ICP-MS vs ESI-MS: Comparison in Detecting Metal Ions in Biological Samples
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Metal Ion Detection Technology Background and Objectives
Metal ion detection in biological samples has evolved significantly over the past decades, driven by the increasing recognition of metals' crucial roles in biological systems. From essential trace elements like zinc, iron, and copper that regulate cellular functions to toxic heavy metals such as lead, mercury, and cadmium that pose serious health risks, accurate detection and quantification of metal ions have become fundamental to various fields including clinical diagnostics, environmental monitoring, and biomedical research.
The technological journey began with colorimetric assays and atomic absorption spectroscopy in the mid-20th century, which provided limited sensitivity and specificity. The introduction of inductively coupled plasma mass spectrometry (ICP-MS) in the 1980s marked a revolutionary advancement, offering multi-element detection capabilities with significantly improved sensitivity. Electrospray ionization mass spectrometry (ESI-MS), initially developed for organic molecule analysis, has more recently emerged as an alternative approach for metal ion detection, particularly valuable for studying metal-protein interactions.
Current technological trends are moving toward higher sensitivity, improved spatial resolution, and the ability to analyze metal speciation rather than just total metal content. The integration of separation techniques with mass spectrometry has enabled researchers to distinguish between different oxidation states and binding forms of metals in biological matrices, providing crucial information about bioavailability and toxicity.
The primary objective of metal ion detection technology development is to achieve comprehensive characterization of the metallome—the entirety of metal and metalloid species within a biological system. This includes quantitative measurement of concentration, determination of spatial distribution, identification of binding partners, and understanding of dynamic changes in response to physiological or pathological conditions.
Specifically for ICP-MS and ESI-MS comparison, the technological goals include: (1) establishing standardized protocols for sample preparation that preserve native metal-biomolecule interactions; (2) developing methods that combine the quantitative strengths of ICP-MS with the structural information provided by ESI-MS; (3) improving detection limits to reach physiologically relevant concentrations of trace metals; and (4) enabling high-throughput analysis for clinical applications.
The ultimate aim is to create analytical platforms that can provide a holistic view of metal homeostasis in biological systems, contributing to our understanding of metal-related diseases, improving diagnostic capabilities, and supporting the development of metal-targeted therapeutics. As these technologies continue to advance, they promise to reveal previously unknown aspects of metal biology and their implications for human health and disease.
The technological journey began with colorimetric assays and atomic absorption spectroscopy in the mid-20th century, which provided limited sensitivity and specificity. The introduction of inductively coupled plasma mass spectrometry (ICP-MS) in the 1980s marked a revolutionary advancement, offering multi-element detection capabilities with significantly improved sensitivity. Electrospray ionization mass spectrometry (ESI-MS), initially developed for organic molecule analysis, has more recently emerged as an alternative approach for metal ion detection, particularly valuable for studying metal-protein interactions.
Current technological trends are moving toward higher sensitivity, improved spatial resolution, and the ability to analyze metal speciation rather than just total metal content. The integration of separation techniques with mass spectrometry has enabled researchers to distinguish between different oxidation states and binding forms of metals in biological matrices, providing crucial information about bioavailability and toxicity.
The primary objective of metal ion detection technology development is to achieve comprehensive characterization of the metallome—the entirety of metal and metalloid species within a biological system. This includes quantitative measurement of concentration, determination of spatial distribution, identification of binding partners, and understanding of dynamic changes in response to physiological or pathological conditions.
Specifically for ICP-MS and ESI-MS comparison, the technological goals include: (1) establishing standardized protocols for sample preparation that preserve native metal-biomolecule interactions; (2) developing methods that combine the quantitative strengths of ICP-MS with the structural information provided by ESI-MS; (3) improving detection limits to reach physiologically relevant concentrations of trace metals; and (4) enabling high-throughput analysis for clinical applications.
The ultimate aim is to create analytical platforms that can provide a holistic view of metal homeostasis in biological systems, contributing to our understanding of metal-related diseases, improving diagnostic capabilities, and supporting the development of metal-targeted therapeutics. As these technologies continue to advance, they promise to reveal previously unknown aspects of metal biology and their implications for human health and disease.
Market Demand Analysis for Biological Sample Metal Analysis
The global market for metal ion detection in biological samples has been experiencing robust growth, driven by increasing applications in clinical diagnostics, pharmaceutical research, environmental monitoring, and food safety. The market size for analytical instruments used in biological metal analysis was valued at approximately $4.2 billion in 2022 and is projected to reach $6.8 billion by 2028, representing a compound annual growth rate of 8.3%.
Healthcare and clinical diagnostics constitute the largest segment of this market, accounting for nearly 42% of the total demand. The growing recognition of metal ions' role in various diseases, including neurodegenerative disorders, cancer, and cardiovascular conditions, has significantly expanded the application of metal analysis techniques in clinical settings. Hospitals and diagnostic laboratories are increasingly adopting advanced analytical methods to detect trace metals in blood, urine, and tissue samples for both diagnostic and therapeutic monitoring purposes.
Pharmaceutical and biotechnology research represents another rapidly growing segment, with a market share of approximately 28%. The need to understand metal-protein interactions, evaluate drug metabolism, and ensure product safety has intensified the demand for high-precision metal detection technologies. This sector particularly values techniques that can provide both qualitative and quantitative analysis with minimal sample preparation.
Environmental and food safety applications collectively account for about 23% of the market. Regulatory bodies worldwide have implemented stricter guidelines regarding metal contamination in food products and environmental samples, driving the adoption of sensitive detection methods. The ability to detect ultra-trace levels of toxic metals like mercury, lead, arsenic, and cadmium in biological matrices has become crucial for compliance and public health protection.
Geographically, North America leads the market with a 38% share, followed by Europe (31%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate of 10.2% during the forecast period, primarily due to increasing healthcare expenditure, expanding research infrastructure, and growing awareness about metal-related health issues in countries like China, India, and Japan.
The demand for more sensitive, accurate, and high-throughput analytical techniques continues to rise. End-users increasingly require methods that can simultaneously detect multiple metal ions, handle complex biological matrices, provide speciation information, and operate with minimal sample volumes. This trend has created a competitive landscape where technologies like ICP-MS and ESI-MS are being continuously refined to meet these evolving market needs.
Healthcare and clinical diagnostics constitute the largest segment of this market, accounting for nearly 42% of the total demand. The growing recognition of metal ions' role in various diseases, including neurodegenerative disorders, cancer, and cardiovascular conditions, has significantly expanded the application of metal analysis techniques in clinical settings. Hospitals and diagnostic laboratories are increasingly adopting advanced analytical methods to detect trace metals in blood, urine, and tissue samples for both diagnostic and therapeutic monitoring purposes.
Pharmaceutical and biotechnology research represents another rapidly growing segment, with a market share of approximately 28%. The need to understand metal-protein interactions, evaluate drug metabolism, and ensure product safety has intensified the demand for high-precision metal detection technologies. This sector particularly values techniques that can provide both qualitative and quantitative analysis with minimal sample preparation.
Environmental and food safety applications collectively account for about 23% of the market. Regulatory bodies worldwide have implemented stricter guidelines regarding metal contamination in food products and environmental samples, driving the adoption of sensitive detection methods. The ability to detect ultra-trace levels of toxic metals like mercury, lead, arsenic, and cadmium in biological matrices has become crucial for compliance and public health protection.
Geographically, North America leads the market with a 38% share, followed by Europe (31%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate of 10.2% during the forecast period, primarily due to increasing healthcare expenditure, expanding research infrastructure, and growing awareness about metal-related health issues in countries like China, India, and Japan.
The demand for more sensitive, accurate, and high-throughput analytical techniques continues to rise. End-users increasingly require methods that can simultaneously detect multiple metal ions, handle complex biological matrices, provide speciation information, and operate with minimal sample volumes. This trend has created a competitive landscape where technologies like ICP-MS and ESI-MS are being continuously refined to meet these evolving market needs.
Current Status and Challenges in Mass Spectrometry Technologies
Mass spectrometry (MS) technologies have evolved significantly over the past decades, establishing themselves as indispensable analytical tools in various scientific fields. Currently, two predominant MS technologies for metal ion detection in biological samples are Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Electrospray Ionization Mass Spectrometry (ESI-MS), each with distinct capabilities and limitations.
ICP-MS has reached maturity as a high-sensitivity technique for elemental analysis, capable of detecting metals at parts-per-trillion levels. Modern ICP-MS systems feature enhanced collision/reaction cells that effectively minimize polyatomic interferences, improving accuracy in complex biological matrices. Recent advancements include single-cell ICP-MS and imaging capabilities, allowing researchers to map metal distributions within tissues and even individual cells with unprecedented spatial resolution.
ESI-MS, while traditionally focused on molecular analysis, has gained traction in metal speciation studies due to its ability to preserve metal-ligand complexes during ionization. Current ESI-MS systems offer high-resolution accurate mass measurements, enabling identification of metal-containing biomolecules with exceptional specificity. The integration of ion mobility spectrometry with ESI-MS has further enhanced separation capabilities for complex biological samples.
Despite these advances, significant challenges persist in both technologies. For ICP-MS, matrix effects from biological samples continue to complicate quantification, requiring extensive sample preparation protocols. The destruction of molecular information during plasma ionization remains an inherent limitation, preventing direct speciation analysis. Additionally, isobaric interferences from biological matrices can mask signals from certain metal isotopes.
ESI-MS faces challenges in ionization efficiency for metal complexes, particularly those with weak coordination bonds that may dissociate during the electrospray process. Quantification remains problematic due to variable ionization efficiencies across different metal species. Furthermore, the complexity of biological samples often necessitates extensive chromatographic separation prior to analysis.
The geographical distribution of these technologies shows concentration in North America, Europe, and East Asia, with limited accessibility in developing regions due to high instrumentation costs and technical expertise requirements. This disparity has created an innovation gap, with most technological advancements originating from established research centers.
Recent hybrid approaches combining the strengths of both technologies, such as LC-ICP-MS and LC-ESI-MS, represent promising developments for comprehensive metal analysis in biological systems. However, standardization of protocols across different platforms remains a significant hurdle for cross-laboratory comparisons and clinical applications.
ICP-MS has reached maturity as a high-sensitivity technique for elemental analysis, capable of detecting metals at parts-per-trillion levels. Modern ICP-MS systems feature enhanced collision/reaction cells that effectively minimize polyatomic interferences, improving accuracy in complex biological matrices. Recent advancements include single-cell ICP-MS and imaging capabilities, allowing researchers to map metal distributions within tissues and even individual cells with unprecedented spatial resolution.
ESI-MS, while traditionally focused on molecular analysis, has gained traction in metal speciation studies due to its ability to preserve metal-ligand complexes during ionization. Current ESI-MS systems offer high-resolution accurate mass measurements, enabling identification of metal-containing biomolecules with exceptional specificity. The integration of ion mobility spectrometry with ESI-MS has further enhanced separation capabilities for complex biological samples.
Despite these advances, significant challenges persist in both technologies. For ICP-MS, matrix effects from biological samples continue to complicate quantification, requiring extensive sample preparation protocols. The destruction of molecular information during plasma ionization remains an inherent limitation, preventing direct speciation analysis. Additionally, isobaric interferences from biological matrices can mask signals from certain metal isotopes.
ESI-MS faces challenges in ionization efficiency for metal complexes, particularly those with weak coordination bonds that may dissociate during the electrospray process. Quantification remains problematic due to variable ionization efficiencies across different metal species. Furthermore, the complexity of biological samples often necessitates extensive chromatographic separation prior to analysis.
The geographical distribution of these technologies shows concentration in North America, Europe, and East Asia, with limited accessibility in developing regions due to high instrumentation costs and technical expertise requirements. This disparity has created an innovation gap, with most technological advancements originating from established research centers.
Recent hybrid approaches combining the strengths of both technologies, such as LC-ICP-MS and LC-ESI-MS, represent promising developments for comprehensive metal analysis in biological systems. However, standardization of protocols across different platforms remains a significant hurdle for cross-laboratory comparisons and clinical applications.
Current Technical Solutions for Metal Ion Detection
01 ICP-MS detection techniques and improvements
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) techniques have been developed with various improvements for enhanced detection capabilities. These include modifications to sample introduction systems, plasma generation, and ion detection methods that allow for lower detection limits and higher sensitivity. Advanced ICP-MS systems can detect trace elements at parts per trillion levels with reduced interference and improved accuracy for complex sample matrices.- ICP-MS detection sensitivity and resolution improvements: Innovations in Inductively Coupled Plasma Mass Spectrometry (ICP-MS) have focused on enhancing detection sensitivity and resolution. These improvements include optimized ion optics, advanced plasma interface designs, and refined sample introduction systems. Such enhancements allow for lower detection limits, better isotope ratio measurements, and reduced interference, making ICP-MS more effective for trace element analysis in complex matrices.
- ESI-MS ionization efficiency and mass accuracy advancements: Electrospray Ionization Mass Spectrometry (ESI-MS) capabilities have been enhanced through improvements in ionization efficiency and mass accuracy. These advancements include modified spray designs, controlled droplet formation techniques, and optimized voltage parameters. Such developments enable better characterization of biomolecules, improved quantification of analytes, and enhanced structural elucidation of complex compounds.
- Hybrid MS systems combining ICP and ESI technologies: Hybrid mass spectrometry systems that integrate both ICP and ESI technologies have been developed to leverage the complementary strengths of each technique. These systems allow for comprehensive analysis of both elemental composition and molecular structure in a single analytical platform. The combination enables enhanced speciation analysis, improved characterization of metalloproteins, and more complete analysis of complex environmental and biological samples.
- Sample preparation and introduction innovations for MS: Novel sample preparation and introduction methods have been developed to enhance the detection capabilities of both ICP-MS and ESI-MS. These innovations include microfluidic sample handling, automated digestion systems, and specialized extraction techniques. Such advancements reduce contamination risks, improve sample throughput, and enhance the recovery of target analytes, ultimately leading to better detection limits and more reliable quantification.
- Data processing and calibration methods for MS detection: Advanced data processing algorithms and calibration methods have been developed to improve the detection capabilities of both ICP-MS and ESI-MS. These computational approaches include machine learning for spectral interpretation, automated interference correction, and advanced statistical methods for quantification. Such techniques enhance the accuracy of isotope ratio measurements, improve detection limits through better signal processing, and enable more reliable identification of compounds in complex matrices.
02 ESI-MS detection methods and sensitivity enhancements
Electrospray Ionization Mass Spectrometry (ESI-MS) detection capabilities have been enhanced through various methodological improvements. These include optimized ionization parameters, interface designs, and mass analyzer configurations that improve the detection of biomolecules, pharmaceuticals, and environmental contaminants. Recent developments focus on increasing sensitivity, reducing matrix effects, and expanding the range of compounds that can be accurately detected and quantified.Expand Specific Solutions03 Combined MS detection systems and hybrid approaches
Hybrid systems combining ICP-MS and ESI-MS technologies or integrating them with other analytical techniques have been developed to expand detection capabilities. These combined approaches leverage the strengths of each technique, allowing for comprehensive analysis of both elemental and molecular species in a single workflow. Such systems provide enhanced versatility for complex analytical challenges requiring both trace element detection and molecular characterization.Expand Specific Solutions04 Sample preparation and introduction innovations
Novel sample preparation and introduction methods have been developed to improve detection capabilities for both ICP-MS and ESI-MS. These innovations include automated sample handling, specialized extraction techniques, and microfluidic devices that enhance sample throughput while minimizing contamination. Advanced sample introduction systems improve ionization efficiency and reduce matrix effects, leading to lower detection limits and more reliable quantification across diverse sample types.Expand Specific Solutions05 Specialized applications and detection of specific compounds
Specialized ICP-MS and ESI-MS methods have been developed for specific applications and compound classes. These include targeted approaches for detecting nanoparticles, pharmaceutical compounds, environmental pollutants, and biological markers. Custom ionization parameters, specialized mass analyzers, and dedicated data processing algorithms enable highly sensitive and selective detection of challenging analytes in complex matrices, expanding the application range of mass spectrometry techniques.Expand Specific Solutions
Key Industry Players in Mass Spectrometry Instrumentation
The ICP-MS vs ESI-MS market for metal ion detection in biological samples is in a growth phase, with increasing adoption across biomedical research and clinical diagnostics. The global market is estimated at approximately $4-5 billion, expanding at 7-8% CAGR. Technologically, ICP-MS has reached maturity with established players like Agilent Technologies and PerkinElmer dominating with comprehensive solutions, while ESI-MS applications are still evolving. Agilent leads with integrated workflows and specialized biological sample preparation technologies, while Hitachi High-Tech and Shimadzu offer competitive alternatives. Academic institutions like Northeastern University and Vanderbilt University are advancing novel applications, particularly in proteomics and metallomics, driving innovation alongside specialized companies like AmberGen that focus on niche biological applications.
Agilent Technologies, Inc.
Technical Solution: Agilent has developed advanced ICP-MS systems like the 7900 and 8900 Triple Quadrupole ICP-MS that offer superior sensitivity for metal ion detection in biological samples. Their ICP-MS technology incorporates helium collision mode to minimize polyatomic interferences, achieving detection limits in the ppt (parts-per-trillion) range for most metals. For biological applications, Agilent has pioneered integrated sample introduction systems that handle high salt/organic matrices while maintaining stability. Their ICP-MS MassHunter software includes specific biological sample workflows with automated quality control protocols. Complementing this, Agilent's 6500 Series ESI-MS systems provide orthogonal analysis capabilities with specialized ionization techniques for metal-protein complexes and metallomics research[1][3].
Strengths: Industry-leading sensitivity (down to sub-ppt levels); robust plasma technology handling complex biological matrices; comprehensive interference management; integrated sample preparation solutions. Weaknesses: Higher acquisition and operational costs compared to competitors; requires specialized training; larger laboratory footprint than some competing systems.
Shimazu KK
Technical Solution: Shimadzu has developed the ICPMS-2030 platform specifically optimized for biological sample analysis, featuring their proprietary mini-torch system that reduces running costs while maintaining sensitivity. Their Development Assistant software includes specialized methods for biological matrices, automating parameter optimization for complex samples. For ESI-MS applications, Shimadzu's LCMS-8060NX offers complementary capabilities for metallomics research with ultra-fast polarity switching. Their LabSolutions software platform provides integrated workflows connecting sample preparation, analysis, and data processing specifically for biological applications. Shimadzu has also pioneered specialized sample introduction systems for handling high-salt biological matrices, including their proprietary nebulizer technology that resists clogging while maintaining analytical performance[6][8].
Strengths: Excellent cost-performance ratio; compact design requiring less laboratory space; intuitive software with guided method development; lower operating costs. Weaknesses: Slightly lower sensitivity for some ultra-trace elements compared to top-tier systems; more limited presence in clinical validation literature; fewer specialized accessories for niche applications.
Core Innovations in ICP-MS and ESI-MS Technologies
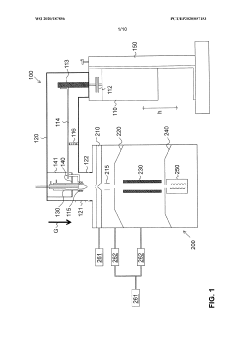
Cooling Plate for ICP-MS
PatentActiveUS20200194247A1
Innovation
- A bronze cooling plate is used, which provides sufficient thermal conductivity and enhanced chemical resistance, eliminating the need for a corrosion-resistant coating and reducing matrix deposition effects, thereby stabilizing the sampling interface.
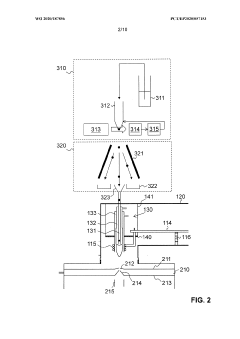
Ion source for inductively coupled plasma mass spectrometry
PatentWO2020187856A1
Innovation
- An ICP source with a vertically oriented plasma torch and injector tube allows sample introduction along a downwards-pointing vertical direction, reducing dependence on carrier gas flow and enabling 100% transport efficiency by utilizing gravity, and includes a metallic cooling plate and electromagnetic coupling element for efficient plasma generation.
Sample Preparation Techniques for Biological Matrices
Sample preparation is a critical determinant of analytical success when detecting metal ions in biological samples using either ICP-MS or ESI-MS. The complex nature of biological matrices necessitates specific preparation techniques to ensure accurate quantification while minimizing interferences and contamination.
Digestion methods represent the primary approach for preparing biological samples. Acid digestion using concentrated nitric acid, often combined with hydrogen peroxide, effectively breaks down organic matter in tissues, blood, and cellular samples. For ICP-MS analysis, complete mineralization is typically required, achieved through closed-vessel microwave-assisted digestion systems that operate under controlled pressure and temperature conditions, reducing volatile element loss.
Extraction procedures offer alternatives when complete digestion is unnecessary or potentially detrimental. Liquid-liquid extraction using chelating agents can selectively isolate metal ions from biological fluids, while solid-phase extraction techniques provide higher selectivity and enrichment factors. These approaches are particularly valuable for ESI-MS applications, where maintaining metal-protein associations may be analytically significant.
Dilution strategies differ markedly between the two analytical platforms. ICP-MS typically requires substantial dilution (10-100 fold) of biological fluids to reduce matrix effects and prevent salt deposition on instrument components. Conversely, ESI-MS often benefits from minimal dilution to maintain analyte concentration, though buffer exchange may be necessary to ensure compatibility with electrospray conditions.
Sample clean-up procedures are essential for both techniques but serve different purposes. For ICP-MS, removal of organic content and particulates prevents plasma instability and cone blockage. For ESI-MS, desalting steps are crucial as high salt concentrations suppress ionization efficiency. Size-exclusion chromatography and ultrafiltration are commonly employed pre-ESI-MS to separate metal-binding proteins from free ions.
Specialized techniques have emerged for specific applications. Laser ablation sample introduction for ICP-MS enables direct solid sample analysis with minimal preparation, preserving spatial information in tissue sections. For ESI-MS, native spray conditions using volatile ammonium acetate or ammonium bicarbonate buffers help maintain metal-protein complexes during ionization, allowing investigation of metalloproteins in their biologically relevant forms.
Standardization approaches also differ significantly between techniques, with isotope dilution methods being particularly powerful for ICP-MS quantification in complex matrices, while ESI-MS often relies on standard addition methods when analyzing metalloproteins or metal-ligand complexes.
Digestion methods represent the primary approach for preparing biological samples. Acid digestion using concentrated nitric acid, often combined with hydrogen peroxide, effectively breaks down organic matter in tissues, blood, and cellular samples. For ICP-MS analysis, complete mineralization is typically required, achieved through closed-vessel microwave-assisted digestion systems that operate under controlled pressure and temperature conditions, reducing volatile element loss.
Extraction procedures offer alternatives when complete digestion is unnecessary or potentially detrimental. Liquid-liquid extraction using chelating agents can selectively isolate metal ions from biological fluids, while solid-phase extraction techniques provide higher selectivity and enrichment factors. These approaches are particularly valuable for ESI-MS applications, where maintaining metal-protein associations may be analytically significant.
Dilution strategies differ markedly between the two analytical platforms. ICP-MS typically requires substantial dilution (10-100 fold) of biological fluids to reduce matrix effects and prevent salt deposition on instrument components. Conversely, ESI-MS often benefits from minimal dilution to maintain analyte concentration, though buffer exchange may be necessary to ensure compatibility with electrospray conditions.
Sample clean-up procedures are essential for both techniques but serve different purposes. For ICP-MS, removal of organic content and particulates prevents plasma instability and cone blockage. For ESI-MS, desalting steps are crucial as high salt concentrations suppress ionization efficiency. Size-exclusion chromatography and ultrafiltration are commonly employed pre-ESI-MS to separate metal-binding proteins from free ions.
Specialized techniques have emerged for specific applications. Laser ablation sample introduction for ICP-MS enables direct solid sample analysis with minimal preparation, preserving spatial information in tissue sections. For ESI-MS, native spray conditions using volatile ammonium acetate or ammonium bicarbonate buffers help maintain metal-protein complexes during ionization, allowing investigation of metalloproteins in their biologically relevant forms.
Standardization approaches also differ significantly between techniques, with isotope dilution methods being particularly powerful for ICP-MS quantification in complex matrices, while ESI-MS often relies on standard addition methods when analyzing metalloproteins or metal-ligand complexes.
Clinical and Toxicological Applications and Implications
The clinical applications of metal ion detection in biological samples have expanded significantly with the advancement of analytical techniques like ICP-MS and ESI-MS. In clinical diagnostics, these technologies enable precise measurement of essential elements such as zinc, copper, and iron, which serve as biomarkers for various pathological conditions. ICP-MS demonstrates superior sensitivity for trace metal quantification in blood and urine samples, making it invaluable for monitoring patients with metal implants or those undergoing chelation therapy. Conversely, ESI-MS offers advantages in speciation analysis, providing insights into metal-protein interactions crucial for understanding diseases like Alzheimer's and Parkinson's.
In toxicological assessments, both techniques play complementary roles. ICP-MS excels in screening for heavy metal poisoning, offering detection limits in the parts-per-trillion range for elements like lead, mercury, and arsenic. This capability is particularly valuable in public health emergencies involving environmental contamination. ESI-MS, with its ability to preserve metal-ligand complexes, provides critical information about the biological activity of toxic metals, helping clinicians understand the mechanisms of toxicity and develop targeted treatments.
The implications for personalized medicine are profound. Metal homeostasis varies significantly between individuals, and abnormal metal profiles can indicate disease states before clinical symptoms appear. ICP-MS-based screening protocols are increasingly incorporated into routine health assessments, while ESI-MS helps characterize metalloproteins and metalloenzymes involved in drug metabolism. This dual approach enables tailored therapeutic strategies based on a patient's unique metallome profile.
Forensic toxicology has also benefited from these technologies. ICP-MS provides quantitative evidence of acute or chronic metal poisoning in legal investigations, while ESI-MS can reveal the specific chemical forms of metals, offering insights into exposure sources and timelines. This complementary information strengthens the evidentiary value in medicolegal cases.
Occupational health monitoring represents another critical application. Workers in industries with metal exposure risks undergo regular biomonitoring using ICP-MS to ensure compliance with safety regulations. ESI-MS adds value by identifying specific metalloprotein adducts that may serve as early biomarkers of occupational disease, potentially allowing for intervention before irreversible health effects occur.
In toxicological assessments, both techniques play complementary roles. ICP-MS excels in screening for heavy metal poisoning, offering detection limits in the parts-per-trillion range for elements like lead, mercury, and arsenic. This capability is particularly valuable in public health emergencies involving environmental contamination. ESI-MS, with its ability to preserve metal-ligand complexes, provides critical information about the biological activity of toxic metals, helping clinicians understand the mechanisms of toxicity and develop targeted treatments.
The implications for personalized medicine are profound. Metal homeostasis varies significantly between individuals, and abnormal metal profiles can indicate disease states before clinical symptoms appear. ICP-MS-based screening protocols are increasingly incorporated into routine health assessments, while ESI-MS helps characterize metalloproteins and metalloenzymes involved in drug metabolism. This dual approach enables tailored therapeutic strategies based on a patient's unique metallome profile.
Forensic toxicology has also benefited from these technologies. ICP-MS provides quantitative evidence of acute or chronic metal poisoning in legal investigations, while ESI-MS can reveal the specific chemical forms of metals, offering insights into exposure sources and timelines. This complementary information strengthens the evidentiary value in medicolegal cases.
Occupational health monitoring represents another critical application. Workers in industries with metal exposure risks undergo regular biomonitoring using ICP-MS to ensure compliance with safety regulations. ESI-MS adds value by identifying specific metalloprotein adducts that may serve as early biomarkers of occupational disease, potentially allowing for intervention before irreversible health effects occur.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!