Enhancing ICP-MS Sensitivity with Optimized Plasma Conditions
SEP 19, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ICP-MS Technology Evolution and Sensitivity Goals
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has evolved significantly since its commercial introduction in the early 1980s. This analytical technique combines the high-temperature ICP source with a mass spectrometer to identify and quantify elements at extremely low concentrations. The evolution of ICP-MS technology has been driven by the increasing demand for lower detection limits, higher sensitivity, and reduced interference across various applications including environmental monitoring, clinical analysis, food safety, and semiconductor manufacturing.
The initial ICP-MS systems utilized quadrupole mass analyzers with detection limits in the parts per billion (ppb) range. By the 1990s, technological advancements led to the development of high-resolution ICP-MS instruments capable of achieving parts per trillion (ppt) detection limits. The introduction of collision/reaction cell technology in the early 2000s represented a significant breakthrough, allowing for the reduction of polyatomic interferences that had previously limited analytical capabilities.
Recent developments have focused on enhancing plasma stability and energy transfer efficiency. Modern ICP-MS systems incorporate advanced radio frequency (RF) generators that provide more stable plasma conditions, contributing to improved sensitivity and reproducibility. The optimization of plasma conditions, including gas flow rates, RF power, and torch design, has become a critical area of research for pushing detection limits even lower.
The current sensitivity goals for ICP-MS technology center around achieving consistent sub-part per trillion (sub-ppt) detection limits across the periodic table while maintaining high sample throughput. Researchers are particularly focused on improving sensitivity for traditionally difficult elements such as arsenic, selenium, and mercury, which are critical in environmental and clinical applications.
Another important trend is the miniaturization of ICP-MS systems without compromising analytical performance. This direction aims to make the technology more accessible for field applications and smaller laboratories. Parallel to this, there is significant interest in developing plasma conditions that require less argon gas consumption, addressing both economic and environmental concerns.
The integration of ICP-MS with other analytical techniques, such as chromatography and laser ablation, has expanded its application scope. These hyphenated techniques demand optimized plasma conditions to maintain sensitivity when dealing with transient signals and complex matrices. Consequently, adaptive plasma control systems that can automatically adjust to changing sample compositions represent an emerging technological goal.
Looking forward, the field is moving toward "smart" plasma systems that utilize artificial intelligence to predict and adjust optimal conditions based on sample characteristics, potentially revolutionizing how sensitivity is maximized in routine analysis.
The initial ICP-MS systems utilized quadrupole mass analyzers with detection limits in the parts per billion (ppb) range. By the 1990s, technological advancements led to the development of high-resolution ICP-MS instruments capable of achieving parts per trillion (ppt) detection limits. The introduction of collision/reaction cell technology in the early 2000s represented a significant breakthrough, allowing for the reduction of polyatomic interferences that had previously limited analytical capabilities.
Recent developments have focused on enhancing plasma stability and energy transfer efficiency. Modern ICP-MS systems incorporate advanced radio frequency (RF) generators that provide more stable plasma conditions, contributing to improved sensitivity and reproducibility. The optimization of plasma conditions, including gas flow rates, RF power, and torch design, has become a critical area of research for pushing detection limits even lower.
The current sensitivity goals for ICP-MS technology center around achieving consistent sub-part per trillion (sub-ppt) detection limits across the periodic table while maintaining high sample throughput. Researchers are particularly focused on improving sensitivity for traditionally difficult elements such as arsenic, selenium, and mercury, which are critical in environmental and clinical applications.
Another important trend is the miniaturization of ICP-MS systems without compromising analytical performance. This direction aims to make the technology more accessible for field applications and smaller laboratories. Parallel to this, there is significant interest in developing plasma conditions that require less argon gas consumption, addressing both economic and environmental concerns.
The integration of ICP-MS with other analytical techniques, such as chromatography and laser ablation, has expanded its application scope. These hyphenated techniques demand optimized plasma conditions to maintain sensitivity when dealing with transient signals and complex matrices. Consequently, adaptive plasma control systems that can automatically adjust to changing sample compositions represent an emerging technological goal.
Looking forward, the field is moving toward "smart" plasma systems that utilize artificial intelligence to predict and adjust optimal conditions based on sample characteristics, potentially revolutionizing how sensitivity is maximized in routine analysis.
Market Demand for High-Sensitivity Elemental Analysis
The global market for high-sensitivity elemental analysis has experienced significant growth over the past decade, driven primarily by increasing demands in environmental monitoring, pharmaceutical research, food safety, and semiconductor manufacturing. ICP-MS (Inductively Coupled Plasma Mass Spectrometry) technology has emerged as the gold standard for trace element analysis due to its exceptional detection capabilities, often reaching parts-per-trillion levels.
Environmental regulations worldwide have become increasingly stringent, requiring more precise detection of toxic elements in air, water, and soil samples. The European Union's Water Framework Directive and the U.S. EPA's regulations on heavy metals have created substantial demand for advanced analytical instruments capable of detecting contaminants at ultra-low concentrations. This regulatory landscape has expanded the market for high-sensitivity ICP-MS systems by approximately 8% annually in the environmental sector alone.
The pharmaceutical industry represents another significant market driver, with growing requirements for elemental impurity testing in drug products following the implementation of ICH Q3D guidelines. These guidelines have established permitted daily exposure limits for 24 elements, necessitating analytical methods with exceptional sensitivity. The pharmaceutical analytical testing market has consequently shown robust growth, with elemental analysis segments expanding particularly rapidly.
Food safety concerns have intensified globally, especially regarding heavy metal contamination in various food products. Major food safety incidents in recent years have prompted regulatory bodies to implement more comprehensive testing protocols. Countries like China, Japan, and South Korea have established some of the world's most rigorous standards for food contaminants, creating substantial demand for high-sensitivity analytical instruments in the Asia-Pacific region.
The semiconductor industry presents perhaps the most demanding application for ultra-trace elemental analysis. As chip architectures continue to shrink, even minute elemental contamination can significantly impact device performance. The need to detect contaminants at ever-lower concentrations has pushed the development of increasingly sensitive ICP-MS technologies, with the semiconductor sector willing to invest in premium analytical solutions that offer enhanced detection capabilities.
Clinical diagnostics represents an emerging market with considerable growth potential. The analysis of trace elements in biological samples for disease biomarkers requires exceptional sensitivity and precision. Research linking trace element imbalances to various health conditions has expanded the application of ICP-MS in clinical settings, though this market segment remains in its early development stages.
Market forecasts indicate continued growth for high-sensitivity elemental analysis technologies, with particular emphasis on instruments offering improved plasma stability, reduced interference, and enhanced ionization efficiency. The global analytical instrumentation market for trace element analysis is projected to maintain steady growth, with ICP-MS technologies capturing an increasing market share due to their superior sensitivity compared to alternative techniques.
Environmental regulations worldwide have become increasingly stringent, requiring more precise detection of toxic elements in air, water, and soil samples. The European Union's Water Framework Directive and the U.S. EPA's regulations on heavy metals have created substantial demand for advanced analytical instruments capable of detecting contaminants at ultra-low concentrations. This regulatory landscape has expanded the market for high-sensitivity ICP-MS systems by approximately 8% annually in the environmental sector alone.
The pharmaceutical industry represents another significant market driver, with growing requirements for elemental impurity testing in drug products following the implementation of ICH Q3D guidelines. These guidelines have established permitted daily exposure limits for 24 elements, necessitating analytical methods with exceptional sensitivity. The pharmaceutical analytical testing market has consequently shown robust growth, with elemental analysis segments expanding particularly rapidly.
Food safety concerns have intensified globally, especially regarding heavy metal contamination in various food products. Major food safety incidents in recent years have prompted regulatory bodies to implement more comprehensive testing protocols. Countries like China, Japan, and South Korea have established some of the world's most rigorous standards for food contaminants, creating substantial demand for high-sensitivity analytical instruments in the Asia-Pacific region.
The semiconductor industry presents perhaps the most demanding application for ultra-trace elemental analysis. As chip architectures continue to shrink, even minute elemental contamination can significantly impact device performance. The need to detect contaminants at ever-lower concentrations has pushed the development of increasingly sensitive ICP-MS technologies, with the semiconductor sector willing to invest in premium analytical solutions that offer enhanced detection capabilities.
Clinical diagnostics represents an emerging market with considerable growth potential. The analysis of trace elements in biological samples for disease biomarkers requires exceptional sensitivity and precision. Research linking trace element imbalances to various health conditions has expanded the application of ICP-MS in clinical settings, though this market segment remains in its early development stages.
Market forecasts indicate continued growth for high-sensitivity elemental analysis technologies, with particular emphasis on instruments offering improved plasma stability, reduced interference, and enhanced ionization efficiency. The global analytical instrumentation market for trace element analysis is projected to maintain steady growth, with ICP-MS technologies capturing an increasing market share due to their superior sensitivity compared to alternative techniques.
Current Plasma Optimization Challenges in ICP-MS
Despite significant advancements in ICP-MS technology, several critical challenges persist in plasma optimization that limit the technique's sensitivity and performance. The fundamental challenge lies in balancing plasma conditions to simultaneously achieve efficient ionization while minimizing interferences. Current RF generators struggle to maintain stable plasma under varying sample matrices, leading to signal drift and reduced analytical precision during extended analytical runs.
Matrix effects represent another significant obstacle, as complex samples can cause plasma destabilization and ion suppression. High dissolved solid content particularly affects plasma temperature distribution and ionization efficiency, creating unpredictable signal responses across different analytes. This variability necessitates extensive calibration procedures that reduce laboratory throughput.
Plasma-sample interaction optimization remains problematic, with current nebulizer and spray chamber designs failing to deliver consistent sample introduction across diverse sample types. The transition region between plasma and mass analyzer continues to be a critical weak point where ion transmission losses occur, particularly for elements with high ionization potentials or those susceptible to oxide formation.
Energy coupling efficiency presents ongoing challenges, with current systems typically achieving only 70-80% power transfer from the RF generator to the plasma. This inefficiency results in higher operational costs and reduced sensitivity for trace element detection. The inability to dynamically adjust plasma conditions in response to changing sample composition further compounds these issues.
Space-charge effects in the ion beam represent another persistent challenge, particularly affecting low-mass analytes in the presence of high-mass matrix elements. Current lens systems struggle to compensate for these effects across the entire mass range, leading to compromised detection limits for certain elements.
Temperature gradients within the plasma create zones of varying ionization efficiency, with current torch designs unable to establish truly homogeneous conditions. This results in element-dependent sensitivity variations that complicate quantitative analysis, especially for multi-element determinations spanning the periodic table.
Plasma cooling interfaces, while improved in recent years, still introduce significant ion losses during the transition from atmospheric pressure to the vacuum system. The formation of polyatomic interferences during this cooling process remains inadequately addressed by current hardware solutions, necessitating complex mathematical corrections or chemical separation procedures that add complexity to analytical workflows.
Matrix effects represent another significant obstacle, as complex samples can cause plasma destabilization and ion suppression. High dissolved solid content particularly affects plasma temperature distribution and ionization efficiency, creating unpredictable signal responses across different analytes. This variability necessitates extensive calibration procedures that reduce laboratory throughput.
Plasma-sample interaction optimization remains problematic, with current nebulizer and spray chamber designs failing to deliver consistent sample introduction across diverse sample types. The transition region between plasma and mass analyzer continues to be a critical weak point where ion transmission losses occur, particularly for elements with high ionization potentials or those susceptible to oxide formation.
Energy coupling efficiency presents ongoing challenges, with current systems typically achieving only 70-80% power transfer from the RF generator to the plasma. This inefficiency results in higher operational costs and reduced sensitivity for trace element detection. The inability to dynamically adjust plasma conditions in response to changing sample composition further compounds these issues.
Space-charge effects in the ion beam represent another persistent challenge, particularly affecting low-mass analytes in the presence of high-mass matrix elements. Current lens systems struggle to compensate for these effects across the entire mass range, leading to compromised detection limits for certain elements.
Temperature gradients within the plasma create zones of varying ionization efficiency, with current torch designs unable to establish truly homogeneous conditions. This results in element-dependent sensitivity variations that complicate quantitative analysis, especially for multi-element determinations spanning the periodic table.
Plasma cooling interfaces, while improved in recent years, still introduce significant ion losses during the transition from atmospheric pressure to the vacuum system. The formation of polyatomic interferences during this cooling process remains inadequately addressed by current hardware solutions, necessitating complex mathematical corrections or chemical separation procedures that add complexity to analytical workflows.
Current Plasma Parameter Control Methodologies
01 Sample introduction and ionization techniques
Various sample introduction and ionization techniques can significantly enhance ICP-MS sensitivity. These include improved nebulizers, spray chambers, and plasma torch designs that increase ionization efficiency and reduce sample loss. Optimized sample introduction systems can minimize matrix effects and improve analyte transport to the plasma, resulting in lower detection limits and better sensitivity for trace element analysis.- Sample introduction and ionization techniques: Various sample introduction and ionization techniques can significantly enhance ICP-MS sensitivity. These include improved nebulizers, spray chambers, and plasma torch designs that increase sample transport efficiency and ionization. Optimized sample introduction systems reduce sample loss and improve the consistency of ion generation, leading to better detection limits and overall sensitivity for trace element analysis.
- Interface design and ion transmission optimization: The interface between the plasma and mass analyzer is critical for ICP-MS sensitivity. Innovations in sampler and skimmer cone designs, ion optics, and vacuum systems improve ion extraction and transmission efficiency. Advanced ion focusing techniques and reduced space-charge effects help maintain signal integrity, particularly for low-abundance elements, resulting in enhanced sensitivity across the mass range.
- Mass analyzer and detector enhancements: Improvements in mass analyzer technology and detector systems directly impact ICP-MS sensitivity. High-performance quadrupole, time-of-flight, and sector-field mass analyzers offer better mass resolution and transmission. Advanced detector systems with extended dynamic range, faster response times, and lower background noise contribute to improved detection limits and overall instrument sensitivity for trace element analysis.
- Sample preparation and preconcentration methods: Specialized sample preparation techniques can enhance effective ICP-MS sensitivity. Methods such as matrix separation, preconcentration, and chelation can increase analyte concentration while reducing matrix interferences. These approaches effectively lower detection limits by improving signal-to-noise ratios, especially for complex samples where direct analysis would be limited by matrix effects or dilution factors.
- Interference reduction and signal enhancement strategies: Various strategies can be employed to reduce interferences and enhance signal quality in ICP-MS. These include collision/reaction cell technologies, cool plasma techniques, and mathematical correction models that minimize polyatomic and isobaric interferences. Signal enhancement can be achieved through optimized plasma conditions, improved gas flows, and RF power settings, all contributing to better sensitivity and lower detection limits.
02 Interface design and ion transmission optimization
The interface between the plasma and the mass analyzer is critical for ICP-MS sensitivity. Innovations in interface design, including improved sampling cones, skimmer cones, and ion optics, enhance ion extraction and transmission efficiency. Advanced ion focusing systems and reduced interface pressures help minimize ion loss during transmission from the plasma to the mass analyzer, resulting in improved sensitivity for trace element detection.Expand Specific Solutions03 Mass analyzer and detector enhancements
Improvements in mass analyzer technology and detector systems play a crucial role in enhancing ICP-MS sensitivity. High-resolution mass analyzers, such as quadrupole, time-of-flight, and sector field instruments, offer better separation of analyte ions from interferences. Advanced detector systems with higher gain, lower noise, and wider dynamic range enable detection of lower concentrations of elements, improving overall instrument sensitivity.Expand Specific Solutions04 Interference reduction and removal strategies
Various strategies for reducing or removing interferences can significantly improve ICP-MS sensitivity. These include collision/reaction cell technology, cool plasma techniques, and mathematical correction models. By effectively eliminating or reducing polyatomic and isobaric interferences, these approaches enable more accurate detection of target analytes at lower concentrations, enhancing the overall sensitivity of ICP-MS analysis.Expand Specific Solutions05 Sample preparation and preconcentration methods
Advanced sample preparation and preconcentration techniques can significantly enhance ICP-MS sensitivity. These include matrix separation, analyte extraction, and preconcentration methods that increase the effective concentration of target analytes while reducing matrix effects. Techniques such as solid-phase extraction, cloud point extraction, and chelation can improve detection limits by orders of magnitude, enabling ultra-trace analysis of elements in complex samples.Expand Specific Solutions
Leading Manufacturers and Research Institutions in ICP-MS
The ICP-MS sensitivity enhancement market is currently in a growth phase, with increasing demand driven by applications in environmental monitoring, pharmaceuticals, and semiconductor manufacturing. The market size is expanding steadily as analytical requirements become more stringent across industries. Technologically, established players like Agilent Technologies, Thermo Fisher Scientific, and Shimadzu lead with mature solutions, while innovative companies such as Kimia Analytics are disrupting the space with patented torch designs offering superior power density and matrix tolerance. Research institutions including CNRS and University of Tokyo collaborate with industry leaders to advance fundamental plasma physics understanding. The competitive landscape shows a blend of established analytical instrumentation companies and specialized ICP-MS technology providers, with emerging differentiation in sample introduction systems (Elemental Scientific) and specialized applications for semiconductor manufacturing (Micron Technology, Tokyo Electron).
Agilent Technologies, Inc.
Technical Solution: Agilent has developed advanced ICP-MS systems featuring Ultra High Matrix Introduction (UHMI) technology that significantly enhances plasma robustness for high-matrix samples. Their ICP-MS instruments incorporate optimized plasma generation systems with precisely controlled RF generators operating at 27 MHz, allowing for fine-tuning of plasma conditions. Agilent's proprietary ShieldTorch System creates a plasma potential control that reduces matrix effects and enhances sensitivity for challenging elements. The company has implemented automated plasma condition optimization through intelligent software algorithms that adjust torch position, gas flows, and RF power based on sample characteristics. Their latest systems feature helium collision cell technology that works synergistically with optimized plasma conditions to minimize polyatomic interferences while maintaining high sensitivity[1][2]. Agilent has also pioneered the use of temperature-controlled spray chambers to stabilize sample introduction and improve reproducibility of measurements at ultra-trace levels.
Strengths: Superior matrix tolerance allowing analysis of complex samples without significant dilution; excellent sensitivity for difficult elements like As, Se, and Hg; comprehensive software control for automated optimization. Weaknesses: Higher power consumption compared to some competitors; requires more extensive training for operators to fully utilize advanced plasma optimization features.
Thermo Fisher Scientific (Bremen) GmbH
Technical Solution: Thermo Fisher Scientific has developed the iCAP RQ ICP-MS platform featuring their proprietary QCell collision/reaction cell technology that works in tandem with optimized plasma conditions to achieve superior sensitivity. Their approach focuses on plasma stability through digital RF generators that maintain precise control over plasma formation and energy transfer. The company has implemented a unique plasma interface design with a recessed plasma torch and optimized sampling cone geometry that enhances ion extraction efficiency while reducing matrix effects. Thermo Fisher's systems incorporate intelligent plasma monitoring with real-time adjustment capabilities that automatically respond to changing sample matrices. Their Enhanced Matrix Tolerance (EMT) technology allows for direct analysis of samples containing up to 25% total dissolved solids while maintaining sensitivity[3]. The company has also developed specialized low-flow nebulizers and cyclonic spray chambers that improve sample introduction efficiency, working synergistically with optimized plasma conditions to enhance overall sensitivity for trace element detection.
Strengths: Exceptional plasma stability across varying matrices; superior interference removal capabilities while maintaining sensitivity; intuitive software for plasma condition optimization. Weaknesses: Higher initial investment cost compared to some competitors; optimization process can be complex for non-expert users; requires regular maintenance of interface components to maintain optimal performance.
Key Innovations in Plasma Interface Design
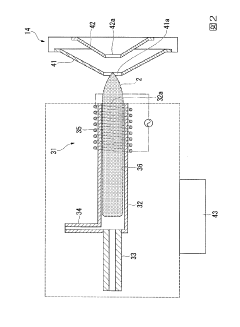
Method and apparatus to increase sensitivity of inductively coupled plasma mass spectrometry
PatentPendingUS20250226199A1
Innovation
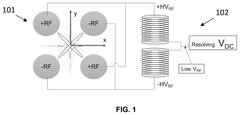
- Implementing time-varying RF and DC fields in the ion guide to selectively eject unwanted ions, reduce charge density, and enhance the transmission of desired ions by applying auxiliary excitation methods such as resolving DC potential, RF dipolar/quadrupolar fields, and segmenting the ion guide to separate and trap ions of interest.
Inductively coupled plasma mass spectrometry
PatentInactiveJP2020027038A
Innovation
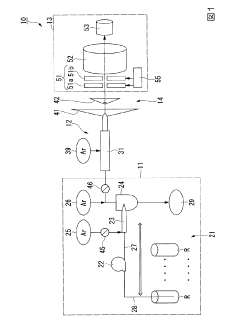
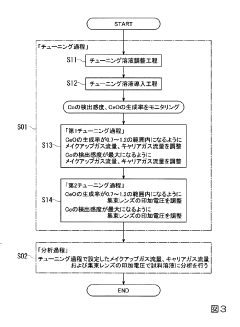
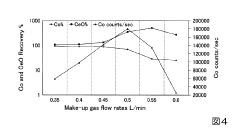
- The method involves tuning the ICP-MS system using a sample solution with a high-concentration acid matrix as a tuning liquid, adjusting carrier gas flow rates and focusing lens settings to control the production rate of coexisting element oxides within a specific range, thereby maximizing detection sensitivity.
Environmental and Sample Matrix Interference Mitigation
Environmental and sample matrix interferences represent significant challenges in ICP-MS analysis, often limiting sensitivity and accuracy. These interferences can originate from various sources including atmospheric contaminants, sample preparation reagents, and the sample matrix itself. The complexity of environmental samples particularly exacerbates these challenges, necessitating robust mitigation strategies.
Matrix-induced interferences typically manifest as signal suppression or enhancement, affecting quantification reliability. Common interfering species include polyatomic ions formed from combinations of argon, oxygen, nitrogen, and elements present in the sample matrix. For instance, 40Ar16O+ can interfere with 56Fe+ determination, while 40Ar35Cl+ affects 75As+ measurements.
Several approaches have been developed to mitigate these interferences while maintaining optimal plasma conditions. Collision/reaction cell technology represents a major advancement, employing gases like helium, hydrogen, or ammonia to selectively remove polyatomic interferences through kinetic energy discrimination or chemical reactions. This technology has proven particularly effective for environmental samples containing high levels of chloride, sulfate, or dissolved solids.
Mathematical correction methods offer another solution, utilizing algorithms to subtract the contribution of known interferences based on natural isotopic abundances. However, these methods require precise knowledge of all potential interfering species and their formation rates under specific plasma conditions.
Sample preparation optimization serves as a preventive measure against matrix interferences. Techniques such as matrix matching, internal standardization, and standard addition help compensate for matrix effects. Additionally, chromatographic separation methods can physically remove interfering components prior to ICP-MS analysis, though this increases analytical complexity and time requirements.
High-resolution ICP-MS instruments provide another approach by physically separating analytes from interferences based on slight mass differences. While effective, these instruments are considerably more expensive and complex to operate than conventional quadrupole systems.
Plasma condition optimization plays a crucial role in interference management. Adjusting parameters such as RF power, nebulizer gas flow, and sampling depth can significantly reduce certain interferences. For example, increasing plasma temperature through higher RF power can break down some polyatomic species, while careful tuning of the plasma sampling position can minimize oxide formation.
Recent advancements include the development of specialized sample introduction systems designed to reduce matrix loading into the plasma, such as aerosol dilution devices and desolvating nebulizers. These technologies effectively decrease the formation of oxide and hydroxide interferences while maintaining sensitivity for target analytes.
Matrix-induced interferences typically manifest as signal suppression or enhancement, affecting quantification reliability. Common interfering species include polyatomic ions formed from combinations of argon, oxygen, nitrogen, and elements present in the sample matrix. For instance, 40Ar16O+ can interfere with 56Fe+ determination, while 40Ar35Cl+ affects 75As+ measurements.
Several approaches have been developed to mitigate these interferences while maintaining optimal plasma conditions. Collision/reaction cell technology represents a major advancement, employing gases like helium, hydrogen, or ammonia to selectively remove polyatomic interferences through kinetic energy discrimination or chemical reactions. This technology has proven particularly effective for environmental samples containing high levels of chloride, sulfate, or dissolved solids.
Mathematical correction methods offer another solution, utilizing algorithms to subtract the contribution of known interferences based on natural isotopic abundances. However, these methods require precise knowledge of all potential interfering species and their formation rates under specific plasma conditions.
Sample preparation optimization serves as a preventive measure against matrix interferences. Techniques such as matrix matching, internal standardization, and standard addition help compensate for matrix effects. Additionally, chromatographic separation methods can physically remove interfering components prior to ICP-MS analysis, though this increases analytical complexity and time requirements.
High-resolution ICP-MS instruments provide another approach by physically separating analytes from interferences based on slight mass differences. While effective, these instruments are considerably more expensive and complex to operate than conventional quadrupole systems.
Plasma condition optimization plays a crucial role in interference management. Adjusting parameters such as RF power, nebulizer gas flow, and sampling depth can significantly reduce certain interferences. For example, increasing plasma temperature through higher RF power can break down some polyatomic species, while careful tuning of the plasma sampling position can minimize oxide formation.
Recent advancements include the development of specialized sample introduction systems designed to reduce matrix loading into the plasma, such as aerosol dilution devices and desolvating nebulizers. These technologies effectively decrease the formation of oxide and hydroxide interferences while maintaining sensitivity for target analytes.
Validation and Standardization Protocols for Enhanced Sensitivity
To establish robust validation and standardization protocols for enhanced ICP-MS sensitivity, systematic approaches must be implemented across laboratory environments. These protocols serve as the foundation for ensuring that sensitivity improvements achieved through optimized plasma conditions are reproducible, reliable, and scientifically sound.
The development of validation protocols begins with the establishment of baseline performance metrics. This includes determining limits of detection (LOD), limits of quantification (LOQ), linear dynamic range, and signal stability under standard operating conditions. These baseline measurements provide reference points against which sensitivity enhancements can be objectively evaluated and quantified.
Statistical validation frameworks must be incorporated to assess the significance of sensitivity improvements. This involves the application of appropriate statistical tests such as t-tests, ANOVA, or more advanced multivariate analysis techniques to determine whether observed sensitivity enhancements represent statistically significant improvements over baseline performance. Rigorous statistical validation helps distinguish genuine sensitivity enhancements from random variations or instrumental noise.
Standardized sample preparation procedures constitute another critical component of validation protocols. These procedures must address potential sources of contamination, matrix effects, and analyte loss during sample handling. Standardization in this area ensures that sensitivity improvements attributed to plasma optimization are not confounded by variations in sample preparation techniques.
Inter-laboratory comparison studies represent a higher level of validation necessary for widespread adoption of optimized plasma conditions. These studies involve multiple laboratories implementing identical protocols to verify that sensitivity enhancements are reproducible across different instruments, operators, and laboratory environments. Such collaborative efforts strengthen the credibility of proposed optimization strategies.
Quality control measures must be integrated into daily operational procedures to maintain enhanced sensitivity levels. This includes regular analysis of certified reference materials, internal standards, and control samples to monitor instrument performance and detect any drift in sensitivity. Automated quality control algorithms can be implemented to flag deviations from expected performance metrics.
Documentation standards for reporting enhanced sensitivity results should specify required metadata, including detailed plasma conditions, instrument configurations, and calibration procedures. Comprehensive documentation facilitates reproducibility and enables meaningful comparison of results across different studies and laboratories.
Regulatory considerations must also be addressed in validation protocols, particularly for applications in clinical diagnostics, environmental monitoring, or food safety where analytical results have significant implications for public health and regulatory compliance. Protocols should align with relevant regulatory frameworks such as ISO/IEC 17025, FDA guidelines, or EPA methods as appropriate.
The development of validation protocols begins with the establishment of baseline performance metrics. This includes determining limits of detection (LOD), limits of quantification (LOQ), linear dynamic range, and signal stability under standard operating conditions. These baseline measurements provide reference points against which sensitivity enhancements can be objectively evaluated and quantified.
Statistical validation frameworks must be incorporated to assess the significance of sensitivity improvements. This involves the application of appropriate statistical tests such as t-tests, ANOVA, or more advanced multivariate analysis techniques to determine whether observed sensitivity enhancements represent statistically significant improvements over baseline performance. Rigorous statistical validation helps distinguish genuine sensitivity enhancements from random variations or instrumental noise.
Standardized sample preparation procedures constitute another critical component of validation protocols. These procedures must address potential sources of contamination, matrix effects, and analyte loss during sample handling. Standardization in this area ensures that sensitivity improvements attributed to plasma optimization are not confounded by variations in sample preparation techniques.
Inter-laboratory comparison studies represent a higher level of validation necessary for widespread adoption of optimized plasma conditions. These studies involve multiple laboratories implementing identical protocols to verify that sensitivity enhancements are reproducible across different instruments, operators, and laboratory environments. Such collaborative efforts strengthen the credibility of proposed optimization strategies.
Quality control measures must be integrated into daily operational procedures to maintain enhanced sensitivity levels. This includes regular analysis of certified reference materials, internal standards, and control samples to monitor instrument performance and detect any drift in sensitivity. Automated quality control algorithms can be implemented to flag deviations from expected performance metrics.
Documentation standards for reporting enhanced sensitivity results should specify required metadata, including detailed plasma conditions, instrument configurations, and calibration procedures. Comprehensive documentation facilitates reproducibility and enables meaningful comparison of results across different studies and laboratories.
Regulatory considerations must also be addressed in validation protocols, particularly for applications in clinical diagnostics, environmental monitoring, or food safety where analytical results have significant implications for public health and regulatory compliance. Protocols should align with relevant regulatory frameworks such as ISO/IEC 17025, FDA guidelines, or EPA methods as appropriate.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!