How to Reduce ICP-MS Interference from Matrix Components
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ICP-MS Technology Background and Objectives
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has evolved significantly since its commercial introduction in the early 1980s, becoming an indispensable analytical technique for trace element analysis across various industries including environmental monitoring, pharmaceuticals, food safety, and semiconductor manufacturing. The technology leverages high-temperature plasma to ionize sample atoms, which are subsequently detected and quantified by a mass spectrometer based on their mass-to-charge ratios.
The historical development of ICP-MS has been characterized by continuous improvements in sensitivity, precision, and interference management. Early systems suffered from significant matrix-induced interferences, limiting their application in complex sample analysis. Over the decades, technological advancements have progressively addressed these limitations, with major breakthroughs including the introduction of collision/reaction cells, high-resolution mass analyzers, and sophisticated sample introduction systems.
Matrix components present one of the most persistent challenges in ICP-MS analysis, causing spectral interferences, signal suppression or enhancement, and instrument contamination. These interferences can significantly compromise measurement accuracy and detection limits, particularly when analyzing complex samples such as biological fluids, environmental matrices, or industrial materials.
The primary objective of current ICP-MS technology development is to enhance the ability to accurately quantify trace elements in increasingly complex matrices while minimizing sample preparation requirements. This involves developing more effective strategies to reduce or eliminate matrix-induced interferences without compromising analytical performance or increasing operational complexity.
Key technological trends include the advancement of collision/reaction cell technologies, the integration of artificial intelligence for automated interference recognition and correction, the development of novel sample introduction systems, and the refinement of mathematical correction models. Additionally, there is growing interest in coupling ICP-MS with separation techniques such as chromatography to physically separate analytes from interfering matrix components prior to analysis.
The ultimate goal is to achieve reliable, interference-free measurements across diverse sample types with minimal method development and sample preparation. This would significantly expand the applicability of ICP-MS in routine analytical workflows, enabling more efficient quality control processes, environmental monitoring programs, and clinical diagnostics.
As regulatory requirements for elemental analysis become increasingly stringent across industries, the demand for more robust and interference-resistant ICP-MS technologies continues to grow, driving innovation in this field toward more sophisticated interference management solutions.
The historical development of ICP-MS has been characterized by continuous improvements in sensitivity, precision, and interference management. Early systems suffered from significant matrix-induced interferences, limiting their application in complex sample analysis. Over the decades, technological advancements have progressively addressed these limitations, with major breakthroughs including the introduction of collision/reaction cells, high-resolution mass analyzers, and sophisticated sample introduction systems.
Matrix components present one of the most persistent challenges in ICP-MS analysis, causing spectral interferences, signal suppression or enhancement, and instrument contamination. These interferences can significantly compromise measurement accuracy and detection limits, particularly when analyzing complex samples such as biological fluids, environmental matrices, or industrial materials.
The primary objective of current ICP-MS technology development is to enhance the ability to accurately quantify trace elements in increasingly complex matrices while minimizing sample preparation requirements. This involves developing more effective strategies to reduce or eliminate matrix-induced interferences without compromising analytical performance or increasing operational complexity.
Key technological trends include the advancement of collision/reaction cell technologies, the integration of artificial intelligence for automated interference recognition and correction, the development of novel sample introduction systems, and the refinement of mathematical correction models. Additionally, there is growing interest in coupling ICP-MS with separation techniques such as chromatography to physically separate analytes from interfering matrix components prior to analysis.
The ultimate goal is to achieve reliable, interference-free measurements across diverse sample types with minimal method development and sample preparation. This would significantly expand the applicability of ICP-MS in routine analytical workflows, enabling more efficient quality control processes, environmental monitoring programs, and clinical diagnostics.
As regulatory requirements for elemental analysis become increasingly stringent across industries, the demand for more robust and interference-resistant ICP-MS technologies continues to grow, driving innovation in this field toward more sophisticated interference management solutions.
Market Demand Analysis for High-Precision ICP-MS
The global market for high-precision Inductively Coupled Plasma Mass Spectrometry (ICP-MS) continues to expand significantly, driven by increasing demands for ultra-trace elemental analysis across multiple industries. Current market valuations place the ICP-MS sector at approximately $1.2 billion, with projections indicating a compound annual growth rate of 7.8% through 2028.
Environmental monitoring represents the largest market segment, accounting for nearly 30% of total demand. Stringent regulations worldwide regarding water quality, soil contamination, and air pollution have necessitated more sensitive and accurate analytical methods. The EPA, EU Water Framework Directive, and similar regulatory bodies have progressively lowered acceptable limits for toxic elements, creating substantial demand for advanced ICP-MS systems capable of detecting contaminants at parts-per-trillion levels.
The pharmaceutical and biomedical research sectors demonstrate the fastest growth trajectory, with increasing requirements for elemental impurity testing in drug products following ICH Q3D guidelines implementation. These regulations mandate comprehensive testing for 24 elemental impurities in pharmaceutical products, directly boosting demand for high-precision ICP-MS instruments that can effectively manage complex biological matrices.
Food safety testing constitutes another rapidly expanding application area, particularly in regions implementing stricter food safety regulations. China's recent overhaul of food safety standards and the EU's continuous updates to maximum contaminant levels have created substantial market opportunities for matrix-interference-resistant ICP-MS technologies.
Semiconductor and electronics manufacturing represents a premium market segment where ultra-high purity analysis is critical. As chip architectures continue to shrink, even minute elemental contamination can significantly impact performance, driving demand for ICP-MS systems with enhanced interference management capabilities.
Geographically, North America and Europe currently dominate market share, though Asia-Pacific shows the highest growth rate, particularly in China, Japan, and South Korea. This growth correlates with increasing environmental regulations, expanding pharmaceutical industries, and significant semiconductor manufacturing activities in these regions.
End-users consistently identify matrix interference management as a critical pain point, with 78% of surveyed laboratory managers citing it as their primary technical challenge when using ICP-MS for complex sample analysis. This challenge creates substantial market opportunity for innovations specifically addressing polyatomic and isobaric interferences in complex matrices.
Environmental monitoring represents the largest market segment, accounting for nearly 30% of total demand. Stringent regulations worldwide regarding water quality, soil contamination, and air pollution have necessitated more sensitive and accurate analytical methods. The EPA, EU Water Framework Directive, and similar regulatory bodies have progressively lowered acceptable limits for toxic elements, creating substantial demand for advanced ICP-MS systems capable of detecting contaminants at parts-per-trillion levels.
The pharmaceutical and biomedical research sectors demonstrate the fastest growth trajectory, with increasing requirements for elemental impurity testing in drug products following ICH Q3D guidelines implementation. These regulations mandate comprehensive testing for 24 elemental impurities in pharmaceutical products, directly boosting demand for high-precision ICP-MS instruments that can effectively manage complex biological matrices.
Food safety testing constitutes another rapidly expanding application area, particularly in regions implementing stricter food safety regulations. China's recent overhaul of food safety standards and the EU's continuous updates to maximum contaminant levels have created substantial market opportunities for matrix-interference-resistant ICP-MS technologies.
Semiconductor and electronics manufacturing represents a premium market segment where ultra-high purity analysis is critical. As chip architectures continue to shrink, even minute elemental contamination can significantly impact performance, driving demand for ICP-MS systems with enhanced interference management capabilities.
Geographically, North America and Europe currently dominate market share, though Asia-Pacific shows the highest growth rate, particularly in China, Japan, and South Korea. This growth correlates with increasing environmental regulations, expanding pharmaceutical industries, and significant semiconductor manufacturing activities in these regions.
End-users consistently identify matrix interference management as a critical pain point, with 78% of surveyed laboratory managers citing it as their primary technical challenge when using ICP-MS for complex sample analysis. This challenge creates substantial market opportunity for innovations specifically addressing polyatomic and isobaric interferences in complex matrices.
Matrix Interference Challenges in ICP-MS
Matrix interference represents one of the most significant challenges in Inductively Coupled Plasma Mass Spectrometry (ICP-MS) analysis. These interferences occur when components in the sample matrix affect the measurement of target analytes, leading to inaccurate results. The primary types of matrix interferences include spectral interferences, where matrix components create ions with the same mass-to-charge ratio as the analyte of interest, and non-spectral interferences, which affect the transport, ionization, or extraction of analyte ions.
Spectral interferences can be further categorized into isobaric overlaps, polyatomic ion interferences, and doubly charged ion interferences. Isobaric overlaps occur when different elements have isotopes with nearly identical masses. For example, 114Cd determination is complicated by the presence of 114Sn. Polyatomic interferences arise when plasma gas, matrix components, or solvent molecules combine to form molecular ions. Common examples include 40Ar16O+ interfering with 56Fe+ and 40Ar35Cl+ affecting 75As+ measurements.
Non-spectral interferences manifest as matrix-induced signal suppression or enhancement. These effects typically result from high concentrations of easily ionized elements (like Na, K, Ca) or high dissolved solid content that alters plasma conditions. Such interferences can change the ionization efficiency of analytes, affecting sensitivity and calibration curves. Additionally, physical interferences may occur when high-matrix samples cause deposition on instrument components, leading to signal drift and decreased performance over time.
The severity of matrix interferences depends on several factors, including sample composition, concentration of matrix elements, and the specific analytes being measured. Biological samples often present complex organic matrices that generate numerous polyatomic interferences. Environmental samples may contain high levels of dissolved salts, while geological samples typically present challenges due to their high mineral content and refractory elements.
Modern ICP-MS instruments operate at detection limits in the parts-per-trillion range, making them extremely sensitive to even minor interferences. This sensitivity paradoxically increases vulnerability to matrix effects, as even trace contaminants can significantly impact results. The challenge is particularly acute when analyzing trace elements in complex matrices like seawater, blood, or digested soil samples.
The economic impact of matrix interferences extends beyond analytical accuracy. Laboratories must invest in additional sample preparation procedures, specialized equipment, and increased analysis time to address these challenges. Industries requiring high-precision elemental analysis, such as semiconductor manufacturing, pharmaceutical production, and environmental monitoring, face increased operational costs due to these analytical challenges.
Spectral interferences can be further categorized into isobaric overlaps, polyatomic ion interferences, and doubly charged ion interferences. Isobaric overlaps occur when different elements have isotopes with nearly identical masses. For example, 114Cd determination is complicated by the presence of 114Sn. Polyatomic interferences arise when plasma gas, matrix components, or solvent molecules combine to form molecular ions. Common examples include 40Ar16O+ interfering with 56Fe+ and 40Ar35Cl+ affecting 75As+ measurements.
Non-spectral interferences manifest as matrix-induced signal suppression or enhancement. These effects typically result from high concentrations of easily ionized elements (like Na, K, Ca) or high dissolved solid content that alters plasma conditions. Such interferences can change the ionization efficiency of analytes, affecting sensitivity and calibration curves. Additionally, physical interferences may occur when high-matrix samples cause deposition on instrument components, leading to signal drift and decreased performance over time.
The severity of matrix interferences depends on several factors, including sample composition, concentration of matrix elements, and the specific analytes being measured. Biological samples often present complex organic matrices that generate numerous polyatomic interferences. Environmental samples may contain high levels of dissolved salts, while geological samples typically present challenges due to their high mineral content and refractory elements.
Modern ICP-MS instruments operate at detection limits in the parts-per-trillion range, making them extremely sensitive to even minor interferences. This sensitivity paradoxically increases vulnerability to matrix effects, as even trace contaminants can significantly impact results. The challenge is particularly acute when analyzing trace elements in complex matrices like seawater, blood, or digested soil samples.
The economic impact of matrix interferences extends beyond analytical accuracy. Laboratories must invest in additional sample preparation procedures, specialized equipment, and increased analysis time to address these challenges. Industries requiring high-precision elemental analysis, such as semiconductor manufacturing, pharmaceutical production, and environmental monitoring, face increased operational costs due to these analytical challenges.
Current Matrix Interference Mitigation Strategies
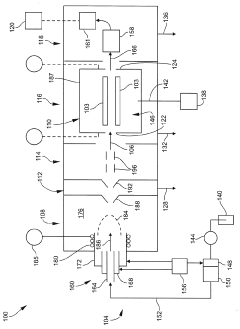
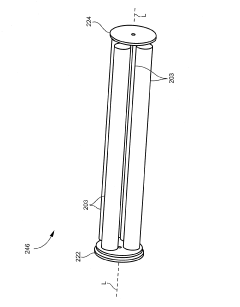
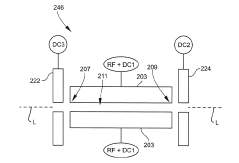
01 Collision/reaction cell technology
Collision/reaction cell technology is used to reduce polyatomic interferences in ICP-MS. This approach involves introducing a collision or reaction gas (such as helium, hydrogen, or ammonia) into a cell positioned before the mass analyzer. The gas selectively reacts with or collides with interfering ions, converting them to different masses or reducing their kinetic energy, while analyte ions pass through relatively unaffected. This technology significantly improves detection limits and accuracy for elements that typically suffer from polyatomic interferences.- Collision/reaction cell technology: Collision/reaction cell technology is used to reduce polyatomic interferences in ICP-MS by introducing a collision or reaction gas into a cell positioned between the ion optics and the mass analyzer. The gas selectively reacts with or collides with interfering ions, converting them to different masses or reducing their kinetic energy, while allowing analyte ions to pass through. This technology significantly improves detection limits and accuracy for elements affected by polyatomic interferences.
- Mathematical correction methods: Mathematical correction algorithms are employed to compensate for spectral interferences in ICP-MS analysis. These methods include equations that account for known interference patterns, isotope ratio calculations, and statistical models that can separate overlapping signals. Advanced software implementations can perform real-time corrections during analysis, improving measurement accuracy without requiring additional hardware modifications to the ICP-MS system.
- Sample preparation techniques: Specialized sample preparation methods can significantly reduce matrix-based interferences in ICP-MS analysis. These techniques include chemical separation procedures, matrix matching, dilution protocols, and digestion methods optimized to minimize the formation of interfering compounds. Proper sample preparation removes or reduces potential interfering elements before they enter the ICP-MS system, resulting in cleaner spectra and more accurate quantification.
- High-resolution mass analyzers: High-resolution mass spectrometers can physically separate ions with very similar mass-to-charge ratios that would otherwise cause interference in conventional quadrupole ICP-MS systems. These analyzers, including magnetic sector and time-of-flight instruments, provide sufficient resolution to distinguish between analyte ions and interfering species with minimal mass differences. This approach allows direct measurement of problematic elements without requiring chemical separation or reaction cell technology.
- Plasma condition optimization: Optimizing plasma conditions can significantly reduce certain types of interferences in ICP-MS. This includes adjusting parameters such as RF power, plasma gas flow rates, torch position, and sample introduction systems. Cold plasma techniques operate at reduced plasma power to minimize argon-based interferences, while robust plasma conditions can break down complex matrix components. These optimizations can be tailored to specific analytical challenges and sample types.
02 Mathematical correction methods
Mathematical correction algorithms can be applied to ICP-MS data to reduce or eliminate spectral interferences. These methods include equations that account for known interference patterns, isotope ratio calculations, and statistical approaches to separate analyte signals from interfering species. Advanced software implementations can perform real-time corrections during analysis, improving accuracy without requiring additional hardware modifications. These mathematical approaches are particularly useful for handling isobaric interferences that cannot be resolved through physical separation methods.Expand Specific Solutions03 Sample preparation techniques
Specialized sample preparation methods can significantly reduce interferences in ICP-MS analysis. These include matrix separation techniques, chromatographic methods, and chemical treatments that remove or transform interfering species before analysis. Techniques such as ion exchange, solid phase extraction, and precipitation can isolate analytes from complex matrices. Additionally, digestion procedures using specific acid combinations or closed-vessel microwave systems can minimize the introduction of contaminants that cause interferences, resulting in cleaner spectra and improved detection limits.Expand Specific Solutions04 High-resolution mass spectrometry
High-resolution mass spectrometry techniques can physically separate interfering ions from analyte ions based on small differences in their mass-to-charge ratios. This approach uses advanced mass analyzers such as magnetic sector or time-of-flight instruments that offer superior mass resolution compared to conventional quadrupole systems. By increasing the resolving power of the instrument, polyatomic interferences can be distinguished from analyte peaks, allowing for accurate quantification even in complex matrices. This technique is particularly valuable for elements that suffer from severe spectral interferences.Expand Specific Solutions05 Alternative sample introduction systems
Modified sample introduction systems can reduce interferences in ICP-MS by altering how the sample enters the plasma. These include specialized nebulizers, desolvation systems, and aerosol filtering devices that can reduce oxide formation and minimize matrix effects. Techniques such as laser ablation, electrothermal vaporization, and flow injection analysis can also be employed to reduce solvent-based interferences. Cold plasma conditions, achieved by reducing the RF power, can suppress argon-based interferences, while specialized spray chambers can reduce the formation of oxides and hydroxides that cause polyatomic interferences.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The ICP-MS interference reduction market is in a growth phase, with increasing demand for accurate trace element analysis across environmental, pharmaceutical, and food safety sectors. The market is expected to reach significant value due to expanding applications in research and industrial quality control. Technologically, solutions are evolving from basic mathematical corrections to sophisticated hardware innovations. Leading players include Thermo Fisher Scientific, offering advanced collision/reaction cell technologies; Agilent Technologies, with helium collision mode systems; Shimadzu, developing unique plasma generation approaches; and specialized innovators like Kimia Analytics with patented ICP torch designs. SPECTRO Analytical and PerkinElmer (Revvity) also contribute significant advancements in interference management through software algorithms and sample introduction systems.
Thermo Fisher Scientific (Bremen) GmbH
Technical Solution: Thermo Fisher Scientific has developed advanced collision/reaction cell technology for their ICP-MS instruments, specifically their Triple Quadrupole ICP-MS systems. Their patented technology utilizes a collision/reaction cell positioned between two quadrupoles, allowing for selective removal of polyatomic interferences through kinetic energy discrimination and chemical reactions. The company's Thermo Scientific iCAP TQ ICP-MS employs three operational modes: Single Quad mode for routine analysis, KED (Kinetic Energy Discrimination) mode using helium gas to reduce polyatomic interferences through collisions, and the advanced Triple Quad mode where the first quadrupole selectively filters ions before they enter the collision/reaction cell, dramatically improving detection limits for challenging elements in complex matrices[1][2]. Their systems also incorporate advanced software algorithms that can predict and correct for spectral overlaps automatically.
Strengths: Superior interference removal through triple quadrupole technology; versatile operation modes for different analytical challenges; excellent sensitivity even in complex matrices; comprehensive software support. Weaknesses: Higher cost compared to single quadrupole systems; requires specialized gases for operation; more complex operation requiring additional training for analysts.
Shimadzu Corp.
Technical Solution: Shimadzu Corporation has developed proprietary interference reduction technology for their ICP-MS systems centered around their patented mini-torch system and collision cell technology. Their ICPMS-2030 platform incorporates a unique octopole collision cell with helium gas that effectively removes polyatomic interferences through kinetic energy discrimination. Shimadzu's approach also includes their Development Assistant software with their patented "LabSolutions ICPMS" that features an interference check function to automatically identify potential spectral interferences and suggest alternative isotopes or correction equations[3]. Their systems employ a unique torch design that reduces sample matrix effects and minimizes oxide formation. Additionally, Shimadzu has implemented their proprietary "IntelliQuant" screening technology that provides semi-quantitative analysis of all elements in a sample simultaneously, helping identify unexpected matrix components that might cause interferences[4].
Strengths: Efficient interference removal with optimized collision cell design; comprehensive software tools for interference identification and correction; reduced sample consumption with mini-torch design; excellent cost-performance ratio. Weaknesses: Limited reaction gas options compared to some competitors; slightly lower sensitivity for certain ultra-trace elements in complex matrices.
Key Patents and Innovations in Interference Reduction
Inductively coupled plasma-mass spectrometry (ICP-ms) with improved signal-to-noise and signal-to-background ratios
PatentActiveJP2023120229A
Innovation
- A method and system utilizing a collision/reaction cell with controlled DC potential barriers and gas interactions to suppress interfering ions by converting them to non-interfering ions or neutral species, and confining ions axially and radially within the cell to enhance signal-to-noise and signal-to-background ratios.
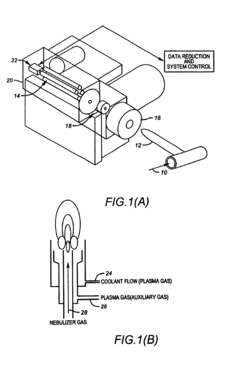
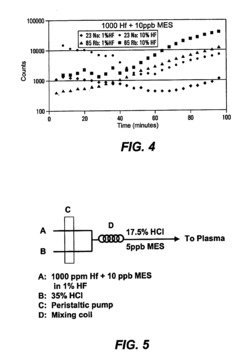
Use of a nebulizer to add gas to eliminate metal deposition on the sampling orifices of an inductively coupled plasma mass spectrometer
PatentInactiveUS6992282B2
Innovation
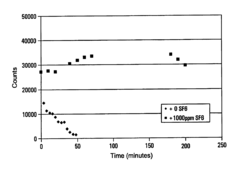
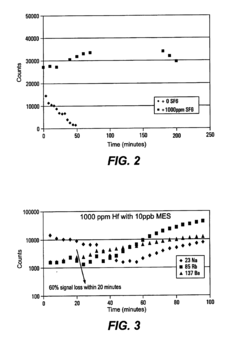
- The use of dilute Sulfur Hexafluoride (SF6) in an inert gas as a nebulizer add-gas to reduce transition metal deposition on the sampling orifices of ICP-MS, with SF6 being ionized to provide free fluoride ions that increase the volatility of transition metals and prevent deposition.
Sample Preparation Optimization Methods
Sample preparation represents a critical first line of defense against matrix interferences in ICP-MS analysis. Optimizing these procedures can significantly reduce or eliminate problematic matrix components before they reach the instrument. Dilution serves as the simplest approach, effectively reducing the concentration of matrix elements while maintaining analyte detectability. For complex samples, a dilution factor of 10-100x can dramatically decrease interference effects, though this requires balancing against detection limit requirements.
Acid digestion protocols can be tailored to specific matrix types, with different combinations of acids (HNO3, HCl, HF, H2O2) selected based on sample composition. Microwave-assisted digestion has emerged as particularly effective, offering complete decomposition of organic matrices while minimizing contamination risks and reducing preparation time from hours to minutes. Temperature-controlled digestion programs further enhance decomposition efficiency while preventing analyte loss.
Separation techniques provide targeted removal of interfering elements. Solid phase extraction (SPE) using specialized resins can selectively retain either analytes or interferents, with chelating resins particularly useful for separating transition metals from complex matrices. Precipitation methods effectively remove high-concentration matrix elements like calcium and magnesium through controlled pH adjustments, while co-precipitation techniques can concentrate trace analytes from dilute solutions.
Matrix matching in calibration standards represents another crucial optimization approach. By preparing calibration standards in solutions that mimic the sample matrix composition, signal suppression or enhancement effects can be normalized across both samples and standards. This technique proves especially valuable when dealing with high-salt matrices or samples with consistent background composition.
Chelation and complexation strategies employ specific reagents to bind interferents, altering their chemical behavior. EDTA and similar chelating agents can effectively sequester polyatomic-forming elements, preventing their participation in interference-generating reactions. For volatile element analysis, chemical modifiers like palladium nitrate or magnesium nitrate stabilize analytes during thermal pretreatment, allowing selective volatilization of matrix components.
Advanced sample introduction systems complement these preparation techniques. Flow injection analysis reduces matrix exposure time, while specialized nebulizers and spray chambers minimize transport of certain matrix components to the plasma. When implemented systematically, these optimized sample preparation methods can dramatically improve measurement accuracy and precision in challenging matrix environments.
Acid digestion protocols can be tailored to specific matrix types, with different combinations of acids (HNO3, HCl, HF, H2O2) selected based on sample composition. Microwave-assisted digestion has emerged as particularly effective, offering complete decomposition of organic matrices while minimizing contamination risks and reducing preparation time from hours to minutes. Temperature-controlled digestion programs further enhance decomposition efficiency while preventing analyte loss.
Separation techniques provide targeted removal of interfering elements. Solid phase extraction (SPE) using specialized resins can selectively retain either analytes or interferents, with chelating resins particularly useful for separating transition metals from complex matrices. Precipitation methods effectively remove high-concentration matrix elements like calcium and magnesium through controlled pH adjustments, while co-precipitation techniques can concentrate trace analytes from dilute solutions.
Matrix matching in calibration standards represents another crucial optimization approach. By preparing calibration standards in solutions that mimic the sample matrix composition, signal suppression or enhancement effects can be normalized across both samples and standards. This technique proves especially valuable when dealing with high-salt matrices or samples with consistent background composition.
Chelation and complexation strategies employ specific reagents to bind interferents, altering their chemical behavior. EDTA and similar chelating agents can effectively sequester polyatomic-forming elements, preventing their participation in interference-generating reactions. For volatile element analysis, chemical modifiers like palladium nitrate or magnesium nitrate stabilize analytes during thermal pretreatment, allowing selective volatilization of matrix components.
Advanced sample introduction systems complement these preparation techniques. Flow injection analysis reduces matrix exposure time, while specialized nebulizers and spray chambers minimize transport of certain matrix components to the plasma. When implemented systematically, these optimized sample preparation methods can dramatically improve measurement accuracy and precision in challenging matrix environments.
Validation and Quality Control Protocols
Validation and quality control protocols are essential components of any ICP-MS methodology aimed at reducing matrix interferences. Robust validation procedures ensure that the analytical methods employed can effectively mitigate interference effects while maintaining accuracy and precision. These protocols must be systematically designed to verify that the chosen interference reduction strategies perform consistently across various sample types and concentrations.
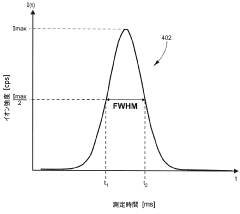
Method validation for ICP-MS interference reduction should include comprehensive assessment of key performance parameters. Linearity testing across the analytical range confirms that the relationship between analyte concentration and instrument response remains consistent despite potential matrix effects. Detection and quantification limits must be established with consideration for how matrix components might elevate background signals or suppress analyte responses, potentially affecting the lowest measurable concentrations.
Accuracy verification through analysis of certified reference materials (CRMs) that closely match the matrix composition of actual samples provides critical evidence of method effectiveness. Recovery studies involving spiked samples at multiple concentration levels help quantify the impact of matrix interferences on analyte recovery and validate correction strategies. Precision testing through replicate analyses evaluates both intra-day and inter-day variability, ensuring that interference reduction techniques deliver consistent results over time.
Robustness testing represents a particularly important aspect of validation for matrix interference reduction. This involves deliberate variation of method parameters such as collision/reaction cell gas flows, plasma conditions, or sample preparation procedures to determine the method's tolerance to operational fluctuations. Successful interference reduction strategies should demonstrate stability across reasonable variations in these parameters.
Ongoing quality control measures must complement initial validation efforts. Regular analysis of quality control samples, including method blanks, laboratory control samples, and matrix-matched standards, provides continuous verification that interference reduction techniques remain effective during routine analysis. Control charts tracking key performance indicators help identify trends that might indicate degradation in interference management capabilities.
System suitability tests performed at the beginning of analytical runs should specifically target known interference challenges. These might include monitoring isotope ratios affected by polyatomic interferences or measuring signal-to-background ratios for elements prone to matrix suppression. Participation in proficiency testing programs offers external validation of a laboratory's ability to manage matrix interferences effectively across challenging sample types.
Documentation of all validation and quality control procedures is essential, creating a comprehensive record that demonstrates the laboratory's capability to produce reliable results despite matrix interference challenges. This documentation should include detailed protocols for interference identification, correction strategies applied, and ongoing monitoring procedures that ensure continued method performance.
Method validation for ICP-MS interference reduction should include comprehensive assessment of key performance parameters. Linearity testing across the analytical range confirms that the relationship between analyte concentration and instrument response remains consistent despite potential matrix effects. Detection and quantification limits must be established with consideration for how matrix components might elevate background signals or suppress analyte responses, potentially affecting the lowest measurable concentrations.
Accuracy verification through analysis of certified reference materials (CRMs) that closely match the matrix composition of actual samples provides critical evidence of method effectiveness. Recovery studies involving spiked samples at multiple concentration levels help quantify the impact of matrix interferences on analyte recovery and validate correction strategies. Precision testing through replicate analyses evaluates both intra-day and inter-day variability, ensuring that interference reduction techniques deliver consistent results over time.
Robustness testing represents a particularly important aspect of validation for matrix interference reduction. This involves deliberate variation of method parameters such as collision/reaction cell gas flows, plasma conditions, or sample preparation procedures to determine the method's tolerance to operational fluctuations. Successful interference reduction strategies should demonstrate stability across reasonable variations in these parameters.
Ongoing quality control measures must complement initial validation efforts. Regular analysis of quality control samples, including method blanks, laboratory control samples, and matrix-matched standards, provides continuous verification that interference reduction techniques remain effective during routine analysis. Control charts tracking key performance indicators help identify trends that might indicate degradation in interference management capabilities.
System suitability tests performed at the beginning of analytical runs should specifically target known interference challenges. These might include monitoring isotope ratios affected by polyatomic interferences or measuring signal-to-background ratios for elements prone to matrix suppression. Participation in proficiency testing programs offers external validation of a laboratory's ability to manage matrix interferences effectively across challenging sample types.
Documentation of all validation and quality control procedures is essential, creating a comprehensive record that demonstrates the laboratory's capability to produce reliable results despite matrix interference challenges. This documentation should include detailed protocols for interference identification, correction strategies applied, and ongoing monitoring procedures that ensure continued method performance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!