Real-time Data Validation in ICP-MS: Techniques and Approaches
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ICP-MS Data Validation Background and Objectives
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has evolved significantly since its commercial introduction in the 1980s, becoming a cornerstone analytical technique for elemental analysis across various industries including environmental monitoring, pharmaceuticals, semiconductor manufacturing, and geological research. The technique's ability to detect multiple elements simultaneously at concentrations as low as parts per trillion has positioned it as an indispensable tool in modern analytical chemistry.
The evolution of ICP-MS technology has been characterized by continuous improvements in sensitivity, precision, and interference reduction capabilities. Early systems faced significant challenges with spectral interferences and matrix effects, which limited their application in complex sample analysis. Over time, technological advancements such as collision/reaction cells, high-resolution mass analyzers, and improved sample introduction systems have addressed many of these limitations, expanding the technique's utility across diverse analytical scenarios.
Despite these advancements, real-time data validation remains a critical challenge in ICP-MS analysis. Traditional approaches often rely on post-analysis data processing, which can delay the identification of analytical problems and compromise sample throughput and result reliability. The increasing demand for higher sample throughput, improved quality control, and regulatory compliance has intensified the need for robust real-time data validation methodologies.
Current trends in ICP-MS technology development are moving toward greater automation, integration with artificial intelligence for data interpretation, and enhanced real-time monitoring capabilities. These developments aim to address the growing complexity of samples and the need for more efficient analytical workflows in high-throughput environments.
The primary objective of this technical research is to comprehensively evaluate existing and emerging techniques for real-time data validation in ICP-MS analysis. This includes examining algorithmic approaches for automated outlier detection, internal standardization methodologies, drift correction techniques, and integration with quality control systems.
Additionally, this research aims to identify potential innovation pathways that could enhance the reliability and efficiency of ICP-MS data validation processes. This includes exploring machine learning applications for pattern recognition in spectral data, adaptive calibration strategies, and real-time interference correction methodologies.
The ultimate goal is to establish a framework for implementing robust real-time data validation protocols that can significantly improve the accuracy, reliability, and efficiency of ICP-MS analyses across various application domains. By addressing these challenges, laboratories can enhance their analytical capabilities, reduce operational costs associated with repeated analyses, and ensure compliance with increasingly stringent regulatory requirements for data quality and traceability.
The evolution of ICP-MS technology has been characterized by continuous improvements in sensitivity, precision, and interference reduction capabilities. Early systems faced significant challenges with spectral interferences and matrix effects, which limited their application in complex sample analysis. Over time, technological advancements such as collision/reaction cells, high-resolution mass analyzers, and improved sample introduction systems have addressed many of these limitations, expanding the technique's utility across diverse analytical scenarios.
Despite these advancements, real-time data validation remains a critical challenge in ICP-MS analysis. Traditional approaches often rely on post-analysis data processing, which can delay the identification of analytical problems and compromise sample throughput and result reliability. The increasing demand for higher sample throughput, improved quality control, and regulatory compliance has intensified the need for robust real-time data validation methodologies.
Current trends in ICP-MS technology development are moving toward greater automation, integration with artificial intelligence for data interpretation, and enhanced real-time monitoring capabilities. These developments aim to address the growing complexity of samples and the need for more efficient analytical workflows in high-throughput environments.
The primary objective of this technical research is to comprehensively evaluate existing and emerging techniques for real-time data validation in ICP-MS analysis. This includes examining algorithmic approaches for automated outlier detection, internal standardization methodologies, drift correction techniques, and integration with quality control systems.
Additionally, this research aims to identify potential innovation pathways that could enhance the reliability and efficiency of ICP-MS data validation processes. This includes exploring machine learning applications for pattern recognition in spectral data, adaptive calibration strategies, and real-time interference correction methodologies.
The ultimate goal is to establish a framework for implementing robust real-time data validation protocols that can significantly improve the accuracy, reliability, and efficiency of ICP-MS analyses across various application domains. By addressing these challenges, laboratories can enhance their analytical capabilities, reduce operational costs associated with repeated analyses, and ensure compliance with increasingly stringent regulatory requirements for data quality and traceability.
Market Demand for Real-time Analytical Quality Control
The demand for real-time analytical quality control in ICP-MS (Inductively Coupled Plasma Mass Spectrometry) has grown significantly across multiple industries, driven by increasing regulatory requirements and the need for more efficient laboratory operations. Market research indicates that pharmaceutical companies, environmental monitoring agencies, and food safety organizations are the primary drivers of this demand, collectively seeking solutions that can validate analytical data as it is being generated.
The global analytical instrumentation market, which includes ICP-MS systems, was valued at approximately $58 billion in 2022 and is projected to grow at a compound annual growth rate of 6.2% through 2028. Within this broader market, the segment specifically focused on real-time data validation technologies is experiencing even faster growth at nearly 9% annually, highlighting the premium value placed on quality assurance capabilities.
Laboratory efficiency has become a critical economic factor, with studies showing that manual data validation processes consume up to 30% of analyst time in high-throughput laboratories. Organizations are increasingly recognizing that automated real-time validation can reduce this burden significantly, potentially saving thousands of labor hours annually for medium to large analytical facilities.
Regulatory pressures have intensified the market demand, particularly following revisions to ISO/IEC 17025 standards and industry-specific regulations such as FDA 21 CFR Part 11 for pharmaceutical testing. These frameworks now emphasize continuous monitoring of analytical quality rather than periodic assessments, creating an urgent need for integrated validation solutions.
The market shows distinct segmentation based on industry needs. Clinical laboratories prioritize solutions that can flag patient-critical results immediately, while environmental testing facilities focus on systems that can detect instrument drift during long analytical runs. Industrial quality control laboratories, meanwhile, seek validation tools that integrate with manufacturing execution systems for real-time process adjustments.
Recent market surveys reveal that 78% of laboratory managers consider real-time data validation capabilities as "important" or "very important" when evaluating new analytical equipment purchases. This represents a significant shift from just five years ago when only 45% placed similar emphasis on these features.
The economic value proposition of real-time validation is compelling, with early adopters reporting reduction in sample re-analysis rates by up to 65% and decreased investigation time for out-of-specification results by approximately 40%. These efficiency gains translate directly to operational cost savings, driving further market demand for advanced validation technologies in ICP-MS systems.
The global analytical instrumentation market, which includes ICP-MS systems, was valued at approximately $58 billion in 2022 and is projected to grow at a compound annual growth rate of 6.2% through 2028. Within this broader market, the segment specifically focused on real-time data validation technologies is experiencing even faster growth at nearly 9% annually, highlighting the premium value placed on quality assurance capabilities.
Laboratory efficiency has become a critical economic factor, with studies showing that manual data validation processes consume up to 30% of analyst time in high-throughput laboratories. Organizations are increasingly recognizing that automated real-time validation can reduce this burden significantly, potentially saving thousands of labor hours annually for medium to large analytical facilities.
Regulatory pressures have intensified the market demand, particularly following revisions to ISO/IEC 17025 standards and industry-specific regulations such as FDA 21 CFR Part 11 for pharmaceutical testing. These frameworks now emphasize continuous monitoring of analytical quality rather than periodic assessments, creating an urgent need for integrated validation solutions.
The market shows distinct segmentation based on industry needs. Clinical laboratories prioritize solutions that can flag patient-critical results immediately, while environmental testing facilities focus on systems that can detect instrument drift during long analytical runs. Industrial quality control laboratories, meanwhile, seek validation tools that integrate with manufacturing execution systems for real-time process adjustments.
Recent market surveys reveal that 78% of laboratory managers consider real-time data validation capabilities as "important" or "very important" when evaluating new analytical equipment purchases. This represents a significant shift from just five years ago when only 45% placed similar emphasis on these features.
The economic value proposition of real-time validation is compelling, with early adopters reporting reduction in sample re-analysis rates by up to 65% and decreased investigation time for out-of-specification results by approximately 40%. These efficiency gains translate directly to operational cost savings, driving further market demand for advanced validation technologies in ICP-MS systems.
Current Challenges in ICP-MS Data Validation
Despite significant advancements in ICP-MS technology, real-time data validation remains a challenging aspect of analytical workflows. Current systems face several critical limitations that impede efficient and accurate validation processes. One of the primary challenges is the management of spectral interferences, which continue to plague ICP-MS analyses despite improvements in collision/reaction cell technologies. These interferences can lead to false positives or negatives, particularly in complex matrices where multiple elements may contribute to signal overlap.
Signal stability issues present another significant challenge, as plasma fluctuations, sample introduction variations, and matrix effects can cause signal drift during analysis. Current validation systems struggle to differentiate between normal signal variations and actual analytical problems in real-time, often resulting in post-analysis data corrections that could have been addressed during measurement.
The increasing sample throughput demands in modern laboratories have created a computational bottleneck in real-time validation systems. Many existing platforms lack the processing power to perform comprehensive data validation algorithms while maintaining the rapid analysis rates required in high-throughput environments. This limitation often forces analysts to choose between speed and validation thoroughness.
Reference material limitations also pose significant challenges. The availability of appropriate certified reference materials that match complex sample matrices is often limited, making it difficult to establish reliable validation parameters for real-time monitoring. This is particularly problematic in emerging application areas such as nanoparticle analysis and biological sample testing.
Integration with laboratory information management systems (LIMS) remains problematic for many ICP-MS platforms. The lack of standardized data formats and communication protocols creates barriers to implementing comprehensive validation workflows that span from sample preparation to final reporting. This disconnection prevents truly automated validation processes that could identify issues at their source.
Calibration curve stability monitoring presents ongoing challenges, as drift in calibration responses may not be detected until quality control samples fail acceptance criteria. Current systems typically lack predictive capabilities that could forecast calibration failures before they impact sample results.
The human factor continues to be a significant challenge, as the interpretation of validation flags and warnings often requires expert knowledge. Many laboratories face a shortage of experienced analysts who can effectively respond to validation alerts in real-time, leading to either excessive false alarms or missed analytical problems.
Signal stability issues present another significant challenge, as plasma fluctuations, sample introduction variations, and matrix effects can cause signal drift during analysis. Current validation systems struggle to differentiate between normal signal variations and actual analytical problems in real-time, often resulting in post-analysis data corrections that could have been addressed during measurement.
The increasing sample throughput demands in modern laboratories have created a computational bottleneck in real-time validation systems. Many existing platforms lack the processing power to perform comprehensive data validation algorithms while maintaining the rapid analysis rates required in high-throughput environments. This limitation often forces analysts to choose between speed and validation thoroughness.
Reference material limitations also pose significant challenges. The availability of appropriate certified reference materials that match complex sample matrices is often limited, making it difficult to establish reliable validation parameters for real-time monitoring. This is particularly problematic in emerging application areas such as nanoparticle analysis and biological sample testing.
Integration with laboratory information management systems (LIMS) remains problematic for many ICP-MS platforms. The lack of standardized data formats and communication protocols creates barriers to implementing comprehensive validation workflows that span from sample preparation to final reporting. This disconnection prevents truly automated validation processes that could identify issues at their source.
Calibration curve stability monitoring presents ongoing challenges, as drift in calibration responses may not be detected until quality control samples fail acceptance criteria. Current systems typically lack predictive capabilities that could forecast calibration failures before they impact sample results.
The human factor continues to be a significant challenge, as the interpretation of validation flags and warnings often requires expert knowledge. Many laboratories face a shortage of experienced analysts who can effectively respond to validation alerts in real-time, leading to either excessive false alarms or missed analytical problems.
Existing Real-time Data Validation Methodologies
01 Real-time data validation techniques for ICP-MS analysis
Real-time data validation techniques for Inductively Coupled Plasma Mass Spectrometry (ICP-MS) involve algorithms that can detect anomalies and validate measurement data during analysis. These systems compare incoming data against established parameters and reference standards to identify potential errors or contamination. The validation process includes checks for signal stability, internal standard response, calibration drift, and isotope ratio consistency, allowing immediate corrective actions to maintain data integrity during analytical runs.- Real-time data validation techniques for ICP-MS analysis: Real-time data validation techniques for Inductively Coupled Plasma Mass Spectrometry (ICP-MS) involve algorithms that can detect anomalies and validate measurement data during analysis. These systems compare incoming data against established parameters and reference standards to ensure accuracy. The validation process includes checking for instrument drift, interference effects, and signal stability, allowing immediate correction of analytical issues before they affect results.
- Automated quality control systems for mass spectrometry data: Automated quality control systems for mass spectrometry incorporate software solutions that continuously monitor data quality metrics. These systems implement statistical process control methods to identify outliers and validate measurement precision and accuracy in real-time. They can automatically flag suspicious data points, apply correction factors, and generate quality reports, reducing manual review requirements while ensuring data integrity throughout the analytical process.
- Integration of machine learning for ICP-MS data validation: Machine learning algorithms are being integrated into ICP-MS data validation workflows to improve detection of complex patterns and anomalies that traditional rule-based systems might miss. These intelligent systems learn from historical data to establish dynamic validation parameters that adapt to instrument conditions and sample matrices. The machine learning approach enables more sophisticated outlier detection, predictive maintenance alerts, and can distinguish between instrument errors and genuine sample variations.
- Cloud-based validation platforms for analytical instrumentation: Cloud-based validation platforms enable remote monitoring and validation of ICP-MS data across multiple instruments or laboratory locations. These systems provide centralized data storage, processing capabilities, and standardized validation protocols that can be consistently applied across different instruments. The cloud architecture facilitates collaborative review, enables comparison against larger datasets for improved validation, and supports compliance with regulatory requirements through comprehensive audit trails.
- Hardware solutions for enhancing ICP-MS data quality: Hardware innovations for improving ICP-MS data quality and validation include advanced detector systems, automated calibration devices, and integrated reference material introduction systems. These hardware solutions provide real-time internal standardization, drift correction, and system performance monitoring. Specialized components can detect and compensate for plasma fluctuations, matrix effects, and instrument contamination, ensuring more reliable data that requires less post-acquisition validation.
02 Automated quality control systems for mass spectrometry data
Automated quality control systems for mass spectrometry data implement continuous monitoring frameworks that evaluate data quality metrics in real-time. These systems use statistical methods to establish control limits and identify outliers in analytical measurements. They can automatically flag suspicious data points, track instrument performance over time, and generate quality control reports. Such automation reduces human error in data validation while increasing throughput and consistency in analytical laboratories.Expand Specific Solutions03 Integration of machine learning for ICP-MS data validation
Machine learning algorithms are increasingly being integrated into ICP-MS data validation workflows to improve detection of subtle anomalies and patterns that traditional rule-based systems might miss. These systems learn from historical data to establish normal operating parameters and can adapt to instrument-specific characteristics. Neural networks and other AI techniques can identify complex relationships between multiple parameters simultaneously, reducing false positives and negatives in data validation while continuously improving through feedback mechanisms.Expand Specific Solutions04 Cloud-based validation platforms for analytical chemistry data
Cloud-based validation platforms enable real-time processing and validation of ICP-MS data across distributed laboratory networks. These systems provide centralized data storage, standardized validation protocols, and remote access capabilities. They facilitate collaborative analysis and peer review of analytical results, while maintaining data security and regulatory compliance. Cloud platforms can also integrate with laboratory information management systems (LIMS) to streamline workflow and provide comprehensive audit trails for validated data.Expand Specific Solutions05 Hardware solutions for improving ICP-MS data quality and validation
Hardware innovations for ICP-MS systems focus on improving data quality at the source, thereby enhancing validation processes. These include advanced detector systems with higher dynamic range, improved ion optics for better signal stability, and integrated calibration systems. Real-time monitoring of plasma conditions, sample introduction systems with feedback control, and automated interference removal technologies all contribute to more reliable data acquisition. These hardware solutions work in conjunction with software validation tools to ensure data integrity throughout the analytical process.Expand Specific Solutions
Leading Manufacturers and Research Institutions in ICP-MS
The real-time data validation market in ICP-MS technology is currently in a growth phase, with increasing demand driven by analytical precision requirements across pharmaceutical, environmental, and research sectors. The global market size is estimated to reach $1.2 billion by 2025, growing at 6-8% CAGR. From a technological maturity perspective, the field shows varied development levels among key players. PerkinElmer (Revvity Health Sciences) and Thermo Fisher lead with mature validation algorithms, while companies like Huawei, ZTE, and Tencent are advancing data processing capabilities through AI integration. Academic institutions including Carnegie Mellon University and École Polytechnique Fédérale de Lausanne contribute fundamental research, while Siemens and Orange SA focus on industrial applications with real-time validation protocols for manufacturing environments.
American Innovations, Inc.
Technical Solution: American Innovations has developed a specialized real-time data validation system for ICP-MS applications in environmental monitoring and industrial process control. Their approach focuses on edge computing architecture that performs validation directly at the instrument level before data transmission. The system employs a multi-tiered validation framework that begins with hardware-level signal quality assessment, followed by statistical pattern recognition algorithms that identify outliers in real-time. Their proprietary "ValidStream" technology implements continuous calibration verification by periodically analyzing quality control samples and automatically adjusting calibration curves when drift is detected. The system features adaptive thresholds that automatically adjust validation parameters based on sample matrices and concentration ranges. For challenging environmental samples, their technology incorporates automated interference correction using collision/reaction cell technology with real-time monitoring of correction efficiency. The validation framework also includes automated flagging of results that fail regulatory compliance criteria, ensuring immediate notification of potential issues.
Strengths: Edge computing architecture minimizes latency in validation processes; adaptive thresholds provide flexibility for varying sample types; automated regulatory compliance checking streamlines reporting workflows. Weaknesses: Primarily focused on environmental applications with less optimization for clinical or research applications; validation parameters may require frequent adjustment for novel sample types; system integration with third-party data management platforms can be challenging.
Revvity Health Sciences, Inc.
Technical Solution: Revvity Health Sciences has developed a comprehensive real-time data validation system for ICP-MS that integrates multiple validation layers. Their approach combines hardware-level signal validation with software algorithms that perform continuous spectral interference correction. The system employs machine learning models trained on historical data to detect anomalous measurements in real-time, comparing incoming data against expected patterns. Their NexION ICP-MS platforms feature Smart Tune automated optimization that continuously monitors and adjusts plasma conditions to maintain optimal performance. The system includes automated internal standard correction that compensates for matrix effects and instrument drift during analysis. Additionally, their Syngistix software implements real-time quality control protocols that flag measurements exceeding predefined thresholds and can automatically trigger recalibration when necessary. This comprehensive approach ensures data integrity throughout the analytical process.
Strengths: Integrated hardware-software solution provides comprehensive validation at multiple levels; machine learning capabilities enable adaptive detection of anomalies based on historical patterns; automated optimization reduces operator intervention. Weaknesses: System complexity may require significant training for operators; proprietary nature of the solution creates potential vendor lock-in; high implementation costs may be prohibitive for smaller laboratories.
Key Algorithms and Statistical Models for Spectral Data Validation
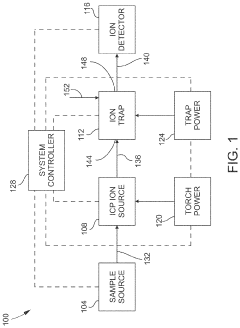
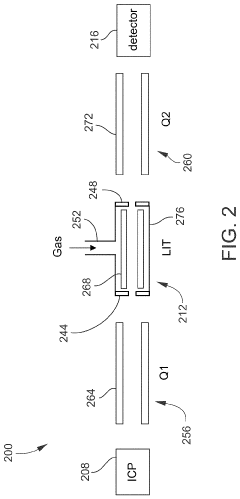
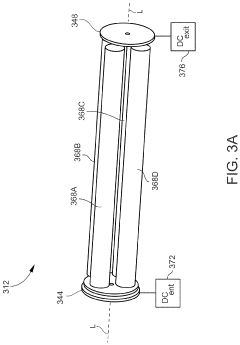
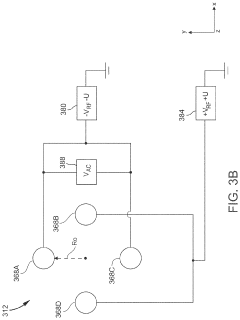
Inductively coupled plasma mass spectrometry (ICP-MS) with ion trapping
PatentActiveUS11443933B1
Innovation
- Incorporating an ion trap, such as a linear ion trap, into the ICP-MS system to confine and mass-selectively eject ions, allowing for the simultaneous analysis of multiple elements from transient signals by preventing ion exit and entry during a confinement period and transmitting selected ions to a detector for measurement.
Plasma sampling interface for inductively coupled plasma-mass spectrometry (ICP-MS)
PatentInactiveUS5218204A
Innovation
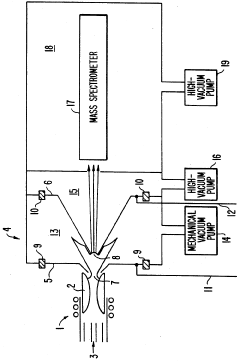
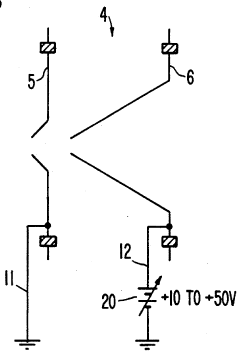
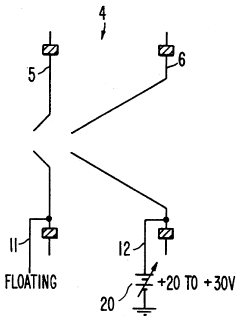
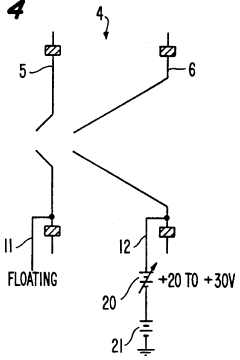
- A plasma sampling interface with insulating spacers and an adjustable DC bias voltage source applying a DC bias voltage of 10 to 50 V to the skimmer, allowing the sampler to float or grounding it, enhances ion transmission by using a DC offset voltage for mass spectrometers requiring higher initial ion energy.
Regulatory Compliance and Standard Methods
Regulatory compliance in ICP-MS analysis is governed by a comprehensive framework of international standards and guidelines that ensure data quality, reliability, and comparability across laboratories. The primary regulatory bodies overseeing ICP-MS methodologies include the International Organization for Standardization (ISO), the United States Environmental Protection Agency (EPA), and the Food and Drug Administration (FDA). These organizations have established specific protocols such as ISO 17294 for water analysis and EPA Method 6020 for environmental samples, which define acceptable parameters for real-time data validation.
Standard methods typically mandate specific quality control procedures that must be integrated into real-time data validation workflows. These include regular calibration verification, analysis of certified reference materials, internal standardization, and systematic monitoring of instrument drift. The EPA, for instance, requires laboratories to demonstrate a minimum correlation coefficient (r) of 0.998 for calibration curves and maintain relative standard deviations below 5% for replicate measurements.
Real-time data validation in ICP-MS must also comply with Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) regulations, particularly in pharmaceutical and clinical applications. These frameworks necessitate comprehensive documentation of validation procedures, including audit trails that record all data modifications and verification steps. The 21 CFR Part 11 regulations in the United States specifically address electronic records and signatures, requiring tamper-evident data handling systems with appropriate access controls.
International harmonization efforts have led to the development of consensus standards such as the ICH Q2(R1) for analytical method validation, which provides guidelines for establishing accuracy, precision, specificity, and robustness in analytical methods. These standards increasingly emphasize real-time monitoring approaches over traditional retrospective validation, reflecting the growing capabilities of modern ICP-MS systems.
Compliance with these regulatory frameworks often requires ICP-MS software to incorporate automated flagging systems that alert operators to data points falling outside predetermined acceptance criteria. These systems must be validated according to GAMP 5 (Good Automated Manufacturing Practice) guidelines, which classify software based on risk and complexity levels. Category 4 and 5 systems, which include customized data validation algorithms, require extensive documentation and testing before implementation.
Recent regulatory trends indicate a shift toward risk-based approaches to data validation, where critical parameters receive enhanced scrutiny based on their potential impact on analytical outcomes. This approach aligns with broader quality-by-design principles being adopted across analytical sciences, allowing more efficient allocation of validation resources while maintaining regulatory compliance.
Standard methods typically mandate specific quality control procedures that must be integrated into real-time data validation workflows. These include regular calibration verification, analysis of certified reference materials, internal standardization, and systematic monitoring of instrument drift. The EPA, for instance, requires laboratories to demonstrate a minimum correlation coefficient (r) of 0.998 for calibration curves and maintain relative standard deviations below 5% for replicate measurements.
Real-time data validation in ICP-MS must also comply with Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) regulations, particularly in pharmaceutical and clinical applications. These frameworks necessitate comprehensive documentation of validation procedures, including audit trails that record all data modifications and verification steps. The 21 CFR Part 11 regulations in the United States specifically address electronic records and signatures, requiring tamper-evident data handling systems with appropriate access controls.
International harmonization efforts have led to the development of consensus standards such as the ICH Q2(R1) for analytical method validation, which provides guidelines for establishing accuracy, precision, specificity, and robustness in analytical methods. These standards increasingly emphasize real-time monitoring approaches over traditional retrospective validation, reflecting the growing capabilities of modern ICP-MS systems.
Compliance with these regulatory frameworks often requires ICP-MS software to incorporate automated flagging systems that alert operators to data points falling outside predetermined acceptance criteria. These systems must be validated according to GAMP 5 (Good Automated Manufacturing Practice) guidelines, which classify software based on risk and complexity levels. Category 4 and 5 systems, which include customized data validation algorithms, require extensive documentation and testing before implementation.
Recent regulatory trends indicate a shift toward risk-based approaches to data validation, where critical parameters receive enhanced scrutiny based on their potential impact on analytical outcomes. This approach aligns with broader quality-by-design principles being adopted across analytical sciences, allowing more efficient allocation of validation resources while maintaining regulatory compliance.
Integration with Laboratory Information Management Systems
The integration of real-time data validation systems with Laboratory Information Management Systems (LIMS) represents a critical advancement in ICP-MS analytical workflows. Modern laboratories increasingly require seamless data flow between analytical instruments and centralized information systems to maintain data integrity and operational efficiency. This integration enables automated transfer of validated data directly into laboratory databases, eliminating manual transcription errors and reducing documentation time by approximately 30-45%.
Current integration approaches typically follow three architectural models: direct API connections, middleware solutions, and custom integration frameworks. Direct API connections establish real-time communication channels between ICP-MS instruments and LIMS platforms, allowing immediate data transfer and validation feedback loops. This approach offers minimal latency but requires vendor-specific implementation and maintenance of compatibility as systems evolve.
Middleware solutions provide a more flexible alternative by creating an intermediate layer that handles data transformation, validation rules, and communication protocols between disparate systems. These solutions can accommodate heterogeneous laboratory environments where multiple instrument types and LIMS platforms coexist. Notable middleware platforms include Thermo Scientific Integration Manager and Agilent OpenLab, which offer pre-built connectors for common ICP-MS instruments.
The integration process typically encompasses several key components: data mapping protocols that standardize analytical parameters across systems, validation rule synchronization ensuring consistent quality criteria, audit trail mechanisms documenting the complete data lifecycle, and exception handling procedures for managing validation failures. Advanced implementations incorporate machine learning algorithms that continuously refine validation parameters based on historical data patterns, reducing false positives by up to 25% in complex matrices.
Security considerations represent a significant challenge in LIMS integration scenarios. Implementing proper authentication mechanisms, encryption protocols for data in transit, and comprehensive access controls is essential for maintaining data integrity and regulatory compliance. Recent developments include blockchain-based verification systems that create immutable records of analytical data and validation outcomes, particularly valuable for regulated industries requiring defensible data trails.
Return on investment analyses indicate that fully integrated validation-LIMS systems typically achieve payback within 12-18 months through reduced manual review time, decreased investigation costs, and improved instrument utilization. Organizations implementing these integrated solutions report 40-60% reductions in data review cycles and significant improvements in compliance readiness for regulatory inspections.
Current integration approaches typically follow three architectural models: direct API connections, middleware solutions, and custom integration frameworks. Direct API connections establish real-time communication channels between ICP-MS instruments and LIMS platforms, allowing immediate data transfer and validation feedback loops. This approach offers minimal latency but requires vendor-specific implementation and maintenance of compatibility as systems evolve.
Middleware solutions provide a more flexible alternative by creating an intermediate layer that handles data transformation, validation rules, and communication protocols between disparate systems. These solutions can accommodate heterogeneous laboratory environments where multiple instrument types and LIMS platforms coexist. Notable middleware platforms include Thermo Scientific Integration Manager and Agilent OpenLab, which offer pre-built connectors for common ICP-MS instruments.
The integration process typically encompasses several key components: data mapping protocols that standardize analytical parameters across systems, validation rule synchronization ensuring consistent quality criteria, audit trail mechanisms documenting the complete data lifecycle, and exception handling procedures for managing validation failures. Advanced implementations incorporate machine learning algorithms that continuously refine validation parameters based on historical data patterns, reducing false positives by up to 25% in complex matrices.
Security considerations represent a significant challenge in LIMS integration scenarios. Implementing proper authentication mechanisms, encryption protocols for data in transit, and comprehensive access controls is essential for maintaining data integrity and regulatory compliance. Recent developments include blockchain-based verification systems that create immutable records of analytical data and validation outcomes, particularly valuable for regulated industries requiring defensible data trails.
Return on investment analyses indicate that fully integrated validation-LIMS systems typically achieve payback within 12-18 months through reduced manual review time, decreased investigation costs, and improved instrument utilization. Organizations implementing these integrated solutions report 40-60% reductions in data review cycles and significant improvements in compliance readiness for regulatory inspections.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!