Achieving High Purity Sample Prep for ICP-MS Analysis
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ICP-MS Sample Prep Evolution and Objectives
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has evolved significantly since its commercial introduction in the early 1980s. This analytical technique combines the high-temperature ICP source with a mass spectrometer, enabling precise detection of metals and several non-metals at concentrations as low as one part per trillion. The evolution of ICP-MS sample preparation techniques has been driven by increasing demands for lower detection limits, higher accuracy, and broader applicability across various industries including environmental monitoring, pharmaceutical analysis, food safety, and semiconductor manufacturing.
Early sample preparation methods for ICP-MS were relatively rudimentary, often involving simple acid digestion procedures that were prone to contamination and incomplete dissolution of complex matrices. The 1990s saw significant advancements with the introduction of microwave-assisted digestion systems, which dramatically improved sample throughput and digestion efficiency while reducing contamination risks.
The 2000s marked a turning point with the development of clean room technologies specifically designed for ultra-trace analysis. These facilities, coupled with high-purity reagents and specialized equipment, enabled researchers to achieve previously unattainable detection limits. Concurrently, automated sample preparation systems emerged, offering improved reproducibility and reduced human error.
Recent technological innovations have focused on miniaturization and integration of sample preparation steps. Microfluidic systems and lab-on-chip technologies have shown promising results in reducing sample and reagent volumes while maintaining or even improving analytical performance. Additionally, novel extraction and preconcentration techniques such as solid-phase extraction (SPE), cloud point extraction (CPE), and dispersive liquid-liquid microextraction (DLLME) have expanded the capabilities of ICP-MS analysis for complex matrices.
The primary objective of modern ICP-MS sample preparation is to achieve complete dissolution of the analytes while minimizing contamination and matrix effects. This involves converting solid samples into homogeneous solutions suitable for nebulization, removing or reducing interfering matrix components, and ensuring stability of the prepared solutions until analysis. Additionally, sample preparation aims to preconcentrate target analytes when necessary to meet required detection limits.
Current research objectives focus on developing environmentally friendly preparation methods that reduce hazardous waste generation while maintaining analytical performance. There is also significant interest in creating universal sample preparation protocols that can be applied across diverse sample types with minimal modification. Furthermore, the integration of artificial intelligence and machine learning approaches for optimizing sample preparation parameters represents an emerging frontier in this field.
As regulatory requirements become increasingly stringent across industries, the evolution of ICP-MS sample preparation continues to be driven by the need for higher purity, greater efficiency, and enhanced reproducibility to ensure reliable ultra-trace elemental analysis.
Early sample preparation methods for ICP-MS were relatively rudimentary, often involving simple acid digestion procedures that were prone to contamination and incomplete dissolution of complex matrices. The 1990s saw significant advancements with the introduction of microwave-assisted digestion systems, which dramatically improved sample throughput and digestion efficiency while reducing contamination risks.
The 2000s marked a turning point with the development of clean room technologies specifically designed for ultra-trace analysis. These facilities, coupled with high-purity reagents and specialized equipment, enabled researchers to achieve previously unattainable detection limits. Concurrently, automated sample preparation systems emerged, offering improved reproducibility and reduced human error.
Recent technological innovations have focused on miniaturization and integration of sample preparation steps. Microfluidic systems and lab-on-chip technologies have shown promising results in reducing sample and reagent volumes while maintaining or even improving analytical performance. Additionally, novel extraction and preconcentration techniques such as solid-phase extraction (SPE), cloud point extraction (CPE), and dispersive liquid-liquid microextraction (DLLME) have expanded the capabilities of ICP-MS analysis for complex matrices.
The primary objective of modern ICP-MS sample preparation is to achieve complete dissolution of the analytes while minimizing contamination and matrix effects. This involves converting solid samples into homogeneous solutions suitable for nebulization, removing or reducing interfering matrix components, and ensuring stability of the prepared solutions until analysis. Additionally, sample preparation aims to preconcentrate target analytes when necessary to meet required detection limits.
Current research objectives focus on developing environmentally friendly preparation methods that reduce hazardous waste generation while maintaining analytical performance. There is also significant interest in creating universal sample preparation protocols that can be applied across diverse sample types with minimal modification. Furthermore, the integration of artificial intelligence and machine learning approaches for optimizing sample preparation parameters represents an emerging frontier in this field.
As regulatory requirements become increasingly stringent across industries, the evolution of ICP-MS sample preparation continues to be driven by the need for higher purity, greater efficiency, and enhanced reproducibility to ensure reliable ultra-trace elemental analysis.
Market Demand for High-Purity Analytical Solutions
The global market for high-purity analytical solutions has experienced significant growth in recent years, driven by increasing demands for precise elemental analysis across various industries. The ICP-MS (Inductively Coupled Plasma Mass Spectrometry) market specifically has expanded at a compound annual growth rate of 7.2% between 2018 and 2023, reflecting the growing need for ultra-trace element detection capabilities.
Pharmaceutical and biotechnology sectors represent the largest market segment, accounting for approximately 32% of the total demand for high-purity sample preparation solutions. This dominance stems from stringent regulatory requirements for product safety and quality control, particularly in drug development and manufacturing processes where even trace contaminants can impact efficacy and safety profiles.
Environmental monitoring applications constitute the second-largest market segment, driven by increasingly strict regulations on pollutants and heavy metals in water, soil, and air samples. Government agencies and environmental testing laboratories require sample preparation methods that can reliably detect contaminants at parts-per-trillion levels, creating sustained demand for advanced purification technologies.
The semiconductor and electronics manufacturing industry has emerged as the fastest-growing segment, with demand increasing by nearly 9% annually. As chip architectures continue to shrink, the tolerance for metallic impurities approaches zero, necessitating ultra-pure sample preparation methods for quality control and failure analysis.
Clinical diagnostics and healthcare applications represent another significant market driver, particularly for trace element analysis in biological samples. The growing focus on personalized medicine and biomarker discovery has increased the need for contamination-free sample preparation to ensure accurate detection of elements at physiologically relevant concentrations.
Geographically, North America and Europe currently dominate the market for high-purity analytical solutions, collectively accounting for over 60% of global demand. However, the Asia-Pacific region is experiencing the most rapid growth, particularly in China, Japan, and South Korea, where expanding pharmaceutical, electronics, and environmental testing sectors are driving adoption of advanced analytical technologies.
Market research indicates that end-users are increasingly prioritizing complete sample preparation solutions rather than individual components, with integrated systems that minimize contamination risks throughout the analytical workflow. This trend has prompted major analytical instrument manufacturers to expand their product portfolios to include comprehensive sample preparation technologies alongside their core instrumentation offerings.
Pharmaceutical and biotechnology sectors represent the largest market segment, accounting for approximately 32% of the total demand for high-purity sample preparation solutions. This dominance stems from stringent regulatory requirements for product safety and quality control, particularly in drug development and manufacturing processes where even trace contaminants can impact efficacy and safety profiles.
Environmental monitoring applications constitute the second-largest market segment, driven by increasingly strict regulations on pollutants and heavy metals in water, soil, and air samples. Government agencies and environmental testing laboratories require sample preparation methods that can reliably detect contaminants at parts-per-trillion levels, creating sustained demand for advanced purification technologies.
The semiconductor and electronics manufacturing industry has emerged as the fastest-growing segment, with demand increasing by nearly 9% annually. As chip architectures continue to shrink, the tolerance for metallic impurities approaches zero, necessitating ultra-pure sample preparation methods for quality control and failure analysis.
Clinical diagnostics and healthcare applications represent another significant market driver, particularly for trace element analysis in biological samples. The growing focus on personalized medicine and biomarker discovery has increased the need for contamination-free sample preparation to ensure accurate detection of elements at physiologically relevant concentrations.
Geographically, North America and Europe currently dominate the market for high-purity analytical solutions, collectively accounting for over 60% of global demand. However, the Asia-Pacific region is experiencing the most rapid growth, particularly in China, Japan, and South Korea, where expanding pharmaceutical, electronics, and environmental testing sectors are driving adoption of advanced analytical technologies.
Market research indicates that end-users are increasingly prioritizing complete sample preparation solutions rather than individual components, with integrated systems that minimize contamination risks throughout the analytical workflow. This trend has prompted major analytical instrument manufacturers to expand their product portfolios to include comprehensive sample preparation technologies alongside their core instrumentation offerings.
Current Challenges in ICP-MS Sample Preparation
Despite significant advancements in ICP-MS technology, sample preparation remains a critical bottleneck in achieving accurate and reliable analytical results. Current sample preparation methods face several persistent challenges that impact the quality and efficiency of analyses. Contamination issues continue to plague the preparation process, with sources ranging from laboratory environments and reagents to containers and instruments. Even trace contaminants can significantly skew results when working at parts-per-billion or parts-per-trillion detection levels.
Matrix effects represent another substantial challenge, as complex sample matrices can cause signal suppression or enhancement, leading to inaccurate quantification. The diversity of sample types encountered in modern applications—from environmental samples to biological tissues and advanced materials—requires increasingly sophisticated preparation approaches tailored to specific matrices.
Incomplete dissolution remains problematic, particularly for samples containing refractory elements or complex mineral structures. Traditional acid digestion methods often fail to completely solubilize certain compounds, resulting in analyte loss and underestimation of element concentrations. This is especially challenging when analyzing geological samples, ceramics, or advanced composite materials.
Time and resource intensity of current preparation methods create operational inefficiencies. Many established protocols require lengthy digestion times, multiple processing steps, and significant analyst intervention, limiting sample throughput and increasing costs. The environmental and safety concerns associated with conventional sample preparation techniques also present challenges, as they typically involve concentrated acids, high temperatures, and potentially hazardous reagents.
Standardization and reproducibility issues persist across laboratories, with slight variations in preparation protocols potentially leading to significant differences in analytical outcomes. This hampers inter-laboratory comparisons and method validation efforts, particularly for complex sample types.
Automation integration remains incomplete in many laboratory settings. While automated sample preparation systems exist, their adoption has been limited by cost constraints, flexibility limitations, and integration challenges with existing workflows. The gap between manual preparation methods and fully automated solutions continues to impact laboratory efficiency and result consistency.
Emerging sample types from advanced manufacturing, nanotechnology, and biological research present novel challenges that existing preparation methods are ill-equipped to address, necessitating continuous innovation in sample preparation techniques to keep pace with evolving analytical demands.
Matrix effects represent another substantial challenge, as complex sample matrices can cause signal suppression or enhancement, leading to inaccurate quantification. The diversity of sample types encountered in modern applications—from environmental samples to biological tissues and advanced materials—requires increasingly sophisticated preparation approaches tailored to specific matrices.
Incomplete dissolution remains problematic, particularly for samples containing refractory elements or complex mineral structures. Traditional acid digestion methods often fail to completely solubilize certain compounds, resulting in analyte loss and underestimation of element concentrations. This is especially challenging when analyzing geological samples, ceramics, or advanced composite materials.
Time and resource intensity of current preparation methods create operational inefficiencies. Many established protocols require lengthy digestion times, multiple processing steps, and significant analyst intervention, limiting sample throughput and increasing costs. The environmental and safety concerns associated with conventional sample preparation techniques also present challenges, as they typically involve concentrated acids, high temperatures, and potentially hazardous reagents.
Standardization and reproducibility issues persist across laboratories, with slight variations in preparation protocols potentially leading to significant differences in analytical outcomes. This hampers inter-laboratory comparisons and method validation efforts, particularly for complex sample types.
Automation integration remains incomplete in many laboratory settings. While automated sample preparation systems exist, their adoption has been limited by cost constraints, flexibility limitations, and integration challenges with existing workflows. The gap between manual preparation methods and fully automated solutions continues to impact laboratory efficiency and result consistency.
Emerging sample types from advanced manufacturing, nanotechnology, and biological research present novel challenges that existing preparation methods are ill-equipped to address, necessitating continuous innovation in sample preparation techniques to keep pace with evolving analytical demands.
State-of-the-Art Sample Preparation Techniques
01 Digestion and dissolution methods for ICP-MS sample preparation
Various digestion and dissolution methods are employed to prepare samples for ICP-MS analysis to ensure accurate purity determination. These include acid digestion using reagents like nitric acid, hydrochloric acid, or hydrofluoric acid, as well as microwave-assisted digestion techniques. The choice of digestion method depends on the sample matrix and the elements to be analyzed. Proper dissolution ensures complete breakdown of the sample matrix, allowing for accurate quantification of trace elements and impurities.- Digestion and dissolution methods for ICP-MS sample preparation: Various digestion and dissolution methods are employed to prepare samples for ICP-MS analysis to ensure accurate purity determination. These include acid digestion using reagents like nitric acid, hydrochloric acid, or hydrofluoric acid, as well as microwave-assisted digestion techniques. The choice of digestion method depends on the sample matrix, with complete dissolution being critical for accurate elemental analysis. These preparation techniques help break down complex matrices into solutions suitable for ICP-MS analysis.
- Automated sample preparation systems for ICP-MS: Automated systems have been developed to streamline and standardize the sample preparation process for ICP-MS analysis. These systems include robotic handling, automated dilution, and integrated sample processing workflows that minimize human error and contamination risks. Automation improves reproducibility, throughput, and precision in sample preparation, which is crucial for high-purity determinations. These systems often incorporate multiple preparation steps including weighing, digestion, dilution, and introduction to the ICP-MS instrument.
- Preconcentration and separation techniques for trace element analysis: Preconcentration and separation techniques are essential for enhancing the detection limits of trace elements in high-purity samples. Methods such as solid-phase extraction, ion exchange, chelation, and precipitation are used to isolate and concentrate target analytes from complex matrices. These techniques help eliminate matrix interferences and improve the signal-to-noise ratio in ICP-MS analysis, allowing for more accurate determination of impurities at ultra-trace levels in high-purity materials.
- Clean room protocols and contamination control in sample preparation: Clean room protocols and contamination control measures are critical for accurate purity analysis by ICP-MS. These include the use of ultra-pure reagents, specialized cleaning procedures for labware, controlled environment processing, and blank correction methodologies. Minimizing environmental contamination during sample preparation is essential, particularly when analyzing high-purity materials where even trace contaminants can significantly impact results. Proper handling techniques and dedicated clean facilities help ensure the integrity of analytical data.
- Specialized sample preparation for different material types: Different material types require specialized sample preparation approaches for ICP-MS purity analysis. For example, semiconductor materials may require specific acid combinations to achieve complete dissolution, while biological samples might need enzymatic digestion or ashing procedures. Metallurgical samples often require fusion techniques, and nanoparticles may need specific dispersion methods. These tailored preparation techniques ensure that the sample is appropriately processed based on its physical and chemical properties to achieve accurate purity determination.
02 Automated sample preparation systems for ICP-MS
Automated systems have been developed to streamline the sample preparation process for ICP-MS analysis. These systems include robotic handling, automated dilution, and integrated sample introduction mechanisms. Automation reduces human error, improves reproducibility, and increases throughput in analytical laboratories. These systems can handle multiple samples simultaneously and perform complex preparation steps with high precision, ensuring consistent results for purity analysis.Expand Specific Solutions03 Filtration and separation techniques for sample purification
Prior to ICP-MS analysis, samples often require filtration and separation to remove particulates and interfering substances. Techniques include membrane filtration, centrifugation, and chromatographic separation. These methods help to isolate the analytes of interest and remove matrix components that could interfere with the measurement. Proper filtration and separation enhance the accuracy of purity determinations by reducing matrix effects and spectral interferences in the ICP-MS analysis.Expand Specific Solutions04 Calibration standards and reference materials for ICP-MS purity analysis
Preparation of calibration standards and reference materials is crucial for accurate quantification in ICP-MS purity analysis. This involves creating solutions with known concentrations of target elements, internal standards for drift correction, and matrix-matched calibration curves. The use of certified reference materials ensures traceability and validates the analytical method. Proper calibration compensates for instrument variations and matrix effects, leading to more reliable purity determinations.Expand Specific Solutions05 Specialized sample preparation for challenging matrices
Certain sample types require specialized preparation techniques due to their complex matrices or physical properties. These include high-purity metals, semiconductors, pharmaceuticals, and environmental samples. Specialized approaches may involve fusion techniques, vapor phase digestion, or dilution protocols specific to the sample type. These methods are designed to overcome challenges such as incomplete dissolution, volatile element loss, or contamination risks, ensuring accurate purity assessment of difficult sample matrices.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The ICP-MS sample preparation market is in a growth phase, characterized by increasing demand for high-purity analytical solutions across pharmaceutical, environmental, and industrial sectors. The global market size is expanding steadily, driven by stringent regulatory requirements and technological advancements. Leading players like Thermo Fisher Scientific, Agilent Technologies, and Waters Technology dominate with comprehensive solution portfolios, while PerkinElmer, Shimadzu, and Elemental Scientific offer specialized technologies. The competitive landscape shows varying degrees of technical maturity, with established companies focusing on integrated systems and automation, while newer entrants like Revvity Health Sciences and Standard BioTools target niche applications. Research institutions including University of Tokyo and Swiss Federal Institute of Technology contribute significantly to advancing sample preparation methodologies for challenging matrices.
Thermo Fisher Scientific (Bremen) GmbH
Technical Solution: Thermo Fisher Scientific has developed comprehensive sample preparation solutions for ICP-MS analysis focusing on automated digestion systems and specialized reagents. Their PrepFAST automated sample preparation system integrates inline dilution, internal standard addition, and calibration standard preparation to minimize contamination risks. The company's advanced microwave digestion technology enables complete matrix decomposition while maintaining sample integrity in a closed vessel environment, significantly reducing the risk of volatile element loss and cross-contamination[1]. Their specialized Clean Chemistry protocols employ ultra-pure acids and reagents with sub-ppt level impurities, ensuring minimal background interference. Additionally, Thermo Fisher has pioneered specialized membrane filtration technologies that selectively remove matrix components while retaining analytes of interest, particularly beneficial for complex biological and environmental samples requiring high purity preparation[3].
Strengths: Comprehensive integration with their ICP-MS instruments provides seamless workflow solutions; advanced automation reduces human error and contamination risks; specialized consumables ensure consistent results. Weaknesses: Higher cost compared to manual preparation methods; proprietary consumables create vendor lock-in; some automated systems have limited flexibility for highly specialized applications.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has developed the Integrated Sample Introduction System (ISIS 3) specifically designed to enhance sample preparation purity for ICP-MS analysis. This system employs a vacuum-controlled discrete sampling approach that significantly reduces matrix effects and memory interference between samples. Their technology incorporates a high-efficiency nebulizer system with programmable rinse cycles that demonstrably reduces carryover to sub-ppb levels even for sticky elements like mercury and boron[2]. Agilent's sample preparation workflow includes their patented HMI (High Matrix Introduction) technology that dilutes the sample aerosol with argon gas before it enters the plasma, effectively handling samples with up to 3% total dissolved solids without physical dilution, thereby maintaining sample integrity while reducing matrix effects[4]. Their OpenLAB software platform provides comprehensive sample tracking and preparation documentation to ensure regulatory compliance and data integrity throughout the preparation process.
Strengths: Exceptional matrix tolerance allowing direct analysis of complex samples; reduced sample preparation time while maintaining high purity; integrated software tracking ensures complete chain of custody. Weaknesses: Higher gas consumption increases operational costs; requires specialized training for optimal performance; some complex matrices still require offline preparation steps despite advanced technology.
Critical Patents and Innovations in Contamination Control
Sample preparation method and detection method for determining impurities in ultra-pure aluminum by utilizing ICP-MS
PatentInactiveCN113504291A
Innovation
- ICP-MS technology is used combined with specific sample preparation methods, including drilling aluminum wire, cleaning, drying, dissolving with aqua regia, and diluting to constant volume through the standard curve method to avoid contamination and matrix effects, and achieve rapid quantitative determination of multiple elements.
Inductively coupled plasma mass spectrometry
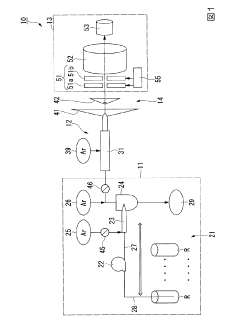
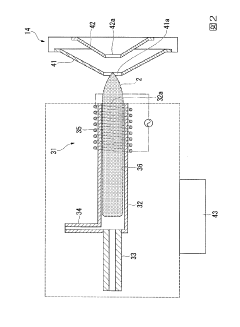
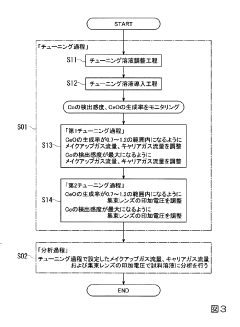
PatentInactiveJP2020027038A
Innovation
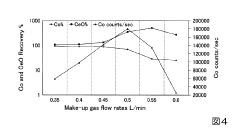
- The method involves tuning the ICP-MS system using a sample solution with a high-concentration acid matrix as a tuning liquid, adjusting carrier gas flow rates and focusing lens settings to control the production rate of coexisting element oxides within a specific range, thereby maximizing detection sensitivity.
Contamination Sources and Mitigation Strategies
Contamination in ICP-MS analysis can originate from multiple sources throughout the sample preparation process, significantly impacting analytical results. Laboratory environments represent a primary contamination source, with airborne particles, dust, and aerosols containing trace elements that can compromise sample integrity. Modern clean room facilities with HEPA filtration systems and positive pressure environments have become essential for ultra-trace analysis, reducing ambient contamination by several orders of magnitude.
Reagent purity constitutes another critical factor, as even high-grade chemicals may contain trace impurities at ppb or ppt levels. The industry has responded with the development of ultra-pure reagents specifically designed for trace element analysis, including sub-boiling distilled acids and high-purity water (18.2 MΩ·cm) produced through multi-stage purification systems. These specialized reagents can reduce background contamination by up to 100-fold compared to analytical grade alternatives.
Laboratory equipment and consumables present significant contamination risks through leaching, adsorption, and direct contact. Materials selection has evolved to favor PTFE, PFA, and quartz for critical sample handling components due to their exceptional chemical resistance and low trace element content. Single-use consumables manufactured under controlled conditions have become standard practice for ultra-trace work, eliminating cross-contamination concerns associated with reusable equipment.
Human operators themselves represent an often-overlooked contamination vector. Skin cells, hair, cosmetics, and clothing fibers can introduce various elements including Na, K, Ca, Mg, and Zn. Comprehensive protocols including specialized clean room garments, powder-free gloves, and strict handling procedures have been developed to minimize anthropogenic contamination during sample preparation.
Effective mitigation strategies employ a multi-layered approach combining preventive measures with contamination monitoring. Blank correction methodologies have advanced significantly, with procedural blanks processed identically to samples enabling accurate quantification of preparation-induced contamination. Statistical process control techniques track blank levels over time, identifying contamination trends before they impact analytical results. Method validation now routinely incorporates contamination assessment through spike recovery experiments and certified reference materials to verify procedure integrity.
Advanced clean techniques including pre-cleaning of containers with dilute acids, sample handling under laminar flow conditions, and minimizing sample transfer steps have become standard practice in high-sensitivity applications. These approaches collectively reduce contamination risks by addressing each potential source systematically, enabling detection limits in the sub-ppt range for many elements.
Reagent purity constitutes another critical factor, as even high-grade chemicals may contain trace impurities at ppb or ppt levels. The industry has responded with the development of ultra-pure reagents specifically designed for trace element analysis, including sub-boiling distilled acids and high-purity water (18.2 MΩ·cm) produced through multi-stage purification systems. These specialized reagents can reduce background contamination by up to 100-fold compared to analytical grade alternatives.
Laboratory equipment and consumables present significant contamination risks through leaching, adsorption, and direct contact. Materials selection has evolved to favor PTFE, PFA, and quartz for critical sample handling components due to their exceptional chemical resistance and low trace element content. Single-use consumables manufactured under controlled conditions have become standard practice for ultra-trace work, eliminating cross-contamination concerns associated with reusable equipment.
Human operators themselves represent an often-overlooked contamination vector. Skin cells, hair, cosmetics, and clothing fibers can introduce various elements including Na, K, Ca, Mg, and Zn. Comprehensive protocols including specialized clean room garments, powder-free gloves, and strict handling procedures have been developed to minimize anthropogenic contamination during sample preparation.
Effective mitigation strategies employ a multi-layered approach combining preventive measures with contamination monitoring. Blank correction methodologies have advanced significantly, with procedural blanks processed identically to samples enabling accurate quantification of preparation-induced contamination. Statistical process control techniques track blank levels over time, identifying contamination trends before they impact analytical results. Method validation now routinely incorporates contamination assessment through spike recovery experiments and certified reference materials to verify procedure integrity.
Advanced clean techniques including pre-cleaning of containers with dilute acids, sample handling under laminar flow conditions, and minimizing sample transfer steps have become standard practice in high-sensitivity applications. These approaches collectively reduce contamination risks by addressing each potential source systematically, enabling detection limits in the sub-ppt range for many elements.
Quality Assurance Standards and Certification Requirements
Quality assurance standards and certification requirements play a pivotal role in ensuring the reliability and accuracy of ICP-MS analysis results. Laboratories conducting high-purity sample preparation must adhere to internationally recognized standards such as ISO/IEC 17025, which establishes general requirements for the competence of testing and calibration laboratories. This standard specifically addresses technical competence, impartiality, and consistent operation of laboratories, making it essential for facilities performing ICP-MS analysis.
The Clean Chemistry Protocol certification, while not universally standardized, has emerged as an important qualification for laboratories specializing in ultra-trace element analysis. This certification focuses on contamination control procedures and requires rigorous documentation of cleaning protocols, reagent purity verification, and environmental monitoring within laboratory spaces.
For pharmaceutical and clinical applications, compliance with Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP) is mandatory. These standards ensure that sample preparation processes are consistently performed according to established protocols and that all procedures are thoroughly documented. The FDA and EMA have established specific guidelines for trace element analysis in pharmaceutical products that must be followed when preparing samples for ICP-MS analysis.
Environmental testing laboratories must comply with standards set by organizations such as the Environmental Protection Agency (EPA) in the United States or equivalent bodies in other regions. Method 200.8 specifically addresses ICP-MS analysis for environmental samples and includes detailed quality control requirements for sample preparation procedures.
Proficiency testing programs, such as those offered by the International Association of Environmental Testing Laboratories (IAETL), provide external validation of a laboratory's sample preparation and analysis capabilities. Regular participation in these programs is often required to maintain accreditation and demonstrate ongoing competence in high-purity sample preparation techniques.
Reference material certification is another critical aspect of quality assurance. Organizations like NIST (USA), BAM (Germany), and NMIJ (Japan) produce certified reference materials that are essential for validating sample preparation methods. These materials have precisely determined elemental compositions and are used to verify the accuracy of the entire analytical process, including the sample preparation steps.
Traceability requirements mandate that all measurements must be traceable to international standards through an unbroken chain of comparisons. This includes the calibration of all equipment used in sample preparation, such as balances, pipettes, and temperature control devices, as well as the validation of reagent purity and blank levels.
The Clean Chemistry Protocol certification, while not universally standardized, has emerged as an important qualification for laboratories specializing in ultra-trace element analysis. This certification focuses on contamination control procedures and requires rigorous documentation of cleaning protocols, reagent purity verification, and environmental monitoring within laboratory spaces.
For pharmaceutical and clinical applications, compliance with Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP) is mandatory. These standards ensure that sample preparation processes are consistently performed according to established protocols and that all procedures are thoroughly documented. The FDA and EMA have established specific guidelines for trace element analysis in pharmaceutical products that must be followed when preparing samples for ICP-MS analysis.
Environmental testing laboratories must comply with standards set by organizations such as the Environmental Protection Agency (EPA) in the United States or equivalent bodies in other regions. Method 200.8 specifically addresses ICP-MS analysis for environmental samples and includes detailed quality control requirements for sample preparation procedures.
Proficiency testing programs, such as those offered by the International Association of Environmental Testing Laboratories (IAETL), provide external validation of a laboratory's sample preparation and analysis capabilities. Regular participation in these programs is often required to maintain accreditation and demonstrate ongoing competence in high-purity sample preparation techniques.
Reference material certification is another critical aspect of quality assurance. Organizations like NIST (USA), BAM (Germany), and NMIJ (Japan) produce certified reference materials that are essential for validating sample preparation methods. These materials have precisely determined elemental compositions and are used to verify the accuracy of the entire analytical process, including the sample preparation steps.
Traceability requirements mandate that all measurements must be traceable to international standards through an unbroken chain of comparisons. This includes the calibration of all equipment used in sample preparation, such as balances, pipettes, and temperature control devices, as well as the validation of reagent purity and blank levels.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!