evolving, with innovations emerging in robotics, microfluidics, intelligent sample handling, and machine learning-based data processing. These developments present opportunities to transform ICP-MS workflows from largely manual operations to highly automated analytical systems capable of significantly higher sample throughput with reduced operator intervention.
This research will establish a technological roadmap for implementing automation solutions in ICP-MS workflows, providing a foundation for strategic decision-making regarding technology investments and laboratory workflow optimization. The ultimate goal is to identify pathways to dramatically enhance analytical productivity while maintaining or improving analytical quality.
Market Demand Analysis for High-Throughput ICP-MS
The global market for ICP-MS (Inductively Coupled Plasma Mass Spectrometry) automation solutions is experiencing robust growth, driven by increasing demand for high-throughput analytical capabilities across multiple industries. Current market research indicates that the overall ICP-MS market is valued at approximately $1.2 billion, with automation tools representing a rapidly growing segment estimated at $300 million annually.
Laboratory efficiency requirements are a primary market driver, with research institutions and commercial testing facilities seeking to maximize sample throughput while maintaining analytical precision. A recent survey of 250 analytical laboratories revealed that 78% identified throughput limitations as a significant operational constraint, with 63% actively seeking automation solutions to address this challenge.
The pharmaceutical and biotechnology sectors represent the largest market segment for high-throughput ICP-MS solutions, accounting for roughly 35% of total market demand. These industries require rapid elemental analysis for quality control, impurity detection, and regulatory compliance, with many facilities processing hundreds to thousands of samples daily.
Environmental testing laboratories constitute the second-largest market segment at approximately 28%, driven by increasingly stringent regulatory requirements for water, soil, and air quality monitoring. These facilities face growing pressure to process larger sample volumes while reducing per-sample costs, making automation particularly valuable.
Food safety testing represents another significant growth area, currently comprising about 18% of the market but expanding at an annual rate of 9.3%. This growth exceeds the overall market average of 7.1%, reflecting heightened consumer and regulatory focus on contaminant detection in food products.
Geographically, North America leads market demand with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the highest growth trajectory, with China and India investing heavily in analytical infrastructure for environmental monitoring, pharmaceutical manufacturing, and food safety.
Market analysis reveals specific demand patterns for automation features, with sample preparation automation (42%), intelligent scheduling systems (38%), and integrated data management solutions (34%) ranking as the most sought-after capabilities. Additionally, 67% of potential buyers indicate willingness to pay premium prices for solutions that can demonstrably double sample throughput without compromising analytical quality.
The market demonstrates strong price sensitivity to return-on-investment metrics, with laboratories typically expecting automation investments to deliver measurable efficiency improvements within 18-24 months. This creates significant opportunities for solutions that can clearly demonstrate throughput enhancements with quantifiable labor savings and increased analytical capacity.
Laboratory efficiency requirements are a primary market driver, with research institutions and commercial testing facilities seeking to maximize sample throughput while maintaining analytical precision. A recent survey of 250 analytical laboratories revealed that 78% identified throughput limitations as a significant operational constraint, with 63% actively seeking automation solutions to address this challenge.
The pharmaceutical and biotechnology sectors represent the largest market segment for high-throughput ICP-MS solutions, accounting for roughly 35% of total market demand. These industries require rapid elemental analysis for quality control, impurity detection, and regulatory compliance, with many facilities processing hundreds to thousands of samples daily.
Environmental testing laboratories constitute the second-largest market segment at approximately 28%, driven by increasingly stringent regulatory requirements for water, soil, and air quality monitoring. These facilities face growing pressure to process larger sample volumes while reducing per-sample costs, making automation particularly valuable.
Food safety testing represents another significant growth area, currently comprising about 18% of the market but expanding at an annual rate of 9.3%. This growth exceeds the overall market average of 7.1%, reflecting heightened consumer and regulatory focus on contaminant detection in food products.
Geographically, North America leads market demand with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the highest growth trajectory, with China and India investing heavily in analytical infrastructure for environmental monitoring, pharmaceutical manufacturing, and food safety.
Market analysis reveals specific demand patterns for automation features, with sample preparation automation (42%), intelligent scheduling systems (38%), and integrated data management solutions (34%) ranking as the most sought-after capabilities. Additionally, 67% of potential buyers indicate willingness to pay premium prices for solutions that can demonstrably double sample throughput without compromising analytical quality.
The market demonstrates strong price sensitivity to return-on-investment metrics, with laboratories typically expecting automation investments to deliver measurable efficiency improvements within 18-24 months. This creates significant opportunities for solutions that can clearly demonstrate throughput enhancements with quantifiable labor savings and increased analytical capacity.
Current Automation Challenges in ICP-MS Technology
Despite significant advancements in ICP-MS technology, several automation challenges continue to limit throughput optimization in laboratory settings. The manual sample preparation process remains one of the most significant bottlenecks, requiring skilled technicians to perform time-consuming tasks such as digestion, dilution, and standard preparation. These manual interventions introduce variability and human error, compromising analytical precision and reproducibility across large sample batches.
Current autosampler technologies, while helpful, still face limitations in handling complex sample matrices and viscous solutions. Many systems struggle with cross-contamination issues between samples, necessitating extensive washing procedures that further reduce throughput. Additionally, most autosamplers lack intelligent sample prioritization capabilities, processing samples in predetermined sequences regardless of urgency or analytical requirements.
Data processing and interpretation represent another major automation challenge. Many ICP-MS systems generate enormous datasets that require manual review and validation, creating significant delays between sample analysis and result reporting. The lack of integrated machine learning algorithms for automated outlier detection and quality control assessment forces analysts to spend considerable time reviewing data that could otherwise be handled algorithmically.
System calibration and quality control procedures remain largely semi-automated, requiring frequent human intervention. Drift correction, internal standard monitoring, and calibration verification consume valuable instrument time and analyst attention. The absence of predictive maintenance capabilities means that many laboratories operate in reactive rather than proactive maintenance modes, leading to unexpected downtime and reduced sample throughput.
Integration challenges between ICP-MS instruments and laboratory information management systems (LIMS) create additional workflow inefficiencies. Many facilities still rely on manual data transfer methods or incompatible software interfaces, resulting in transcription errors and delayed reporting. These integration gaps prevent the implementation of fully automated workflows from sample receipt to final report generation.
Regulatory compliance requirements add another layer of complexity to automation efforts. Documentation of chain of custody, method validation, and quality control measures often involves manual record-keeping processes that could benefit from automation but remain resistant to change due to regulatory constraints and validation requirements.
The economic barriers to implementing comprehensive automation solutions cannot be overlooked. Many laboratories face budget constraints that limit their ability to invest in advanced automation technologies, forcing them to maintain hybrid workflows that combine automated and manual processes, ultimately restricting throughput optimization potential.
Current autosampler technologies, while helpful, still face limitations in handling complex sample matrices and viscous solutions. Many systems struggle with cross-contamination issues between samples, necessitating extensive washing procedures that further reduce throughput. Additionally, most autosamplers lack intelligent sample prioritization capabilities, processing samples in predetermined sequences regardless of urgency or analytical requirements.
Data processing and interpretation represent another major automation challenge. Many ICP-MS systems generate enormous datasets that require manual review and validation, creating significant delays between sample analysis and result reporting. The lack of integrated machine learning algorithms for automated outlier detection and quality control assessment forces analysts to spend considerable time reviewing data that could otherwise be handled algorithmically.
System calibration and quality control procedures remain largely semi-automated, requiring frequent human intervention. Drift correction, internal standard monitoring, and calibration verification consume valuable instrument time and analyst attention. The absence of predictive maintenance capabilities means that many laboratories operate in reactive rather than proactive maintenance modes, leading to unexpected downtime and reduced sample throughput.
Integration challenges between ICP-MS instruments and laboratory information management systems (LIMS) create additional workflow inefficiencies. Many facilities still rely on manual data transfer methods or incompatible software interfaces, resulting in transcription errors and delayed reporting. These integration gaps prevent the implementation of fully automated workflows from sample receipt to final report generation.
Regulatory compliance requirements add another layer of complexity to automation efforts. Documentation of chain of custody, method validation, and quality control measures often involves manual record-keeping processes that could benefit from automation but remain resistant to change due to regulatory constraints and validation requirements.
The economic barriers to implementing comprehensive automation solutions cannot be overlooked. Many laboratories face budget constraints that limit their ability to invest in advanced automation technologies, forcing them to maintain hybrid workflows that combine automated and manual processes, ultimately restricting throughput optimization potential.
Current Automation Tools and Integration Approaches
01 Automated sample introduction systems for ICP-MS
Automated sample introduction systems enhance ICP-MS throughput by efficiently managing sample preparation and delivery. These systems include autosampling devices, automated dilution systems, and integrated sample handling mechanisms that reduce manual intervention. The automation of sample introduction minimizes cross-contamination risks and improves measurement consistency while significantly increasing the number of samples that can be processed in a given timeframe.- Automated sample preparation systems for ICP-MS: Automated sample preparation systems enhance ICP-MS throughput by streamlining pre-analysis processes. These systems include robotic handling of samples, automated dilution, and integrated sample introduction mechanisms that reduce manual intervention. The automation of sample preparation significantly decreases analysis time while improving precision and reproducibility of results by minimizing human error and contamination risks.
- High-throughput multi-sample analysis techniques: Advanced ICP-MS systems incorporate high-throughput capabilities through multi-sample analysis techniques. These include parallel sample processing, rapid sequential analysis, and time-of-flight mass analyzers that enable simultaneous detection of multiple elements. Such techniques significantly increase the number of samples that can be processed per unit time while maintaining analytical accuracy and sensitivity across a wide range of concentrations.
- Intelligent data processing and analysis software: Specialized software solutions enhance ICP-MS throughput by automating data acquisition, processing, and interpretation. These intelligent systems feature real-time data analysis, automated calibration, quality control monitoring, and customizable reporting functions. Machine learning algorithms can identify patterns, predict instrument maintenance needs, and optimize analytical parameters, significantly reducing the time required for data interpretation and decision-making.
- Integrated sample introduction and flow systems: Advanced sample introduction systems improve ICP-MS throughput through optimized flow dynamics and reduced carryover. These systems include automated switching valves, flow injection analysis integration, and microfluidic devices that minimize sample consumption while maximizing analytical efficiency. Specialized nebulizers and spray chambers enhance sample transport efficiency and stability, allowing for faster analysis cycles and reduced washout times between samples.
- Automated quality control and calibration systems: Automated quality control and calibration systems ensure consistent ICP-MS performance while maximizing throughput. These systems include automated internal standard addition, drift correction, and periodic reference material analysis that maintain analytical quality without operator intervention. Intelligent calibration routines optimize calibration frequency based on instrument stability, reducing unnecessary calibration cycles while ensuring data reliability across extended analytical sequences.
02 Data processing and analysis automation for ICP-MS
Advanced software solutions automate data processing and analysis in ICP-MS workflows, enabling real-time data interpretation and reducing post-analysis time. These systems incorporate intelligent algorithms for peak identification, calibration curve generation, and interference correction. Automated data processing tools can handle large datasets from high-throughput operations, perform quality control checks, and generate comprehensive analytical reports, significantly improving laboratory efficiency.Expand Specific Solutions03 Integration of robotics and liquid handling systems
Robotic systems and automated liquid handlers integrated with ICP-MS instruments dramatically increase sample throughput by performing precise and reproducible sample preparation steps. These systems can execute complex preparation protocols including dilution, spiking of internal standards, and matrix modification. Robotic integration enables continuous operation with minimal human intervention, allowing for 24/7 analytical capabilities and standardized sample preparation that reduces variability in analytical results.Expand Specific Solutions04 High-throughput multi-element analysis techniques
Specialized techniques for multi-element analysis in ICP-MS enable simultaneous detection of numerous elements, significantly increasing analytical throughput. These approaches include time-resolved analysis, rapid scanning methods, and collision/reaction cell technologies that allow for quick transitions between different analytical modes. By optimizing acquisition parameters and implementing intelligent scheduling algorithms, these techniques minimize analysis time while maintaining analytical quality for complex sample matrices.Expand Specific Solutions05 Integrated sample preparation and pretreatment automation
Automated sample preparation systems for ICP-MS incorporate microwave digestion, automated filtration, and online dilution capabilities to streamline pretreatment processes. These integrated systems can handle various sample types and perform complex preparation steps such as acid digestion, matrix removal, and preconcentration without manual intervention. By automating these traditionally time-consuming steps, laboratories can process more samples while reducing exposure to hazardous chemicals and improving method reproducibility.Expand Specific Solutions
Leading Manufacturers and Research Institutions in ICP-MS Automation
The ICP-MS automation market is currently in a growth phase, with increasing demand for high-throughput analytical solutions across pharmaceutical, semiconductor, and environmental sectors. The global market size is expanding rapidly as laboratories seek efficiency improvements through automation. Leading players like Thermo Fisher Scientific, Agilent Technologies, and Shimadzu have developed mature automation platforms with integrated sample preparation and analysis workflows. Emerging companies such as Elemental Scientific and Kimia Analytics are introducing innovative solutions focused on specialized applications. The technology landscape shows varying maturity levels, with established manufacturers (PerkinElmer/Revvity, SPECTRO) offering comprehensive systems while newer entrants focus on niche improvements in sample introduction, dilution protocols, and data processing to enhance throughput capabilities.
Shimadzu Corp.
Technical Solution: Shimadzu Corporation has developed innovative automation solutions for enhancing ICP-MS throughput through their ICPMS-2030 platform integrated with their proprietary AS-10 autosampler system. Their approach focuses on intelligent sample scheduling algorithms that optimize the sequence of sample analysis based on matrix complexity and required washout times, minimizing overall analytical time[5]. Shimadzu's LabSolutions ICPMS software incorporates Development Assistant and Diagnosis Assistant functions that automate method development and system maintenance procedures, reducing instrument downtime between analytical runs. Their automation platform includes an advanced rinse solution selection system that automatically chooses appropriate rinse solutions based on previous sample matrices, optimizing washout efficiency. Shimadzu has also implemented their Eco mode technology that automatically reduces gas and power consumption during standby periods between sample batches, improving operational efficiency while maintaining system readiness for rapid startup[6]. Their system incorporates automatic performance validation protocols that ensure consistent analytical quality without manual intervention.
Strengths: Intelligent sample scheduling significantly optimizes overall batch analysis time. Automated method development reduces setup time for new analytical protocols. Eco mode technology reduces operational costs during standby periods. Weaknesses: More limited autodilution capabilities compared to some competitors. Requires careful method development to fully optimize throughput benefits. Integration with third-party laboratory information management systems may require additional customization.
PerkinElmer U.S. LLC
Technical Solution: PerkinElmer (now part of Revvity Health Sciences) has developed comprehensive automation solutions for ICP-MS throughput enhancement through their NexION ICP-MS platform integrated with their Syngistix software and SC-2 DX and SC-4 DX autosamplers. Their Universal Cell Technology (UCT) enables simultaneous multi-element analysis across different interference removal modes without mode switching, significantly reducing analysis time[7]. PerkinElmer's automation approach incorporates their PrepFAST autodilution system that provides inline dilution capabilities, automatically preparing calibration standards and diluting over-range samples without manual intervention. Their Syngistix software platform features Smart Monitoring capabilities that continuously track system performance and automatically trigger maintenance procedures when needed, minimizing unplanned downtime. PerkinElmer has also implemented their All Matrix Solution (AMS) system that introduces a small flow of dilute acid to the nebulizer during the rinse cycle, significantly reducing memory effects and carryover between samples[8]. Their automation platform includes intelligent quality control protocols that can automatically identify and address analytical issues in real-time.
Strengths: Universal Cell Technology enables efficient multi-element analysis without mode switching delays. PrepFAST autodilution system significantly reduces manual sample preparation time. Smart Monitoring capabilities minimize unplanned downtime through predictive maintenance. Weaknesses: Complex system integration may require specialized technical support. Higher initial investment compared to basic systems. Some specialized applications may require custom method development.
Key Innovations in Sample Handling and Data Processing
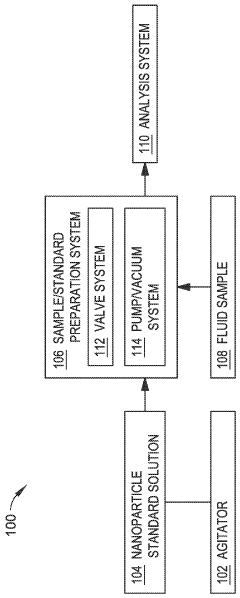
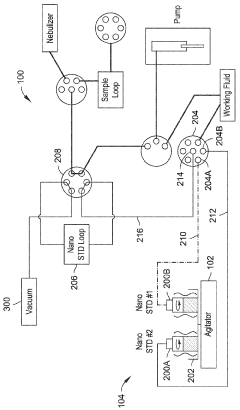
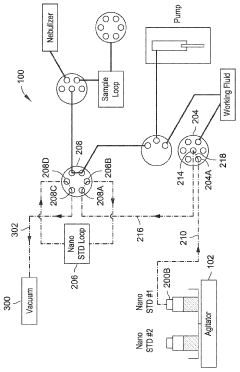
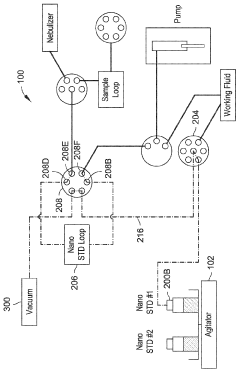
Automated inline nanoparticle standard material addition
PatentWO2023239604A1
Innovation
- An automated system with an agitator and fluid preparation system that mixes nanoparticle standards homogenously and introduces them inline with fluid samples using pumps and valves, minimizing contact time and preventing damage, while also incorporating a rinse and purge process to maintain accuracy.
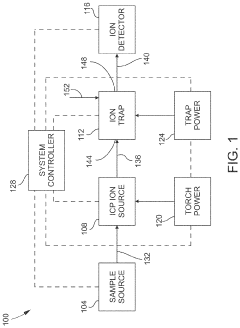
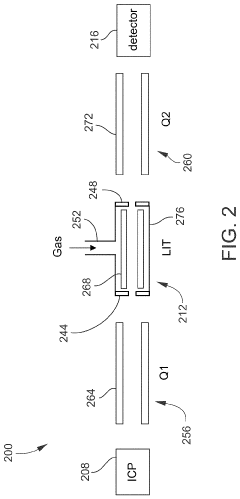
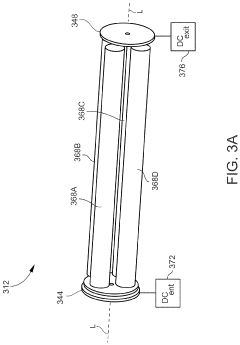
Inductively coupled plasma mass spectrometry (ICP-MS) with ion trapping
PatentActiveUS11443933B1
Innovation
- Incorporating an ion trap, such as a linear ion trap, into the ICP-MS system to confine and mass-selectively eject ions, allowing for the simultaneous analysis of multiple elements from transient signals by preventing ion exit and entry during a confinement period and transmitting selected ions to a detector for measurement.
Quality Control and Validation in Automated ICP-MS Systems
Quality control and validation are critical components in automated ICP-MS systems, ensuring that the enhanced throughput does not compromise analytical accuracy and reliability. Automated systems must incorporate robust quality control protocols that monitor system performance continuously and flag potential issues before they affect results.
Standard reference materials (SRMs) play a pivotal role in validation processes, with automated systems programmed to analyze these materials at predetermined intervals. This approach enables real-time verification of instrument calibration and performance metrics. Modern automated ICP-MS platforms typically incorporate internal standard monitoring systems that can automatically detect and correct for signal drift, matrix effects, and other analytical interferences.
Statistical process control (SPC) methods have been integrated into automated ICP-MS workflows, allowing for continuous monitoring of quality control parameters. These systems generate control charts that visualize trends in instrument performance, enabling early detection of systematic errors or gradual degradation in analytical capabilities. Advanced platforms now implement machine learning algorithms that can predict maintenance needs based on subtle changes in quality control metrics.
Automated validation protocols have evolved to include comprehensive system suitability tests that evaluate multiple performance parameters simultaneously. These tests assess factors such as sensitivity, resolution, precision, and background levels to ensure the entire analytical system meets predefined specifications. Documentation of these validation processes has also been automated, with systems generating detailed reports that satisfy regulatory requirements for industries such as pharmaceuticals, environmental monitoring, and food safety.
Inter-laboratory comparison programs have adapted to accommodate automated systems, with specialized software facilitating the exchange and statistical analysis of quality control data across multiple facilities. This collaborative approach strengthens validation processes by providing broader context for performance evaluation and identifying potential systematic biases in analytical methods.
The integration of automated quality control with laboratory information management systems (LIMS) has created comprehensive data integrity frameworks that track samples from receipt through analysis and reporting. These systems maintain audit trails that document all quality control activities, ensuring traceability and compliance with regulatory standards while supporting the higher sample throughput enabled by automation.
Standard reference materials (SRMs) play a pivotal role in validation processes, with automated systems programmed to analyze these materials at predetermined intervals. This approach enables real-time verification of instrument calibration and performance metrics. Modern automated ICP-MS platforms typically incorporate internal standard monitoring systems that can automatically detect and correct for signal drift, matrix effects, and other analytical interferences.
Statistical process control (SPC) methods have been integrated into automated ICP-MS workflows, allowing for continuous monitoring of quality control parameters. These systems generate control charts that visualize trends in instrument performance, enabling early detection of systematic errors or gradual degradation in analytical capabilities. Advanced platforms now implement machine learning algorithms that can predict maintenance needs based on subtle changes in quality control metrics.
Automated validation protocols have evolved to include comprehensive system suitability tests that evaluate multiple performance parameters simultaneously. These tests assess factors such as sensitivity, resolution, precision, and background levels to ensure the entire analytical system meets predefined specifications. Documentation of these validation processes has also been automated, with systems generating detailed reports that satisfy regulatory requirements for industries such as pharmaceuticals, environmental monitoring, and food safety.
Inter-laboratory comparison programs have adapted to accommodate automated systems, with specialized software facilitating the exchange and statistical analysis of quality control data across multiple facilities. This collaborative approach strengthens validation processes by providing broader context for performance evaluation and identifying potential systematic biases in analytical methods.
The integration of automated quality control with laboratory information management systems (LIMS) has created comprehensive data integrity frameworks that track samples from receipt through analysis and reporting. These systems maintain audit trails that document all quality control activities, ensuring traceability and compliance with regulatory standards while supporting the higher sample throughput enabled by automation.
Cost-Benefit Analysis of ICP-MS Automation Implementation
Implementing automation tools in ICP-MS laboratories requires significant initial investment, but offers substantial long-term financial benefits. The initial capital expenditure typically ranges from $50,000 to $200,000 depending on the level of automation desired, including autosampler systems, automated sample preparation units, and integrated software solutions. These costs may appear prohibitive for smaller laboratories, but must be evaluated against the operational savings they generate.
Labor cost reduction represents the most significant financial benefit, with automated systems reducing hands-on time by 60-75% compared to manual operations. A typical laboratory analyzing 100 samples daily can reallocate 4-6 technician hours per day to other value-adding activities, translating to approximately $40,000-$60,000 annual savings in labor costs alone.
Consumables usage optimization provides additional cost benefits. Automated systems precisely control reagent dispensing, reducing waste by 15-30% compared to manual operations. For laboratories processing high sample volumes, this can result in $5,000-$15,000 annual savings on chemicals and consumables.
Throughput enhancement directly impacts revenue generation capacity. Automated ICP-MS systems can increase sample processing capacity by 40-200% depending on the application, allowing laboratories to accept more client work without proportional increases in operating costs. This improved capacity utilization can generate $100,000+ additional annual revenue for commercial testing facilities.
Maintenance costs must be factored into the equation. While automated systems require specialized maintenance, they typically experience fewer user-induced failures. Annual maintenance contracts for automated ICP-MS systems range from $8,000-$20,000, partially offset by reduced downtime and fewer emergency repairs.
Return on investment calculations indicate that most laboratories achieve full ROI within 18-36 months after implementation. Smaller laboratories with lower sample volumes may experience longer payback periods of 3-5 years, while high-throughput facilities can recoup investments in as little as 12-18 months.
Intangible benefits further enhance the value proposition, including improved data quality, reduced human error rates, enhanced regulatory compliance, and improved staff satisfaction through elimination of repetitive manual tasks. These factors, while difficult to quantify precisely, contribute significantly to the overall positive cost-benefit assessment of ICP-MS automation implementation.
Labor cost reduction represents the most significant financial benefit, with automated systems reducing hands-on time by 60-75% compared to manual operations. A typical laboratory analyzing 100 samples daily can reallocate 4-6 technician hours per day to other value-adding activities, translating to approximately $40,000-$60,000 annual savings in labor costs alone.
Consumables usage optimization provides additional cost benefits. Automated systems precisely control reagent dispensing, reducing waste by 15-30% compared to manual operations. For laboratories processing high sample volumes, this can result in $5,000-$15,000 annual savings on chemicals and consumables.
Throughput enhancement directly impacts revenue generation capacity. Automated ICP-MS systems can increase sample processing capacity by 40-200% depending on the application, allowing laboratories to accept more client work without proportional increases in operating costs. This improved capacity utilization can generate $100,000+ additional annual revenue for commercial testing facilities.
Maintenance costs must be factored into the equation. While automated systems require specialized maintenance, they typically experience fewer user-induced failures. Annual maintenance contracts for automated ICP-MS systems range from $8,000-$20,000, partially offset by reduced downtime and fewer emergency repairs.
Return on investment calculations indicate that most laboratories achieve full ROI within 18-36 months after implementation. Smaller laboratories with lower sample volumes may experience longer payback periods of 3-5 years, while high-throughput facilities can recoup investments in as little as 12-18 months.
Intangible benefits further enhance the value proposition, including improved data quality, reduced human error rates, enhanced regulatory compliance, and improved staff satisfaction through elimination of repetitive manual tasks. These factors, while difficult to quantify precisely, contribute significantly to the overall positive cost-benefit assessment of ICP-MS automation implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!