Practical Steps to Optimize ICP-MS Throughput Without Compromising Quality
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ICP-MS Technology Evolution and Optimization Goals
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has evolved significantly since its commercial introduction in the 1980s. This analytical technique combines the high-temperature ICP source with a mass spectrometer to detect metals and several non-metals at concentrations as low as one part per trillion. The evolution of ICP-MS technology has been driven by the increasing demands for higher sensitivity, lower detection limits, and improved interference management.
Early ICP-MS systems were primarily focused on achieving basic analytical capabilities with limited sample throughput. The 1990s saw significant improvements in ion optics, interface design, and vacuum systems, which enhanced instrument stability and sensitivity. By the early 2000s, collision/reaction cell technology emerged as a breakthrough innovation, effectively addressing polyatomic interferences that had previously limited the technique's application range.
The past decade has witnessed remarkable advancements in ICP-MS hardware and software integration. Modern systems incorporate sophisticated auto-samplers, intelligent dilution systems, and automated quality control protocols. These developments have substantially improved sample throughput capabilities while maintaining analytical precision and accuracy.
Current technological trends in ICP-MS focus on enhancing throughput without sacrificing data quality. This includes the development of rapid multi-element analysis protocols, faster washout systems, and intelligent scheduling algorithms that optimize sample sequencing. Additionally, manufacturers are implementing advanced collision/reaction cell technologies that allow for faster analysis while maintaining effective interference removal.
The optimization goals for modern ICP-MS applications center around several key parameters: maximizing sample throughput, minimizing carryover between samples, reducing argon and other consumable usage, extending calibration stability, and ensuring consistent data quality across large sample batches. These goals must be balanced against practical laboratory constraints including staffing levels, regulatory requirements, and cost considerations.
Looking forward, the ICP-MS technology roadmap aims to further enhance throughput capabilities through innovations in sample introduction systems, plasma generation efficiency, and detector technology. Machine learning algorithms are increasingly being integrated to predict maintenance needs, optimize method parameters, and identify potential quality issues before they impact results.
The ultimate objective for ICP-MS optimization is to achieve the highest possible sample throughput while maintaining or improving analytical performance metrics such as detection limits, precision, and accuracy. This requires a holistic approach that considers all aspects of the analytical workflow, from sample preparation to data processing and reporting.
Early ICP-MS systems were primarily focused on achieving basic analytical capabilities with limited sample throughput. The 1990s saw significant improvements in ion optics, interface design, and vacuum systems, which enhanced instrument stability and sensitivity. By the early 2000s, collision/reaction cell technology emerged as a breakthrough innovation, effectively addressing polyatomic interferences that had previously limited the technique's application range.
The past decade has witnessed remarkable advancements in ICP-MS hardware and software integration. Modern systems incorporate sophisticated auto-samplers, intelligent dilution systems, and automated quality control protocols. These developments have substantially improved sample throughput capabilities while maintaining analytical precision and accuracy.
Current technological trends in ICP-MS focus on enhancing throughput without sacrificing data quality. This includes the development of rapid multi-element analysis protocols, faster washout systems, and intelligent scheduling algorithms that optimize sample sequencing. Additionally, manufacturers are implementing advanced collision/reaction cell technologies that allow for faster analysis while maintaining effective interference removal.
The optimization goals for modern ICP-MS applications center around several key parameters: maximizing sample throughput, minimizing carryover between samples, reducing argon and other consumable usage, extending calibration stability, and ensuring consistent data quality across large sample batches. These goals must be balanced against practical laboratory constraints including staffing levels, regulatory requirements, and cost considerations.
Looking forward, the ICP-MS technology roadmap aims to further enhance throughput capabilities through innovations in sample introduction systems, plasma generation efficiency, and detector technology. Machine learning algorithms are increasingly being integrated to predict maintenance needs, optimize method parameters, and identify potential quality issues before they impact results.
The ultimate objective for ICP-MS optimization is to achieve the highest possible sample throughput while maintaining or improving analytical performance metrics such as detection limits, precision, and accuracy. This requires a holistic approach that considers all aspects of the analytical workflow, from sample preparation to data processing and reporting.
Market Demand for High-Throughput Analytical Methods
The analytical testing market has witnessed substantial growth in recent years, with the global market value reaching $83.1 billion in 2022 and projected to exceed $111.8 billion by 2027, growing at a CAGR of 6.1%. Within this expanding landscape, ICP-MS (Inductively Coupled Plasma Mass Spectrometry) has emerged as a critical technology for elemental analysis across multiple industries, driving significant demand for higher throughput capabilities.
Environmental monitoring represents one of the largest market segments demanding high-throughput ICP-MS solutions. Regulatory bodies worldwide have implemented increasingly stringent requirements for monitoring heavy metals and trace elements in water, soil, and air samples. The EPA, EU Water Framework Directive, and similar global regulations have expanded the list of elements requiring routine monitoring, creating pressure on laboratories to process more samples while maintaining detection limits in the parts-per-trillion range.
The pharmaceutical and biomedical sectors demonstrate particularly strong growth in demand for high-throughput analytical methods. With the rise of personalized medicine and biologics, manufacturers require more comprehensive elemental impurity testing as mandated by USP <232>, <233>, and ICH Q3D guidelines. Market research indicates that pharmaceutical companies are willing to invest 15-20% more in analytical technologies that can reduce sample processing time by at least 30% without compromising accuracy.
Food safety testing represents another significant market driver, with global food recalls due to contamination increasing by 22% between 2019 and 2022. Regulatory frameworks such as the FDA's Food Safety Modernization Act and the EU's General Food Law have intensified requirements for routine testing, creating bottlenecks in traditional analytical workflows. Industry surveys indicate that food testing laboratories seek solutions that can increase sample throughput by 40-50% to meet current demand.
The semiconductor and electronics manufacturing industries face unprecedented pressure for higher throughput elemental analysis as chip production scales to meet global demand. With feature sizes continuing to shrink, contamination control becomes increasingly critical, requiring more frequent and comprehensive testing. Market analysis shows that semiconductor manufacturers prioritize analytical methods that can deliver results within hours rather than days, with 78% of industry respondents citing testing turnaround time as a critical factor in supplier selection.
Contract research organizations (CROs) and environmental testing laboratories report average backlogs of 2-3 weeks for routine elemental analysis, indicating significant unmet demand for higher throughput solutions. Laboratory managers consistently identify instrument throughput as among their top three considerations when making capital equipment purchases, alongside sensitivity and reliability.
Environmental monitoring represents one of the largest market segments demanding high-throughput ICP-MS solutions. Regulatory bodies worldwide have implemented increasingly stringent requirements for monitoring heavy metals and trace elements in water, soil, and air samples. The EPA, EU Water Framework Directive, and similar global regulations have expanded the list of elements requiring routine monitoring, creating pressure on laboratories to process more samples while maintaining detection limits in the parts-per-trillion range.
The pharmaceutical and biomedical sectors demonstrate particularly strong growth in demand for high-throughput analytical methods. With the rise of personalized medicine and biologics, manufacturers require more comprehensive elemental impurity testing as mandated by USP <232>, <233>, and ICH Q3D guidelines. Market research indicates that pharmaceutical companies are willing to invest 15-20% more in analytical technologies that can reduce sample processing time by at least 30% without compromising accuracy.
Food safety testing represents another significant market driver, with global food recalls due to contamination increasing by 22% between 2019 and 2022. Regulatory frameworks such as the FDA's Food Safety Modernization Act and the EU's General Food Law have intensified requirements for routine testing, creating bottlenecks in traditional analytical workflows. Industry surveys indicate that food testing laboratories seek solutions that can increase sample throughput by 40-50% to meet current demand.
The semiconductor and electronics manufacturing industries face unprecedented pressure for higher throughput elemental analysis as chip production scales to meet global demand. With feature sizes continuing to shrink, contamination control becomes increasingly critical, requiring more frequent and comprehensive testing. Market analysis shows that semiconductor manufacturers prioritize analytical methods that can deliver results within hours rather than days, with 78% of industry respondents citing testing turnaround time as a critical factor in supplier selection.
Contract research organizations (CROs) and environmental testing laboratories report average backlogs of 2-3 weeks for routine elemental analysis, indicating significant unmet demand for higher throughput solutions. Laboratory managers consistently identify instrument throughput as among their top three considerations when making capital equipment purchases, alongside sensitivity and reliability.
Current ICP-MS Throughput Limitations and Challenges
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) faces several significant throughput limitations that impact laboratory efficiency and productivity. The most prominent challenge is the time-consuming sample preparation process, which typically involves digestion, dilution, and addition of internal standards. This preparatory phase can constitute up to 70% of the total analysis time, creating a substantial bottleneck in high-volume testing environments.
Instrument calibration and quality control procedures further constrain throughput capabilities. Standard ICP-MS protocols require frequent calibration checks and quality control samples, often consuming 15-20% of available instrument time. While essential for maintaining analytical integrity, these procedures significantly reduce the number of samples that can be processed within a given timeframe.
Sample introduction systems present another critical limitation. Conventional nebulizers and spray chambers have inherent inefficiencies, with typical sample transport efficiencies of only 1-3%. This inefficiency necessitates longer sample introduction times and contributes to increased washout periods between samples to prevent cross-contamination, particularly when analyzing samples with varying concentration levels.
Data acquisition and processing constraints also impact overall throughput. Multi-element analysis requires sequential or quasi-simultaneous measurement of different masses, extending analysis time per sample. Additionally, complex matrices often require longer dwell times at specific masses to achieve adequate detection limits, further reducing sample throughput.
Instrument maintenance requirements constitute a significant operational challenge. Regular cleaning of sampling cones, ion lenses, and other components is necessary to maintain optimal performance, resulting in substantial downtime. Cone orifices frequently become clogged when analyzing samples with high dissolved solid content, necessitating more frequent maintenance interventions.
Memory effects represent another persistent challenge, particularly when analyzing elements like mercury, boron, and iodine. These elements tend to adhere to sample introduction components, requiring extended washout times between samples to prevent carryover, which directly impacts throughput capacity.
Laboratory workflow inefficiencies further compound these technical limitations. Many facilities still rely on manual sample handling and data transfer processes, introducing delays and potential errors. The integration of ICP-MS systems with laboratory information management systems (LIMS) often remains suboptimal, creating additional workflow bottlenecks.
Instrument calibration and quality control procedures further constrain throughput capabilities. Standard ICP-MS protocols require frequent calibration checks and quality control samples, often consuming 15-20% of available instrument time. While essential for maintaining analytical integrity, these procedures significantly reduce the number of samples that can be processed within a given timeframe.
Sample introduction systems present another critical limitation. Conventional nebulizers and spray chambers have inherent inefficiencies, with typical sample transport efficiencies of only 1-3%. This inefficiency necessitates longer sample introduction times and contributes to increased washout periods between samples to prevent cross-contamination, particularly when analyzing samples with varying concentration levels.
Data acquisition and processing constraints also impact overall throughput. Multi-element analysis requires sequential or quasi-simultaneous measurement of different masses, extending analysis time per sample. Additionally, complex matrices often require longer dwell times at specific masses to achieve adequate detection limits, further reducing sample throughput.
Instrument maintenance requirements constitute a significant operational challenge. Regular cleaning of sampling cones, ion lenses, and other components is necessary to maintain optimal performance, resulting in substantial downtime. Cone orifices frequently become clogged when analyzing samples with high dissolved solid content, necessitating more frequent maintenance interventions.
Memory effects represent another persistent challenge, particularly when analyzing elements like mercury, boron, and iodine. These elements tend to adhere to sample introduction components, requiring extended washout times between samples to prevent carryover, which directly impacts throughput capacity.
Laboratory workflow inefficiencies further compound these technical limitations. Many facilities still rely on manual sample handling and data transfer processes, introducing delays and potential errors. The integration of ICP-MS systems with laboratory information management systems (LIMS) often remains suboptimal, creating additional workflow bottlenecks.
Current Throughput Optimization Strategies and Methodologies
01 Automated sample introduction systems
Automated sample introduction systems can significantly increase ICP-MS throughput by reducing manual handling and enabling continuous operation. These systems include autosampling devices, automated dilution systems, and robotic sample preparation platforms that can prepare and introduce samples in sequence. By minimizing human intervention and standardizing sample preparation, these systems not only increase throughput but also improve reproducibility and reduce the risk of contamination.- Automated sample introduction systems: Automated sample introduction systems enhance ICP-MS throughput by streamlining the sample preparation and introduction process. These systems include autosampling devices, automated dilution systems, and integrated sample preparation platforms that reduce manual handling and increase the number of samples that can be processed in a given time period. The automation reduces operator intervention, minimizes contamination risks, and ensures consistent sample delivery to the plasma source.
- Multi-element analysis optimization: Techniques for optimizing multi-element analysis in ICP-MS significantly improve throughput by enabling simultaneous detection of multiple elements in a single analytical run. These innovations include advanced detector systems, optimized mass spectrometer configurations, and specialized software algorithms that can process complex spectral data efficiently. By analyzing multiple elements concurrently rather than sequentially, analysis time per sample is substantially reduced while maintaining analytical precision.
- High-speed data acquisition and processing: High-speed data acquisition and processing systems enhance ICP-MS throughput by reducing the time required for signal collection and analysis. These systems incorporate advanced electronics, parallel processing capabilities, and specialized algorithms for rapid spectral interpretation. Real-time data processing allows for immediate quality control checks and reduces the overall analysis cycle time, enabling higher sample throughput without compromising analytical quality.
- Plasma source and interface optimization: Innovations in plasma source design and sample-plasma interface optimization improve ICP-MS throughput by enhancing ionization efficiency and ion transmission. These developments include modified torch designs, improved ion optics, and optimized interface configurations that increase sensitivity while reducing matrix effects. By improving the efficiency of sample ionization and ion extraction, these innovations allow for shorter acquisition times and faster sample-to-sample transitions.
- Integrated sample preparation techniques: Integrated sample preparation techniques enhance ICP-MS throughput by reducing the time and labor required for pre-analysis sample processing. These innovations include online digestion systems, automated extraction techniques, and microfluidic sample preparation platforms that can be directly coupled to the ICP-MS instrument. By minimizing manual sample handling steps and integrating preparation with analysis, these techniques significantly reduce the overall time from sample receipt to result reporting.
02 Multi-element analysis optimization
Techniques for optimizing multi-element analysis in ICP-MS focus on improving the instrument's ability to detect multiple elements simultaneously or in rapid succession. This includes optimized scanning methods, improved ion optics, and specialized software algorithms that can process multi-element data efficiently. By analyzing multiple elements in a single run, these techniques significantly reduce the time required per sample and increase overall throughput while maintaining analytical accuracy.Expand Specific Solutions03 High-throughput sample preparation methods
Advanced sample preparation methods specifically designed for high-throughput ICP-MS analysis include microwave digestion systems, automated extraction techniques, and miniaturized sample preparation platforms. These methods reduce sample preparation time, which is often the bottleneck in ICP-MS workflows. By optimizing digestion parameters, reducing reagent volumes, and parallelizing preparation steps, these approaches can significantly increase the number of samples that can be processed and analyzed in a given time period.Expand Specific Solutions04 Data processing and analysis automation
Advanced data processing and analysis automation systems for ICP-MS include specialized software algorithms for peak identification, calibration, and quantification. These systems can automatically process large datasets, apply correction factors, identify outliers, and generate comprehensive reports. By reducing the time required for data interpretation and reporting, these systems significantly increase the overall throughput of ICP-MS analysis workflows while maintaining data quality and integrity.Expand Specific Solutions05 Integrated high-throughput analytical platforms
Integrated analytical platforms combine ICP-MS with other analytical techniques or sample processing systems to create comprehensive high-throughput solutions. These platforms may incorporate liquid chromatography, automated sample preparation, and multi-detector systems within a single workflow. By eliminating manual transfer steps between different analytical stages and optimizing the integration of various components, these systems maximize sample throughput while providing comprehensive analytical information.Expand Specific Solutions
Leading Manufacturers and Research Institutions in ICP-MS
The ICP-MS throughput optimization market is in a growth phase, driven by increasing demand for high-throughput analytical solutions across pharmaceutical, environmental, and industrial sectors. The market is characterized by established analytical instrumentation leaders like Thermo Fisher Scientific, PerkinElmer (Revvity), and Shimadzu dominating with comprehensive solutions, while specialized players such as Kimia Analytics focus on innovative torch technologies. Technical maturity varies, with major players offering advanced automation, intelligent sample handling, and software integration capabilities. Emerging companies like Suzhou Semiken and Jiangsu Skyray are expanding the competitive landscape with cost-effective alternatives. The market is evolving toward AI-driven optimization, multi-element analysis acceleration, and enhanced sample preparation workflows to balance throughput with analytical quality.
Thermo Fisher Scientific (Bremen) GmbH
Technical Solution: Thermo Fisher Scientific has developed comprehensive ICP-MS throughput optimization solutions centered around their iCAP RQ and iCAP TQ ICP-MS systems. Their approach integrates hardware innovations with advanced software capabilities. The company's Qtegra Intelligent Scientific Data Solution (ISDS) software platform features intelligent dilution protocols that automatically adjust sample concentrations to fall within calibration ranges, eliminating manual re-runs[1]. Their kinetic energy discrimination (KED) technology with helium collision cells effectively removes polyatomic interferences while maintaining sensitivity, reducing the need for complex correction equations[2]. Thermo Fisher has also implemented high-throughput sample introduction systems with rapid rinse capabilities that reduce carryover and minimize washout times between samples. Their PrepFAST autosampling technology can prepare standards and perform intelligent dilutions in real-time, cutting manual preparation time by up to 70%[3]. Additionally, their instruments feature intelligent scheduling algorithms that optimize sequence runs based on analyte groups and required washout protocols.
Strengths: Comprehensive integration of hardware and software solutions provides end-to-end throughput optimization. Advanced interference management maintains data quality while increasing speed. Weaknesses: Higher initial investment costs compared to some competitors. Some advanced features require significant operator training to fully utilize their potential.
PerkinElmer U.S. LLC
Technical Solution: PerkinElmer's approach to ICP-MS throughput optimization centers on their NexION series instruments with patented Universal Cell Technology (UCT). Their solution incorporates a triple-quadrupole design that enables simultaneous multi-element analysis while maintaining high sensitivity and selectivity[1]. The company has developed Syngistix software with built-in Smart Tune automated optimization routines that reduce instrument setup time by up to 75% compared to manual tuning procedures[2]. Their All Matrix Solution (AMS) system employs a specialized sample introduction system that handles high dissolved solid content without dilution, eliminating time-consuming sample preparation steps for complex matrices. PerkinElmer's proprietary LumiCoil RF generator technology provides exceptional plasma stability, reducing the need for recalibration during long analytical runs[3]. Additionally, their SC-FAST autosampling system reduces sample-to-sample analysis time to less than 90 seconds while maintaining washout efficiency. The company has also implemented intelligent QC protocols that automatically trigger corrective actions when quality control samples fall outside specified parameters, eliminating manual intervention during analytical runs.
Strengths: Superior matrix tolerance reduces sample preparation requirements. Automated optimization routines minimize setup time and operator intervention. Weaknesses: Higher gas consumption compared to some competitors. System complexity may require more specialized maintenance support.
Key Innovations in Sample Introduction and Data Processing
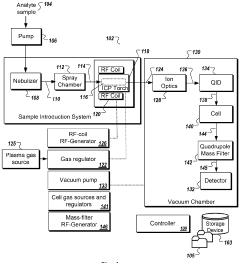
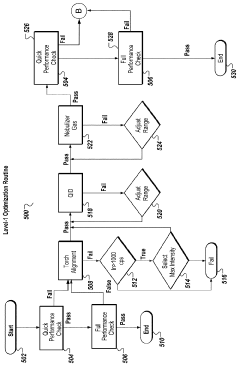
Systems and methods for automated optimization of a multi-mode inductively coupled plasma mass spectrometer
PatentActiveCA2938675C
Innovation
- An automated optimization system for multi-mode ICP-MS that allows for 'single click' operation, using a processor and non-transitory computer-readable medium to execute user inputs for automated tuning routines, including performance assessments and dynamic range optimization, to adjust settings such as torch alignment, quadrupole ion deflector calibration, and nebulizer gas flow, ensuring optimal instrument performance across various modes.
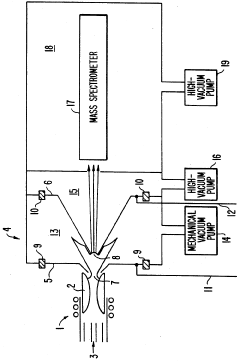
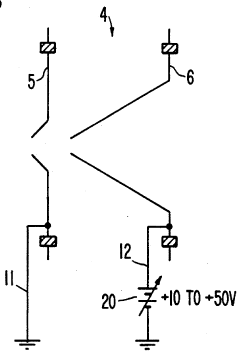
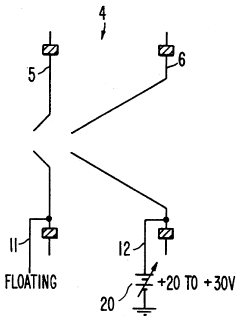
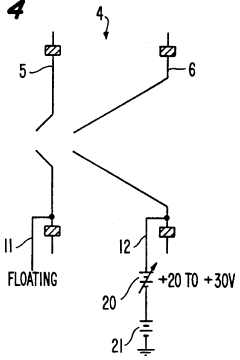
Plasma sampling interface for inductively coupled plasma-mass spectrometry (ICP-MS)
PatentInactiveUS5218204A
Innovation
- A plasma sampling interface with insulating spacers and an adjustable DC bias voltage source applying a DC bias voltage of 10 to 50 V to the skimmer, allowing the sampler to float or grounding it, enhances ion transmission by using a DC offset voltage for mass spectrometers requiring higher initial ion energy.
Quality Control Frameworks for High-Throughput Analysis
Quality control frameworks are essential components for maintaining analytical integrity in high-throughput ICP-MS operations. These frameworks must balance the dual imperatives of maximizing sample processing capacity while ensuring consistent analytical quality. Effective frameworks typically incorporate multi-tiered monitoring systems that operate at different temporal scales—from real-time quality checks to periodic system-wide evaluations.
The foundation of any robust quality control framework begins with standardized operating procedures (SOPs) specifically designed for high-throughput environments. These SOPs must detail calibration frequencies, acceptance criteria for quality control samples, and decision trees for addressing analytical deviations. For ICP-MS specifically, these procedures should account for the unique challenges of mass spectral interferences and instrument drift that can become more pronounced during extended analytical runs.
Statistical process control (SPC) represents another critical element in quality frameworks for ICP-MS. Implementation of control charts for key performance indicators—such as internal standard recovery, calibration stability, and duplicate sample agreement—provides objective metrics for monitoring analytical performance. Modern laboratory information management systems (LIMS) can automate the generation of these control charts, enabling real-time quality assessment without impeding throughput.
Risk-based approaches to quality control have gained prominence in high-throughput environments. This methodology allocates quality control resources proportionally to the analytical risk, with more stringent controls applied to critical samples or those with challenging matrices. For instance, environmental samples with complex matrices might require more frequent quality checks than simpler pharmaceutical preparations, even within the same analytical batch.
Integration of automated quality control samples at predetermined intervals represents a practical strategy for maintaining vigilance without sacrificing efficiency. These automated insertions—typically including method blanks, continuing calibration verifications, and duplicate samples—serve as sentinels that can trigger corrective actions when necessary. The optimal frequency of these insertions should be determined through statistical evaluation of historical performance data rather than arbitrary conventions.
Peer review processes constitute the final layer in comprehensive quality frameworks. Regular independent verification of analytical results by qualified personnel helps identify systematic errors that automated systems might miss. In high-throughput environments, this review can be streamlined through targeted examination of flagged results rather than comprehensive review of all data, thereby preserving efficiency while maintaining quality assurance.
The foundation of any robust quality control framework begins with standardized operating procedures (SOPs) specifically designed for high-throughput environments. These SOPs must detail calibration frequencies, acceptance criteria for quality control samples, and decision trees for addressing analytical deviations. For ICP-MS specifically, these procedures should account for the unique challenges of mass spectral interferences and instrument drift that can become more pronounced during extended analytical runs.
Statistical process control (SPC) represents another critical element in quality frameworks for ICP-MS. Implementation of control charts for key performance indicators—such as internal standard recovery, calibration stability, and duplicate sample agreement—provides objective metrics for monitoring analytical performance. Modern laboratory information management systems (LIMS) can automate the generation of these control charts, enabling real-time quality assessment without impeding throughput.
Risk-based approaches to quality control have gained prominence in high-throughput environments. This methodology allocates quality control resources proportionally to the analytical risk, with more stringent controls applied to critical samples or those with challenging matrices. For instance, environmental samples with complex matrices might require more frequent quality checks than simpler pharmaceutical preparations, even within the same analytical batch.
Integration of automated quality control samples at predetermined intervals represents a practical strategy for maintaining vigilance without sacrificing efficiency. These automated insertions—typically including method blanks, continuing calibration verifications, and duplicate samples—serve as sentinels that can trigger corrective actions when necessary. The optimal frequency of these insertions should be determined through statistical evaluation of historical performance data rather than arbitrary conventions.
Peer review processes constitute the final layer in comprehensive quality frameworks. Regular independent verification of analytical results by qualified personnel helps identify systematic errors that automated systems might miss. In high-throughput environments, this review can be streamlined through targeted examination of flagged results rather than comprehensive review of all data, thereby preserving efficiency while maintaining quality assurance.
Environmental and Regulatory Considerations for ICP-MS Operations
Environmental regulations significantly impact ICP-MS operations across various industries. Laboratories must comply with strict guidelines regarding waste disposal, particularly for samples containing heavy metals and other hazardous elements. The Environmental Protection Agency (EPA) in the United States and similar regulatory bodies worldwide have established specific protocols for handling, storing, and disposing of waste generated during ICP-MS analysis. These regulations often require detailed documentation of waste management practices and may necessitate specialized disposal services.
Air quality considerations also play a crucial role in ICP-MS facility design. Proper ventilation systems must be installed to manage argon gas emissions and potential release of volatile compounds during sample preparation. Many jurisdictions require permits for facilities operating instruments that generate specific types of emissions, with regular monitoring and reporting requirements.
Water consumption represents another environmental concern for high-throughput ICP-MS operations. The cooling systems and sample preparation processes can consume significant volumes of water. Implementing water recycling systems and optimizing rinse cycles can substantially reduce environmental impact while simultaneously improving operational efficiency.
Energy efficiency has become increasingly important in laboratory operations. Modern ICP-MS instruments offer various power-saving features that can be activated during idle periods without compromising analytical readiness. Implementing automated shutdown procedures during extended non-operational periods can further reduce energy consumption.
Regulatory compliance extends to data management practices as well. Many industries require adherence to specific data integrity standards such as GLP (Good Laboratory Practice) or ISO 17025. These standards mandate proper documentation of all analytical procedures, including quality control measures and instrument performance verification.
Safety regulations for laboratory personnel must be integrated into throughput optimization strategies. Proper training on handling hazardous materials, emergency procedures, and instrument operation is not only a regulatory requirement but also enhances operational efficiency by reducing accidents and downtime.
Forward-thinking laboratories are increasingly adopting green chemistry principles in their ICP-MS workflows. This includes minimizing sample and reagent volumes, selecting less hazardous alternatives when possible, and designing analytical methods that generate less waste while maintaining analytical quality.
Air quality considerations also play a crucial role in ICP-MS facility design. Proper ventilation systems must be installed to manage argon gas emissions and potential release of volatile compounds during sample preparation. Many jurisdictions require permits for facilities operating instruments that generate specific types of emissions, with regular monitoring and reporting requirements.
Water consumption represents another environmental concern for high-throughput ICP-MS operations. The cooling systems and sample preparation processes can consume significant volumes of water. Implementing water recycling systems and optimizing rinse cycles can substantially reduce environmental impact while simultaneously improving operational efficiency.
Energy efficiency has become increasingly important in laboratory operations. Modern ICP-MS instruments offer various power-saving features that can be activated during idle periods without compromising analytical readiness. Implementing automated shutdown procedures during extended non-operational periods can further reduce energy consumption.
Regulatory compliance extends to data management practices as well. Many industries require adherence to specific data integrity standards such as GLP (Good Laboratory Practice) or ISO 17025. These standards mandate proper documentation of all analytical procedures, including quality control measures and instrument performance verification.
Safety regulations for laboratory personnel must be integrated into throughput optimization strategies. Proper training on handling hazardous materials, emergency procedures, and instrument operation is not only a regulatory requirement but also enhances operational efficiency by reducing accidents and downtime.
Forward-thinking laboratories are increasingly adopting green chemistry principles in their ICP-MS workflows. This includes minimizing sample and reagent volumes, selecting less hazardous alternatives when possible, and designing analytical methods that generate less waste while maintaining analytical quality.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!