Measurement Precision in ICP-MS: Influencing Factors
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ICP-MS Technology Evolution and Precision Goals
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has evolved significantly since its commercial introduction in the early 1980s. This analytical technique combines the high-temperature ICP source with a mass spectrometer to detect and quantify elements at ultra-trace concentrations. The evolution of ICP-MS technology has been driven by the increasing demand for higher precision, lower detection limits, and broader elemental coverage across various scientific and industrial applications.
The initial ICP-MS systems faced significant challenges in measurement precision, primarily due to limitations in ion optics, interface design, and detector technology. Early instruments typically achieved relative standard deviations (RSDs) of 2-5% for most elements, which was adequate for many applications but insufficient for high-precision isotopic analysis and ultra-trace detection.
By the 1990s, the introduction of collision/reaction cell technology marked a pivotal advancement in addressing polyatomic interferences, a major factor affecting measurement precision. This innovation significantly improved the accuracy of measurements for elements previously affected by spectral overlaps, reducing RSDs to 1-2% for most applications.
The early 2000s witnessed the development of high-resolution ICP-MS systems, capable of resolving spectral interferences through improved mass separation. Simultaneously, advancements in sample introduction systems, including desolvation nebulizers and laser ablation techniques, enhanced sample transport efficiency and stability, further contributing to measurement precision.
Current precision goals in ICP-MS technology focus on achieving sub-1% RSDs for routine analyses and approaching 0.1% for specialized applications such as isotope ratio measurements. These goals are driven by emerging applications in fields including geochronology, nuclear forensics, and biomedical research, where minute differences in elemental or isotopic composition can yield significant scientific insights.
The pursuit of higher precision continues to drive innovation in several key areas: improved plasma stability through advanced RF generators and gas control systems; enhanced ion transmission efficiency via refined ion optics; more stable and sensitive detector systems; and sophisticated software algorithms for signal processing and drift correction.
Looking forward, the integration of artificial intelligence and machine learning approaches for real-time data processing and instrument optimization represents a promising frontier for further enhancing measurement precision. Additionally, miniaturization efforts aim to maintain high precision while reducing instrument footprint and resource requirements, potentially expanding ICP-MS applications to field and point-of-care settings.
The initial ICP-MS systems faced significant challenges in measurement precision, primarily due to limitations in ion optics, interface design, and detector technology. Early instruments typically achieved relative standard deviations (RSDs) of 2-5% for most elements, which was adequate for many applications but insufficient for high-precision isotopic analysis and ultra-trace detection.
By the 1990s, the introduction of collision/reaction cell technology marked a pivotal advancement in addressing polyatomic interferences, a major factor affecting measurement precision. This innovation significantly improved the accuracy of measurements for elements previously affected by spectral overlaps, reducing RSDs to 1-2% for most applications.
The early 2000s witnessed the development of high-resolution ICP-MS systems, capable of resolving spectral interferences through improved mass separation. Simultaneously, advancements in sample introduction systems, including desolvation nebulizers and laser ablation techniques, enhanced sample transport efficiency and stability, further contributing to measurement precision.
Current precision goals in ICP-MS technology focus on achieving sub-1% RSDs for routine analyses and approaching 0.1% for specialized applications such as isotope ratio measurements. These goals are driven by emerging applications in fields including geochronology, nuclear forensics, and biomedical research, where minute differences in elemental or isotopic composition can yield significant scientific insights.
The pursuit of higher precision continues to drive innovation in several key areas: improved plasma stability through advanced RF generators and gas control systems; enhanced ion transmission efficiency via refined ion optics; more stable and sensitive detector systems; and sophisticated software algorithms for signal processing and drift correction.
Looking forward, the integration of artificial intelligence and machine learning approaches for real-time data processing and instrument optimization represents a promising frontier for further enhancing measurement precision. Additionally, miniaturization efforts aim to maintain high precision while reducing instrument footprint and resource requirements, potentially expanding ICP-MS applications to field and point-of-care settings.
Market Demand for High-Precision Elemental Analysis
The global market for high-precision elemental analysis has experienced substantial growth over the past decade, driven primarily by increasing demands across multiple industries for more accurate and reliable analytical methods. ICP-MS (Inductively Coupled Plasma Mass Spectrometry) technology has emerged as a cornerstone analytical tool due to its exceptional sensitivity, multi-element capabilities, and wide dynamic range.
The pharmaceutical sector represents one of the largest market segments, with stringent regulatory requirements necessitating precise elemental analysis for drug safety and quality control. Recent regulatory changes by the FDA and EMA have lowered permissible limits for elemental impurities in pharmaceutical products, creating heightened demand for high-precision ICP-MS instrumentation capable of detecting contaminants at increasingly lower concentrations.
Environmental monitoring constitutes another significant market driver, with government agencies and research institutions requiring precise measurement of trace elements in various environmental matrices. The growing public concern over water quality, soil contamination, and air pollution has led to more comprehensive monitoring programs worldwide, expanding the market for advanced analytical technologies.
The semiconductor and electronics manufacturing industries have become increasingly dependent on high-precision elemental analysis as component miniaturization continues. With circuit features now measured in nanometers, even minute elemental contamination can significantly impact product performance and reliability, driving demand for ultra-sensitive analytical capabilities.
Food safety testing represents a rapidly expanding application area, particularly in developed economies where consumers and regulatory bodies demand comprehensive testing for heavy metals and other potentially harmful elements. The global food safety testing market has been growing at approximately 8% annually, with elemental analysis forming a critical component of this expansion.
Clinical and biomedical research applications have also contributed significantly to market growth, with increasing focus on the role of trace elements in human health and disease. The growing field of metallomics has created new opportunities for high-precision elemental analysis technologies.
Market analysis indicates that the global ICP-MS instrument market is projected to maintain strong growth through 2028, with particular emphasis on instruments offering enhanced precision, lower detection limits, and improved interference management capabilities. The Asia-Pacific region, especially China and India, represents the fastest-growing market segment due to expanding industrial bases and strengthening environmental regulations.
Customer demands increasingly focus on instruments that not only provide exceptional precision but also offer improved usability, reduced maintenance requirements, and lower operating costs. This has driven manufacturers to develop more sophisticated systems with advanced interference removal technologies and improved sample introduction systems to enhance measurement precision.
The pharmaceutical sector represents one of the largest market segments, with stringent regulatory requirements necessitating precise elemental analysis for drug safety and quality control. Recent regulatory changes by the FDA and EMA have lowered permissible limits for elemental impurities in pharmaceutical products, creating heightened demand for high-precision ICP-MS instrumentation capable of detecting contaminants at increasingly lower concentrations.
Environmental monitoring constitutes another significant market driver, with government agencies and research institutions requiring precise measurement of trace elements in various environmental matrices. The growing public concern over water quality, soil contamination, and air pollution has led to more comprehensive monitoring programs worldwide, expanding the market for advanced analytical technologies.
The semiconductor and electronics manufacturing industries have become increasingly dependent on high-precision elemental analysis as component miniaturization continues. With circuit features now measured in nanometers, even minute elemental contamination can significantly impact product performance and reliability, driving demand for ultra-sensitive analytical capabilities.
Food safety testing represents a rapidly expanding application area, particularly in developed economies where consumers and regulatory bodies demand comprehensive testing for heavy metals and other potentially harmful elements. The global food safety testing market has been growing at approximately 8% annually, with elemental analysis forming a critical component of this expansion.
Clinical and biomedical research applications have also contributed significantly to market growth, with increasing focus on the role of trace elements in human health and disease. The growing field of metallomics has created new opportunities for high-precision elemental analysis technologies.
Market analysis indicates that the global ICP-MS instrument market is projected to maintain strong growth through 2028, with particular emphasis on instruments offering enhanced precision, lower detection limits, and improved interference management capabilities. The Asia-Pacific region, especially China and India, represents the fastest-growing market segment due to expanding industrial bases and strengthening environmental regulations.
Customer demands increasingly focus on instruments that not only provide exceptional precision but also offer improved usability, reduced maintenance requirements, and lower operating costs. This has driven manufacturers to develop more sophisticated systems with advanced interference removal technologies and improved sample introduction systems to enhance measurement precision.
Current Limitations and Challenges in ICP-MS Precision
Despite significant advancements in ICP-MS technology, several critical limitations continue to challenge measurement precision. Sample introduction systems remain a primary source of instability, with conventional nebulizers typically achieving only 1-3% precision under optimal conditions. Fluctuations in sample uptake rates, droplet size distribution variability, and plasma loading effects contribute significantly to measurement uncertainty. Even modern cyclonic spray chambers cannot completely eliminate these variations, particularly when analyzing complex matrices.
Matrix effects represent another substantial challenge, as high-concentration matrix elements can suppress or enhance analyte signals unpredictably. This phenomenon becomes particularly problematic when analyzing environmental or biological samples where matrix composition varies widely between specimens. Current mathematical correction models often fail to fully compensate for these effects when matrix complexity exceeds certain thresholds.
Spectral interferences continue to plague ICP-MS precision despite advances in collision/reaction cell technologies. Polyatomic interferences (such as 40Ar16O+ interfering with 56Fe+) and isobaric overlaps remain particularly challenging for certain element combinations. While high-resolution instruments can separate many interferences, this approach sacrifices sensitivity and increases cost substantially, creating a precision-sensitivity tradeoff that limits practical applications.
Instrument drift represents a persistent challenge, with signal intensity typically changing by 1-5% per hour during routine operation. This necessitates frequent recalibration and internal standardization, which introduces additional variables and potential error sources. Thermal instability in the sample introduction system and gradual changes in plasma conditions contribute significantly to this drift phenomenon.
Detector limitations further constrain precision, particularly when measuring elements across wide concentration ranges. Pulse-counting detectors offer excellent precision at low concentrations but suffer from dead time corrections at higher counts, while analog detection modes introduce additional noise. The crossover region between these detection modes often exhibits degraded precision, with relative standard deviations increasing by 2-3 times compared to single-mode measurements.
Fundamental counting statistics impose theoretical limits on achievable precision, particularly at ultra-trace levels. For elements present at sub-ppb concentrations, even perfect instrumental conditions cannot overcome the statistical limitations of ion counting, resulting in practical precision limits of 3-5% for many real-world applications. This statistical barrier represents a fundamental challenge that technological improvements alone cannot fully address.
Matrix effects represent another substantial challenge, as high-concentration matrix elements can suppress or enhance analyte signals unpredictably. This phenomenon becomes particularly problematic when analyzing environmental or biological samples where matrix composition varies widely between specimens. Current mathematical correction models often fail to fully compensate for these effects when matrix complexity exceeds certain thresholds.
Spectral interferences continue to plague ICP-MS precision despite advances in collision/reaction cell technologies. Polyatomic interferences (such as 40Ar16O+ interfering with 56Fe+) and isobaric overlaps remain particularly challenging for certain element combinations. While high-resolution instruments can separate many interferences, this approach sacrifices sensitivity and increases cost substantially, creating a precision-sensitivity tradeoff that limits practical applications.
Instrument drift represents a persistent challenge, with signal intensity typically changing by 1-5% per hour during routine operation. This necessitates frequent recalibration and internal standardization, which introduces additional variables and potential error sources. Thermal instability in the sample introduction system and gradual changes in plasma conditions contribute significantly to this drift phenomenon.
Detector limitations further constrain precision, particularly when measuring elements across wide concentration ranges. Pulse-counting detectors offer excellent precision at low concentrations but suffer from dead time corrections at higher counts, while analog detection modes introduce additional noise. The crossover region between these detection modes often exhibits degraded precision, with relative standard deviations increasing by 2-3 times compared to single-mode measurements.
Fundamental counting statistics impose theoretical limits on achievable precision, particularly at ultra-trace levels. For elements present at sub-ppb concentrations, even perfect instrumental conditions cannot overcome the statistical limitations of ion counting, resulting in practical precision limits of 3-5% for many real-world applications. This statistical barrier represents a fundamental challenge that technological improvements alone cannot fully address.
Current Methodologies for Enhancing ICP-MS Precision
01 Calibration methods for improving ICP-MS precision
Various calibration techniques are employed to enhance the precision of ICP-MS measurements. These include internal standardization, standard addition methods, and matrix-matched calibration. These approaches compensate for matrix effects, instrument drift, and signal fluctuations that can affect measurement accuracy. Advanced calibration algorithms and reference materials are used to establish reliable calibration curves, ensuring consistent and precise analytical results across different sample types.- Calibration methods for improving ICP-MS precision: Various calibration techniques are employed to enhance the precision of ICP-MS measurements. These include internal standardization, isotope dilution, and matrix-matched calibration approaches. By implementing proper calibration protocols, systematic errors can be minimized, leading to more accurate and precise elemental analysis. Advanced calibration methods can compensate for matrix effects and instrument drift, which are common sources of measurement variability in ICP-MS.
- Sample preparation techniques for enhanced measurement precision: Proper sample preparation is crucial for achieving high precision in ICP-MS analysis. This includes digestion methods, dilution protocols, and matrix modification techniques that minimize interferences. Standardized sample preparation procedures help reduce contamination and ensure homogeneity, leading to more consistent and precise measurements. Techniques such as microwave digestion and clean room processing can significantly improve the quality of analytical results.
- Interference reduction strategies in ICP-MS: Various approaches are employed to reduce spectral and non-spectral interferences in ICP-MS, thereby improving measurement precision. These include collision/reaction cell technology, high-resolution mass spectrometry, and mathematical correction models. By effectively managing interferences from polyatomic species, isobars, and matrix effects, the signal-to-noise ratio can be optimized, resulting in more precise quantification of trace elements across diverse sample types.
- Instrumental optimization for precision enhancement: Optimizing instrumental parameters is essential for maximizing ICP-MS measurement precision. This includes fine-tuning plasma conditions, ion optics, detector settings, and sample introduction systems. Advanced hardware configurations such as specialized nebulizers, spray chambers, and torch designs can significantly improve stability and sensitivity. Regular maintenance protocols and performance checks ensure consistent instrument operation, contributing to reproducible and precise analytical results.
- Statistical methods and quality control for precision assessment: Statistical approaches and quality control procedures are implemented to evaluate and improve ICP-MS measurement precision. These include uncertainty estimation, replicate analysis, control charts, and proficiency testing. By applying robust statistical tools to assess measurement variability and identify sources of error, analysts can establish confidence intervals and validate analytical methods. Continuous monitoring through quality control samples helps maintain high precision levels during routine analysis.
02 Sample preparation techniques for enhanced precision
Proper sample preparation is crucial for achieving high precision in ICP-MS analysis. This includes digestion methods, dilution protocols, and matrix modification techniques that minimize interferences and contamination. Specialized preparation procedures for different sample types (biological, environmental, industrial) help maintain measurement consistency. Techniques such as microwave digestion, acid dissolution, and clean room protocols significantly improve measurement precision by reducing sample heterogeneity and background contamination.Expand Specific Solutions03 Interference reduction technologies
Various technologies are implemented to reduce spectral and non-spectral interferences in ICP-MS, thereby improving measurement precision. These include collision/reaction cell technologies, high-resolution mass analyzers, and mathematical correction models. By effectively eliminating or compensating for polyatomic interferences, isobaric overlaps, and matrix effects, these technologies enable more precise quantification of trace elements, especially in complex sample matrices.Expand Specific Solutions04 Instrument optimization and stability control
Achieving high precision in ICP-MS measurements requires careful optimization of instrument parameters and maintaining system stability. This involves tuning of plasma conditions, ion optics, detector settings, and sample introduction systems. Automated monitoring and feedback systems help control instrument drift and fluctuations. Temperature stabilization, vacuum system management, and regular performance checks ensure consistent analytical performance over time, significantly improving measurement precision.Expand Specific Solutions05 Data processing and statistical methods
Advanced data processing and statistical methods play a crucial role in enhancing ICP-MS measurement precision. These include signal integration techniques, outlier detection algorithms, and uncertainty estimation models. Statistical approaches such as weighted regression, robust statistics, and multivariate analysis help in extracting reliable quantitative information from raw measurement data. Software solutions that incorporate these methods enable automated quality control and improved precision reporting for complex analytical scenarios.Expand Specific Solutions
Leading Manufacturers and Research Institutions in ICP-MS
The ICP-MS measurement precision landscape is currently in a mature growth phase, with the global market valued at approximately $4.5 billion and projected to expand at a CAGR of 7-8% through 2028. Leading the technological innovation are established analytical instrumentation companies including Thermo Fisher Scientific, Agilent Technologies, and Shimadzu Corporation, who have developed high-precision systems with detection limits in the parts-per-trillion range. Academic institutions like University of Tokyo and EPFL are advancing fundamental research, while specialized firms such as Kimia Analytics and Cerno Bioscience are developing complementary software and components to enhance measurement accuracy. The competitive environment is characterized by continuous improvements in interference reduction, calibration methodologies, and sample introduction systems, with increasing focus on automation and AI-driven data processing to minimize human error factors.
Thermo Fisher Scientific (Bremen) GmbH
Technical Solution: Thermo Fisher Scientific has developed advanced ICP-MS systems featuring their patented Triple Quadrupole (TQ-ICP-MS) technology that significantly improves measurement precision. Their iCAP TQ ICP-MS platform incorporates a collision/reaction cell system with three quadrupoles that effectively removes polyatomic and isobaric interferences[1]. The company has implemented advanced plasma temperature control mechanisms that maintain stable plasma conditions, reducing matrix effects and improving long-term stability. Their systems utilize intelligent autodilution technology that automatically adjusts sample concentrations to optimal measurement ranges, minimizing detector saturation issues[2]. Thermo Fisher has also developed specialized software algorithms for real-time drift correction and internal standardization that compensate for signal fluctuations during analysis. Their instruments feature high-precision sample introduction systems with low dead volumes and optimized nebulizer designs that reduce sample-to-sample memory effects and improve washout characteristics[3].
Strengths: Superior interference removal capabilities through triple quadrupole technology, excellent sensitivity and detection limits, comprehensive software for data quality control, and robust plasma stability. Weaknesses: Higher acquisition and operational costs compared to competitors, more complex operation requiring advanced user training, and larger physical footprint requiring more laboratory space.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has pioneered several innovations in ICP-MS precision measurement with their 8900 Triple Quadrupole ICP-MS system. Their approach focuses on controlling key influencing factors through integrated hardware and software solutions. Agilent's patented High Matrix Introduction (HMI) technology dilutes the plasma with argon, allowing analysis of samples with up to 3% total dissolved solids without manual dilution[1]. Their Ultra High Matrix Introduction (UHMI) system extends this capability to 25% TDS, significantly reducing matrix effects. The company's Octopole Reaction System (ORS) provides effective interference removal using helium collision mode and reactive gases for problematic elements[2]. Agilent has developed advanced temperature-controlled spray chambers that minimize drift by maintaining consistent sample introduction conditions. Their instruments incorporate real-time monitoring of plasma conditions with automated correction algorithms that adjust RF power and gas flows to maintain optimal ionization efficiency[3]. Additionally, Agilent's ICP-MS MassHunter software includes comprehensive quality control tools for monitoring and improving measurement precision.
Strengths: Exceptional matrix tolerance through HMI/UHMI technology, excellent interference management via the ORS system, intuitive software interface with comprehensive QC tools, and robust long-term stability. Weaknesses: Higher gas consumption compared to some competitors, more complex maintenance requirements for the collision/reaction cell, and premium pricing that may be prohibitive for smaller laboratories.
Critical Innovations in Interference Reduction Technologies
Inductively coupled plasma mass spectroscopy apparatus and measured data processing method in the inductively coupled plasma mass spectroscopy apparatus
PatentActiveUS8530829B2
Innovation
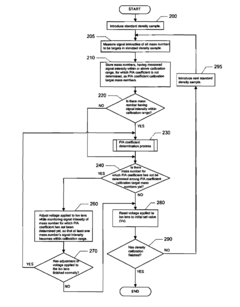
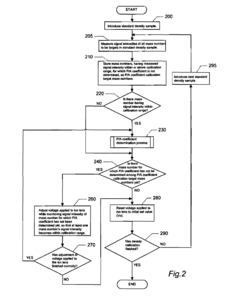
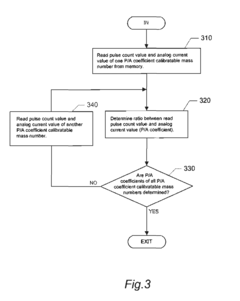
- An ICP-MS apparatus and method that uses standard density samples to generate a working curve and adjusts ion lens voltage to ensure signal intensities fall within a calibration range, combined with a P/A coefficient estimation method that correlates P/A coefficients from different measurements to accurately determine coefficients for mass numbers outside the initial calibration range.
Determination Method of Trace Impurity Elements Using Inductively Coupled Plasma Mass Spectrometer
PatentInactiveJP4512605B2
Innovation
- A method involving stable isotope dilution mass spectrometry is developed, utilizing a general-purpose quadrupole ICP-MS to quantify trace elements by adding enriched stable isotopes, establishing isotope equilibrium, and correcting for recovery rates and contamination through a series of signal intensity measurements and calibration curves, without the need for precise isotope ratio measurements.
Regulatory Standards for Analytical Instrumentation
Regulatory standards for analytical instrumentation in the field of ICP-MS (Inductively Coupled Plasma Mass Spectrometry) are governed by several international bodies that establish guidelines to ensure measurement precision and reliability. The United States Food and Drug Administration (FDA) has implemented specific requirements for analytical methods used in pharmaceutical analysis, including ICP-MS, through its guidance documents on method validation. These guidelines emphasize the importance of precision, accuracy, specificity, and robustness in analytical measurements.
The International Organization for Standardization (ISO) has developed ISO 17025, which provides general requirements for the competence of testing and calibration laboratories. This standard is particularly relevant for laboratories utilizing ICP-MS technology, as it establishes protocols for quality management systems and technical requirements that directly impact measurement precision. Laboratories must demonstrate their ability to consistently produce valid results with stated uncertainties.
European Medicines Agency (EMA) has established guidelines on bioanalytical method validation that include specific considerations for elemental analysis using ICP-MS. These guidelines address critical factors affecting measurement precision, such as matrix effects, spectral interferences, and calibration procedures. Compliance with these standards is mandatory for laboratories conducting analyses for regulatory submissions in Europe.
The United States Environmental Protection Agency (EPA) has developed Method 6020 specifically for ICP-MS analysis of environmental samples. This method outlines detailed procedures for sample preparation, instrument calibration, and quality control measures designed to minimize factors that negatively impact measurement precision. The method specifies acceptable limits for relative standard deviation (RSD) in replicate measurements.
In Japan, the Ministry of Health, Labour and Welfare (MHLW) has established guidelines for elemental impurity testing in pharmaceutical products that incorporate ICP-MS methodologies. These guidelines align with the International Council for Harmonisation (ICH) Q3D Elemental Impurities guideline, which specifies permitted daily exposure limits for various elements and appropriate analytical procedures.
Regulatory bodies also mandate regular performance verification of ICP-MS instruments through system suitability tests. These tests evaluate key performance parameters such as sensitivity, resolution, precision, and accuracy. Documentation of these verification procedures is essential for regulatory compliance and serves as evidence of the instrument's capability to deliver precise measurements.
The International Organization for Standardization (ISO) has developed ISO 17025, which provides general requirements for the competence of testing and calibration laboratories. This standard is particularly relevant for laboratories utilizing ICP-MS technology, as it establishes protocols for quality management systems and technical requirements that directly impact measurement precision. Laboratories must demonstrate their ability to consistently produce valid results with stated uncertainties.
European Medicines Agency (EMA) has established guidelines on bioanalytical method validation that include specific considerations for elemental analysis using ICP-MS. These guidelines address critical factors affecting measurement precision, such as matrix effects, spectral interferences, and calibration procedures. Compliance with these standards is mandatory for laboratories conducting analyses for regulatory submissions in Europe.
The United States Environmental Protection Agency (EPA) has developed Method 6020 specifically for ICP-MS analysis of environmental samples. This method outlines detailed procedures for sample preparation, instrument calibration, and quality control measures designed to minimize factors that negatively impact measurement precision. The method specifies acceptable limits for relative standard deviation (RSD) in replicate measurements.
In Japan, the Ministry of Health, Labour and Welfare (MHLW) has established guidelines for elemental impurity testing in pharmaceutical products that incorporate ICP-MS methodologies. These guidelines align with the International Council for Harmonisation (ICH) Q3D Elemental Impurities guideline, which specifies permitted daily exposure limits for various elements and appropriate analytical procedures.
Regulatory bodies also mandate regular performance verification of ICP-MS instruments through system suitability tests. These tests evaluate key performance parameters such as sensitivity, resolution, precision, and accuracy. Documentation of these verification procedures is essential for regulatory compliance and serves as evidence of the instrument's capability to deliver precise measurements.
Environmental Impact of ICP-MS Sample Preparation Methods
The environmental impact of ICP-MS sample preparation methods represents a significant concern in analytical chemistry laboratories worldwide. Traditional sample preparation techniques often involve hazardous chemicals, including strong acids like nitric, hydrochloric, and hydrofluoric acids, which pose substantial environmental risks when improperly disposed. These chemicals can contaminate water systems, damage soil quality, and negatively impact aquatic ecosystems when released into the environment.
Acid digestion procedures, commonly employed for solid sample preparation, generate acidic waste streams that require specialized neutralization and disposal protocols. The environmental footprint extends beyond chemical waste to include high energy consumption during microwave-assisted digestion and other thermal decomposition methods, contributing to increased carbon emissions and resource depletion.
Recent advancements in green analytical chemistry have led to the development of more environmentally friendly sample preparation alternatives. These include the use of diluted acids, smaller sample volumes (microsampling), and non-traditional solvents derived from renewable resources. Such approaches significantly reduce hazardous waste generation while maintaining measurement precision essential for ICP-MS analysis.
Solid phase extraction (SPE) techniques have emerged as environmentally superior alternatives to traditional liquid-liquid extraction methods. SPE reduces solvent consumption by up to 90% while simultaneously enhancing analyte concentration factors, thereby improving detection limits without increasing environmental burden. Similarly, ultrasound-assisted extraction methods offer reduced extraction times and lower solvent requirements compared to conventional techniques.
Laboratory waste management practices directly influence the environmental impact of ICP-MS operations. Implementing proper segregation, neutralization, and disposal protocols for acid wastes can substantially mitigate environmental harm. Additionally, recycling and recovery systems for certain reagents, particularly expensive or highly toxic substances, present both economic and environmental benefits.
The environmental consequences of sample preparation extend to measurement precision in ICP-MS. Contamination from environmental sources during extensive sample handling can introduce variability and compromise analytical results. Therefore, streamlined "green" preparation methods not only reduce environmental impact but often enhance measurement precision by minimizing contamination opportunities and sample manipulation steps.
Regulatory frameworks increasingly emphasize environmental considerations in laboratory operations, with many jurisdictions implementing stricter controls on chemical waste disposal. Laboratories adopting environmentally responsible sample preparation methods gain advantages in regulatory compliance while contributing to broader sustainability goals in scientific research and analytical testing.
Acid digestion procedures, commonly employed for solid sample preparation, generate acidic waste streams that require specialized neutralization and disposal protocols. The environmental footprint extends beyond chemical waste to include high energy consumption during microwave-assisted digestion and other thermal decomposition methods, contributing to increased carbon emissions and resource depletion.
Recent advancements in green analytical chemistry have led to the development of more environmentally friendly sample preparation alternatives. These include the use of diluted acids, smaller sample volumes (microsampling), and non-traditional solvents derived from renewable resources. Such approaches significantly reduce hazardous waste generation while maintaining measurement precision essential for ICP-MS analysis.
Solid phase extraction (SPE) techniques have emerged as environmentally superior alternatives to traditional liquid-liquid extraction methods. SPE reduces solvent consumption by up to 90% while simultaneously enhancing analyte concentration factors, thereby improving detection limits without increasing environmental burden. Similarly, ultrasound-assisted extraction methods offer reduced extraction times and lower solvent requirements compared to conventional techniques.
Laboratory waste management practices directly influence the environmental impact of ICP-MS operations. Implementing proper segregation, neutralization, and disposal protocols for acid wastes can substantially mitigate environmental harm. Additionally, recycling and recovery systems for certain reagents, particularly expensive or highly toxic substances, present both economic and environmental benefits.
The environmental consequences of sample preparation extend to measurement precision in ICP-MS. Contamination from environmental sources during extensive sample handling can introduce variability and compromise analytical results. Therefore, streamlined "green" preparation methods not only reduce environmental impact but often enhance measurement precision by minimizing contamination opportunities and sample manipulation steps.
Regulatory frameworks increasingly emphasize environmental considerations in laboratory operations, with many jurisdictions implementing stricter controls on chemical waste disposal. Laboratories adopting environmentally responsible sample preparation methods gain advantages in regulatory compliance while contributing to broader sustainability goals in scientific research and analytical testing.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!