Optimizing ICP-MS Nebulizer Efficiency for Consistent Spray
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ICP-MS Nebulizer Technology Background and Objectives
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has evolved significantly since its commercial introduction in the early 1980s, becoming an essential analytical technique for trace element analysis across various industries including environmental monitoring, pharmaceuticals, semiconductor manufacturing, and geological research. The nebulizer component, responsible for converting liquid samples into aerosols suitable for plasma ionization, represents a critical element in the ICP-MS analytical chain that directly impacts measurement accuracy, precision, and sensitivity.
The evolution of nebulizer technology has progressed from simple pneumatic designs to sophisticated microfluidic systems. Early concentric and cross-flow nebulizers suffered from inconsistent spray patterns and high sample consumption, while modern designs incorporate advanced materials and precision engineering to enhance performance. This technological progression reflects the growing demand for higher sensitivity, lower detection limits, and improved analytical reliability in increasingly complex sample matrices.
Current market trends indicate a shift toward nebulizers capable of handling difficult sample types, including those with high dissolved solid content, organic solvents, and hydrofluoric acid. Additionally, there is increasing emphasis on nebulizers that can operate efficiently at lower flow rates, reducing sample consumption and waste generation while maintaining analytical performance.
The primary technical objective in nebulizer optimization centers on achieving consistent, reproducible aerosol generation regardless of sample composition or matrix effects. This consistency is fundamental to reliable quantitative analysis, as variations in droplet size distribution and transport efficiency directly affect signal stability and analytical precision. Secondary objectives include minimizing sample consumption, reducing memory effects, preventing clogging, and extending operational lifetimes.
Recent technological innovations have introduced computational fluid dynamics modeling to optimize nebulizer design, resulting in more efficient aerosol generation and transport. Advanced manufacturing techniques, including 3D printing and microfabrication, have enabled novel geometries previously impossible to produce using conventional methods.
The optimization of nebulizer efficiency represents a critical frontier in advancing ICP-MS capabilities, particularly as analytical demands increase for lower detection limits, higher throughput, and more challenging sample types. Achieving consistent spray patterns under varying sample conditions remains a significant challenge that, when addressed, could substantially improve analytical performance across numerous scientific and industrial applications.
This technical investigation aims to comprehensively evaluate current nebulizer technologies, identify key performance limitations, and explore innovative approaches to optimize spray consistency, ultimately enhancing the reliability and sensitivity of ICP-MS analysis across diverse analytical scenarios.
The evolution of nebulizer technology has progressed from simple pneumatic designs to sophisticated microfluidic systems. Early concentric and cross-flow nebulizers suffered from inconsistent spray patterns and high sample consumption, while modern designs incorporate advanced materials and precision engineering to enhance performance. This technological progression reflects the growing demand for higher sensitivity, lower detection limits, and improved analytical reliability in increasingly complex sample matrices.
Current market trends indicate a shift toward nebulizers capable of handling difficult sample types, including those with high dissolved solid content, organic solvents, and hydrofluoric acid. Additionally, there is increasing emphasis on nebulizers that can operate efficiently at lower flow rates, reducing sample consumption and waste generation while maintaining analytical performance.
The primary technical objective in nebulizer optimization centers on achieving consistent, reproducible aerosol generation regardless of sample composition or matrix effects. This consistency is fundamental to reliable quantitative analysis, as variations in droplet size distribution and transport efficiency directly affect signal stability and analytical precision. Secondary objectives include minimizing sample consumption, reducing memory effects, preventing clogging, and extending operational lifetimes.
Recent technological innovations have introduced computational fluid dynamics modeling to optimize nebulizer design, resulting in more efficient aerosol generation and transport. Advanced manufacturing techniques, including 3D printing and microfabrication, have enabled novel geometries previously impossible to produce using conventional methods.
The optimization of nebulizer efficiency represents a critical frontier in advancing ICP-MS capabilities, particularly as analytical demands increase for lower detection limits, higher throughput, and more challenging sample types. Achieving consistent spray patterns under varying sample conditions remains a significant challenge that, when addressed, could substantially improve analytical performance across numerous scientific and industrial applications.
This technical investigation aims to comprehensively evaluate current nebulizer technologies, identify key performance limitations, and explore innovative approaches to optimize spray consistency, ultimately enhancing the reliability and sensitivity of ICP-MS analysis across diverse analytical scenarios.
Market Demand Analysis for High-Efficiency Nebulizers
The global market for high-efficiency nebulizers in ICP-MS (Inductively Coupled Plasma Mass Spectrometry) applications has been experiencing robust growth, driven by increasing demand for precise elemental analysis across multiple industries. Current market estimates value the analytical instrument sector at approximately $5.7 billion, with ICP-MS systems representing a significant segment showing annual growth rates of 6-8%.
Healthcare and pharmaceutical sectors constitute the largest market segment, accounting for nearly 35% of the total demand for high-efficiency nebulizers. This is primarily due to stringent regulatory requirements for trace element analysis in drug development and quality control processes. The need for consistent spray performance directly impacts measurement accuracy, which is critical for compliance with FDA and EMA guidelines.
Environmental monitoring represents the second-largest market segment, contributing about 28% of the demand. Government regulations worldwide are becoming increasingly stringent regarding the detection and quantification of heavy metals and other toxic elements in soil, water, and air samples. This regulatory pressure has created sustained demand for more efficient nebulizer technologies that can deliver lower detection limits and higher reproducibility.
The semiconductor and electronics manufacturing industry has emerged as the fastest-growing segment, with a compound annual growth rate of approximately 9.2%. As miniaturization continues and component purity requirements become more demanding, manufacturers require increasingly sensitive analytical tools for quality control. High-efficiency nebulizers that minimize sample consumption while maximizing signal stability are particularly valued in this sector.
Market research indicates that end-users are willing to pay premium prices for nebulizers that demonstrate superior spray consistency, reduced sample consumption, and resistance to high dissolved solids. A survey of laboratory managers revealed that 73% consider nebulizer performance a critical factor in their purchasing decisions for ICP-MS systems, ranking it above initial acquisition cost.
Regional analysis shows North America and Europe currently dominating the market with a combined share of 62%, though Asia-Pacific is projected to show the highest growth rate over the next five years. China and India, in particular, are investing heavily in analytical infrastructure for environmental monitoring and pharmaceutical quality control.
The market is also witnessing a shift toward nebulizers that can handle microvolume samples, reflecting the broader trend of miniaturization in analytical chemistry. This is particularly evident in biomedical research, where sample availability is often limited. Nebulizers capable of efficient operation with sample volumes below 100 μL are experiencing demand growth rates exceeding 12% annually.
Healthcare and pharmaceutical sectors constitute the largest market segment, accounting for nearly 35% of the total demand for high-efficiency nebulizers. This is primarily due to stringent regulatory requirements for trace element analysis in drug development and quality control processes. The need for consistent spray performance directly impacts measurement accuracy, which is critical for compliance with FDA and EMA guidelines.
Environmental monitoring represents the second-largest market segment, contributing about 28% of the demand. Government regulations worldwide are becoming increasingly stringent regarding the detection and quantification of heavy metals and other toxic elements in soil, water, and air samples. This regulatory pressure has created sustained demand for more efficient nebulizer technologies that can deliver lower detection limits and higher reproducibility.
The semiconductor and electronics manufacturing industry has emerged as the fastest-growing segment, with a compound annual growth rate of approximately 9.2%. As miniaturization continues and component purity requirements become more demanding, manufacturers require increasingly sensitive analytical tools for quality control. High-efficiency nebulizers that minimize sample consumption while maximizing signal stability are particularly valued in this sector.
Market research indicates that end-users are willing to pay premium prices for nebulizers that demonstrate superior spray consistency, reduced sample consumption, and resistance to high dissolved solids. A survey of laboratory managers revealed that 73% consider nebulizer performance a critical factor in their purchasing decisions for ICP-MS systems, ranking it above initial acquisition cost.
Regional analysis shows North America and Europe currently dominating the market with a combined share of 62%, though Asia-Pacific is projected to show the highest growth rate over the next five years. China and India, in particular, are investing heavily in analytical infrastructure for environmental monitoring and pharmaceutical quality control.
The market is also witnessing a shift toward nebulizers that can handle microvolume samples, reflecting the broader trend of miniaturization in analytical chemistry. This is particularly evident in biomedical research, where sample availability is often limited. Nebulizers capable of efficient operation with sample volumes below 100 μL are experiencing demand growth rates exceeding 12% annually.
Current Nebulizer Designs and Technical Challenges
Current nebulizer designs for ICP-MS systems primarily fall into several categories, each with distinct operational principles and performance characteristics. Pneumatic nebulizers, including concentric, cross-flow, and micro-concentric designs, remain the most widely utilized due to their relative simplicity and cost-effectiveness. Concentric nebulizers operate by introducing sample liquid through a capillary surrounded by high-velocity gas, creating aerosol through shear forces. Cross-flow designs position the gas flow perpendicular to the sample capillary, offering improved tolerance for high dissolved solids but generally lower efficiency.
Ultrasonic nebulizers represent a significant advancement, utilizing piezoelectric transducers to generate aerosols through high-frequency vibrations. These systems achieve substantially higher sample transport efficiency (15-30%) compared to pneumatic designs (1-3%), resulting in enhanced sensitivity. However, their complexity, higher maintenance requirements, and susceptibility to matrix effects limit widespread adoption in routine analytical environments.
Direct injection nebulizers and high-efficiency nebulizers have emerged as specialized solutions for particular analytical challenges. These designs minimize sample dilution and dead volume while improving transport efficiency, but often require precise operational parameters and regular maintenance to maintain optimal performance.
Despite technological advancements, current nebulizer designs face several persistent technical challenges. Sample transport efficiency remains a critical limitation, with most conventional systems delivering only a small fraction of the sample to the plasma. This inefficiency necessitates larger sample volumes and contributes to signal instability. The formation of consistent aerosol particle size distribution represents another significant challenge, as variations directly impact ionization efficiency and signal stability.
Matrix effects pose substantial difficulties, particularly with high-salt or high-organic content samples that can cause physical blockages and signal suppression. Current designs struggle to maintain consistent performance across diverse sample matrices without significant method development or sample preparation. Memory effects and carryover between samples remain problematic, especially for trace analysis applications requiring high sensitivity and low detection limits.
Temperature stability presents another challenge, as nebulizer performance can vary significantly with ambient temperature fluctuations or heat transfer from the plasma. This thermal sensitivity affects spray consistency and ultimately analytical precision. Additionally, most current designs exhibit limited operational lifespans under routine analytical conditions, requiring frequent replacement or maintenance that increases operational costs and downtime.
The integration of nebulizers with modern ICP-MS systems presents compatibility challenges, particularly as instrument manufacturers pursue higher sensitivity and lower detection limits. Existing nebulizer technologies often become limiting factors in achieving the theoretical performance capabilities of advanced mass spectrometry systems.
Ultrasonic nebulizers represent a significant advancement, utilizing piezoelectric transducers to generate aerosols through high-frequency vibrations. These systems achieve substantially higher sample transport efficiency (15-30%) compared to pneumatic designs (1-3%), resulting in enhanced sensitivity. However, their complexity, higher maintenance requirements, and susceptibility to matrix effects limit widespread adoption in routine analytical environments.
Direct injection nebulizers and high-efficiency nebulizers have emerged as specialized solutions for particular analytical challenges. These designs minimize sample dilution and dead volume while improving transport efficiency, but often require precise operational parameters and regular maintenance to maintain optimal performance.
Despite technological advancements, current nebulizer designs face several persistent technical challenges. Sample transport efficiency remains a critical limitation, with most conventional systems delivering only a small fraction of the sample to the plasma. This inefficiency necessitates larger sample volumes and contributes to signal instability. The formation of consistent aerosol particle size distribution represents another significant challenge, as variations directly impact ionization efficiency and signal stability.
Matrix effects pose substantial difficulties, particularly with high-salt or high-organic content samples that can cause physical blockages and signal suppression. Current designs struggle to maintain consistent performance across diverse sample matrices without significant method development or sample preparation. Memory effects and carryover between samples remain problematic, especially for trace analysis applications requiring high sensitivity and low detection limits.
Temperature stability presents another challenge, as nebulizer performance can vary significantly with ambient temperature fluctuations or heat transfer from the plasma. This thermal sensitivity affects spray consistency and ultimately analytical precision. Additionally, most current designs exhibit limited operational lifespans under routine analytical conditions, requiring frequent replacement or maintenance that increases operational costs and downtime.
The integration of nebulizers with modern ICP-MS systems presents compatibility challenges, particularly as instrument manufacturers pursue higher sensitivity and lower detection limits. Existing nebulizer technologies often become limiting factors in achieving the theoretical performance capabilities of advanced mass spectrometry systems.
Current Approaches to Nebulizer Spray Optimization
01 Nebulizer design optimization for improved efficiency
Various design improvements in nebulizers can significantly enhance the efficiency of ICP-MS analysis. These include modifications to the nebulizer geometry, spray chamber configuration, and flow dynamics. Optimized designs can reduce sample consumption, improve aerosol quality, and increase the transport efficiency of analytes to the plasma, resulting in better sensitivity and lower detection limits for ICP-MS applications.- Nebulizer design optimization for improved efficiency: Various design improvements in nebulizers can significantly enhance the efficiency of ICP-MS analysis. These include modifications to the nebulizer geometry, spray chamber configurations, and flow dynamics. Optimized designs can reduce sample consumption, improve aerosol quality, and increase the transport efficiency of analytes to the plasma, resulting in better sensitivity and lower detection limits for ICP-MS applications.
- Ultrasonic and high-efficiency nebulization techniques: Ultrasonic nebulization technologies offer advantages over conventional pneumatic nebulizers for ICP-MS applications. These systems use high-frequency vibrations to generate fine aerosols with more uniform droplet size distribution, resulting in higher sample introduction efficiency. Advanced ultrasonic nebulizers can achieve improved analyte transport efficiency, reduced matrix effects, and enhanced sensitivity for trace element analysis in various sample types.
- Microfluidic and low-flow nebulization systems: Microfluidic and low-flow nebulization systems are designed to operate efficiently with minimal sample volumes. These systems incorporate precise flow control mechanisms, specialized capillary designs, and optimized gas-liquid interaction zones to maximize nebulization efficiency at low flow rates. The benefits include reduced sample consumption, improved stability, and enhanced sensitivity for precious or limited sample analysis in ICP-MS applications.
- Temperature and pressure control for nebulizer efficiency: Controlling temperature and pressure parameters in the nebulization process can significantly impact ICP-MS efficiency. Systems that incorporate precise temperature regulation of the sample, nebulizer gas, and spray chamber can optimize aerosol generation and transport. Additionally, pressure-controlled nebulization systems can maintain consistent droplet formation and improve the stability of analytical signals, leading to more reliable quantitative results in ICP-MS analysis.
- Desolvation and aerosol modification techniques: Desolvation and aerosol modification techniques can enhance nebulizer efficiency in ICP-MS by improving the quality of the sample aerosol before it reaches the plasma. These approaches include membrane desolvation, heated spray chambers, and aerosol filtering systems that remove larger droplets. By reducing solvent load and optimizing particle size distribution, these techniques minimize plasma loading, reduce interferences, and improve ionization efficiency, resulting in enhanced sensitivity and precision in ICP-MS measurements.
02 Ultrasonic and high-efficiency nebulization techniques
Ultrasonic nebulization technologies offer advantages over conventional pneumatic nebulizers for ICP-MS applications. These systems use high-frequency vibrations to generate fine aerosols with more uniform droplet size distribution, leading to improved sample introduction efficiency. The enhanced nebulization efficiency results in better sensitivity, reduced matrix effects, and improved precision in elemental analysis, particularly for samples with complex matrices or limited volume.Expand Specific Solutions03 Temperature and pressure control systems for nebulizers
Controlling temperature and pressure parameters in the nebulization process can significantly impact ICP-MS efficiency. Systems that incorporate precise temperature regulation of the spray chamber and nebulizer gas, along with optimized pressure control mechanisms, can enhance aerosol generation and transport efficiency. These control systems help maintain stable analytical conditions, reduce signal drift, and improve the overall reliability of ICP-MS measurements.Expand Specific Solutions04 Microfluidic and low-flow nebulization systems
Microfluidic and low-flow nebulization technologies have been developed to address the challenges of analyzing small sample volumes in ICP-MS. These systems incorporate precise fluid handling components, specialized capillaries, and optimized gas flow paths to efficiently nebulize microliter or nanoliter sample volumes. The improved efficiency of these systems enables analysis of precious samples, reduces waste generation, and decreases matrix effects while maintaining analytical performance.Expand Specific Solutions05 Automated cleaning and maintenance systems for sustained nebulizer efficiency
Automated cleaning and maintenance systems have been developed to maintain optimal nebulizer performance in ICP-MS instruments. These systems incorporate mechanisms for regular cleaning of nebulizer components, prevention of salt buildup, and monitoring of nebulizer efficiency parameters. By ensuring consistent nebulizer performance over time, these systems improve long-term stability, reduce downtime, and enhance the reproducibility of analytical results in routine ICP-MS applications.Expand Specific Solutions
Leading Manufacturers and Research Institutions in ICP-MS
The ICP-MS nebulizer efficiency optimization market is in a growth phase, with increasing demand driven by analytical chemistry advancements across pharmaceutical, environmental, and materials science sectors. The competitive landscape features established analytical instrument manufacturers like Thermo Fisher Scientific, Bruker, and Shimadzu dominating with comprehensive solutions, while specialized players such as Glass Expansion and Micromass UK focus on nebulizer innovations. Academic institutions including Keio University and Clemson University Research Foundation contribute significant research. The technology is reaching maturity in standard applications but continues evolving for specialized uses, with companies like LECO and Hitachi advancing integration with broader analytical platforms to enhance spray consistency and sample introduction efficiency.
Thermo Fisher Scientific (Bremen) GmbH
Technical Solution: Thermo Fisher Scientific has developed advanced nebulizer systems for ICP-MS featuring their proprietary PFA (perfluoroalkoxy) microflow nebulizers that operate at low sample consumption rates (50-400 μL/min). Their technology incorporates precision-engineered spray chambers with temperature control capabilities that maintain consistent aerosol generation regardless of ambient conditions. The company's latest innovations include the FAST (Flow Injection Analysis for Speciation Technology) system which integrates with their nebulizers to provide automated sample introduction with minimal washout times. Their nebulizers feature computer-controlled gas flow regulators that dynamically adjust argon pressure to maintain optimal droplet size distribution (typically 5-10 μm) even when sample viscosity changes. Thermo Fisher has also implemented self-diagnostic capabilities that monitor nebulizer performance in real-time and alert operators when spray efficiency decreases below predetermined thresholds.
Strengths: Superior precision engineering allows for consistent droplet formation across a wide range of sample types. Their integrated temperature control systems minimize drift during long analytical runs. Weaknesses: Higher cost compared to conventional nebulizers, and their proprietary systems often require specific Thermo Fisher components for optimal performance.
Glass Expansion Pty Ltd.
Technical Solution: Glass Expansion has pioneered specialized nebulizer technology specifically optimized for ICP-MS applications with their SeaSpray and MicroMist nebulizer series. Their patented designs feature unique glass-to-glass connections that eliminate potential contamination sources from traditional o-ring seals. The company's nebulizers incorporate precision-machined sapphire orifices (typically 50-200 μm) that produce highly uniform droplet distributions with relative standard deviations below 2% across analytical runs. Their innovative VitriCone spray chamber design creates a cyclonic flow pattern that efficiently removes larger droplets while maintaining high transport efficiency (typically 15-20% compared to conventional 1-2%). Glass Expansion has also developed the Assist gas adaptation system that introduces a secondary gas flow around the primary nebulizer gas, creating a sheath effect that stabilizes the spray pattern even with challenging high-salt matrices. Their nebulizers feature self-aspirating capabilities that maintain consistent sample uptake rates independent of peristaltic pump variations.
Strengths: Exceptional chemical resistance across the entire pH range and with aggressive organic solvents. Their nebulizers demonstrate superior long-term stability with minimal drift even during extended analytical sequences. Weaknesses: More fragile than PEEK or PFA alternatives, requiring careful handling, and their specialized designs can be more difficult to clean when clogged.
Key Patents and Innovations in Nebulizer Design
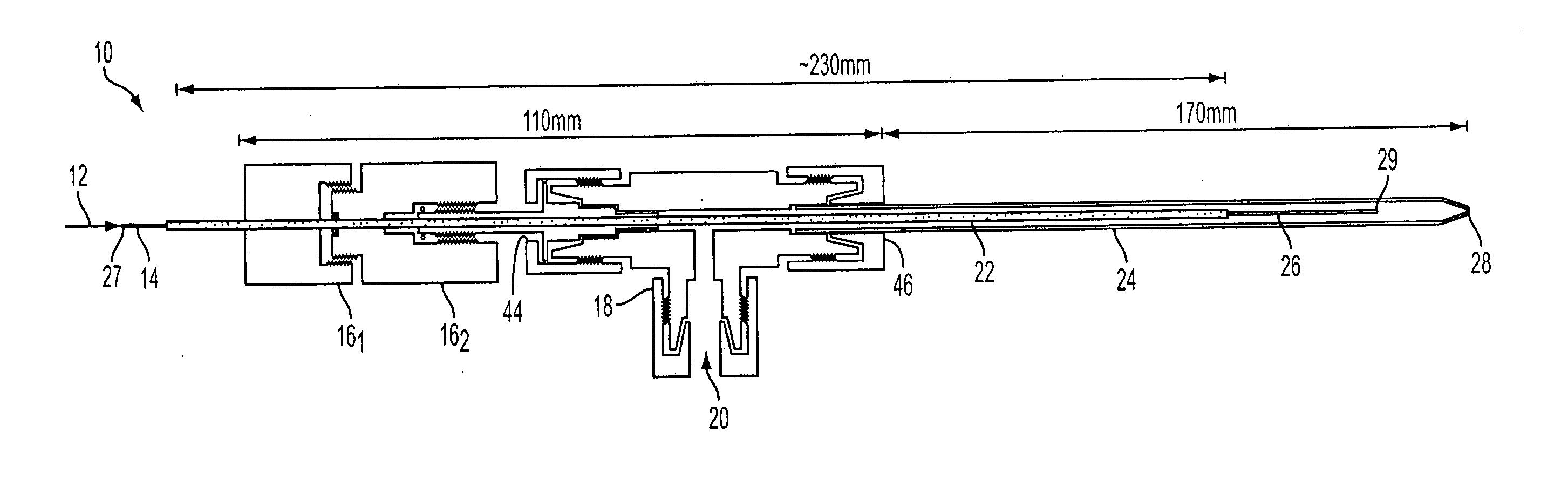
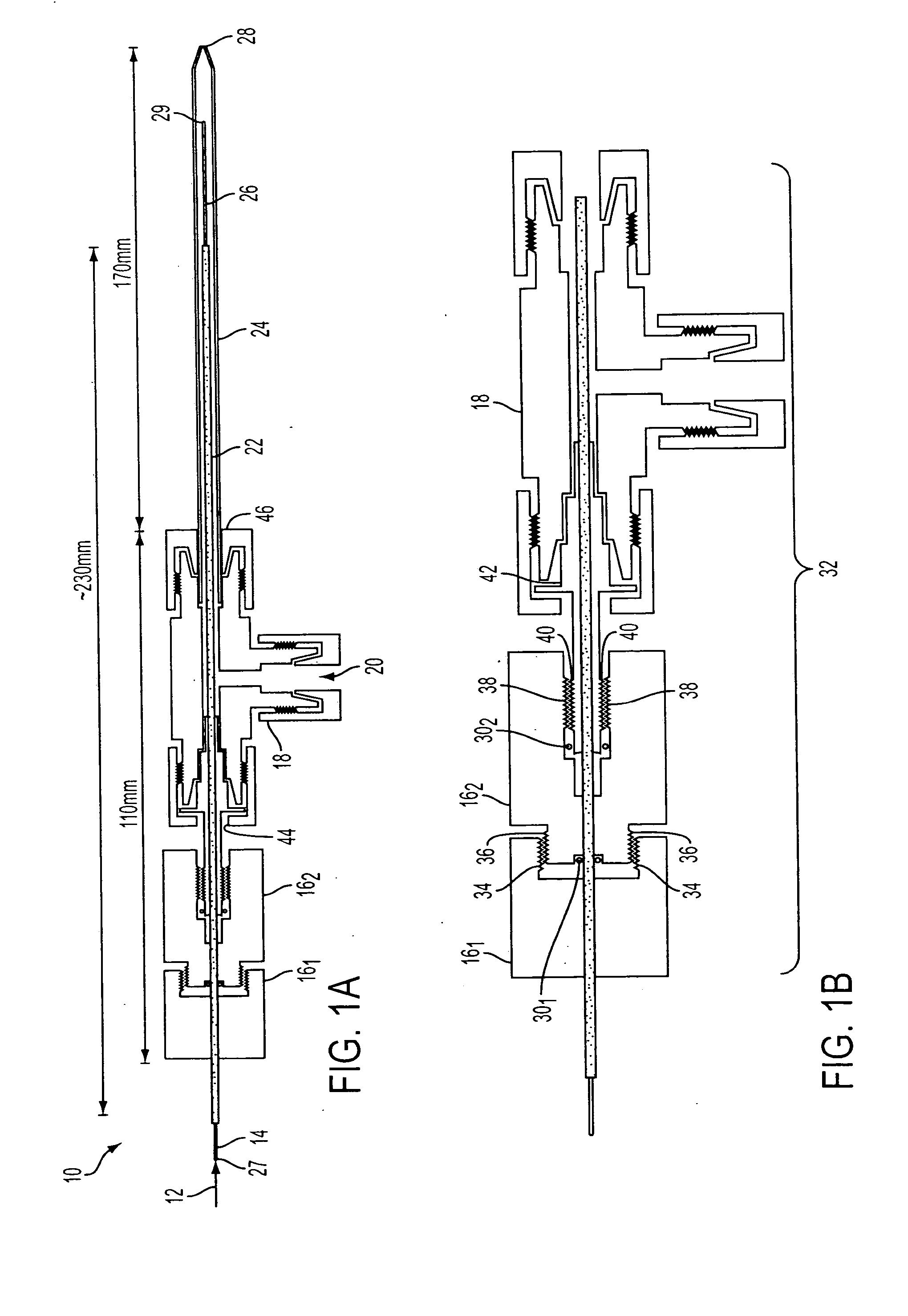
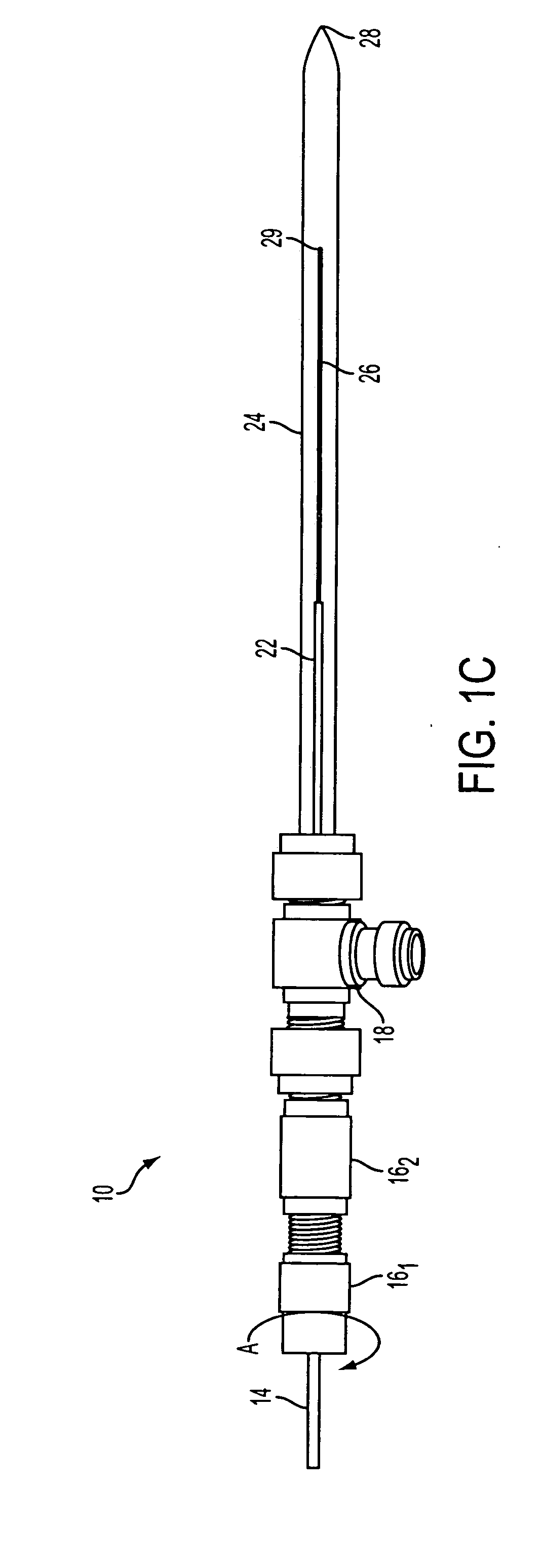
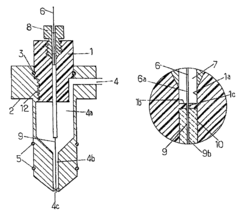
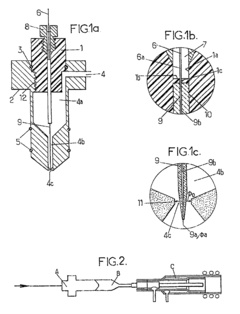
Demountable direct injection high efficiency nebulizer for inductively coupled plasma mass spectrometry
PatentInactiveUS20050230617A1
Innovation
- A demountable direct injection high efficiency nebulizer with an adjustable capillary position and modular design, featuring a capillary adjustment adapter for precise positioning and a connector system, allowing for easy capillary replacement and improved aerosol properties.
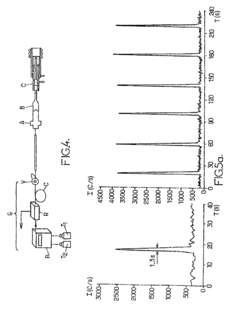
Nebulizer with nanometric flow rate of a liquid effluent and nebulizing installation comprising same
PatentInactiveUS7863560B2
Innovation
- A nebulizer with a capillary tube and nebulizing needle arrangement that creates a stable, laminar flow regime, allowing for a spray of liquid effluent at flow rates between 10 nl/min and 1 μl/min, with a nebulizing gas nozzle and chamber configuration that minimizes turbulence and ensures optimal contact between the liquid and gas, enabling efficient nebulization at nanoscale.
Environmental Impact and Waste Reduction Strategies
The optimization of ICP-MS nebulizer efficiency not only impacts analytical performance but also has significant environmental implications. Traditional nebulizer systems typically utilize only 1-2% of the sample solution for analysis, with the remaining 98-99% becoming waste. This inefficiency translates to substantial chemical waste generation, particularly problematic when analyzing samples containing toxic elements or when using hazardous reagents for sample preparation.
Implementing waste reduction strategies in ICP-MS nebulization processes offers multiple environmental and economic benefits. Micro-flow and nano-flow nebulizers represent a significant advancement, reducing sample consumption from traditional 1-2 mL/min to as low as 20-50 μL/min. This 20-50 fold reduction in sample volume directly correlates to proportional decreases in waste generation, minimizing environmental impact and reducing disposal costs.
Recycling systems for nebulizer waste present another viable approach. Advanced systems can collect, filter, and recirculate unused sample solution, particularly valuable for precious or limited samples. Some laboratories have reported up to 80% reduction in waste volume through implementation of closed-loop recycling systems, though these require careful monitoring to prevent cross-contamination.
The environmental footprint extends beyond waste volume to include energy consumption. Optimized nebulizer designs that achieve consistent spray patterns at lower gas pressures can reduce argon consumption by 15-30%. Given that argon production is energy-intensive, this reduction translates to significant decreases in the carbon footprint of analytical operations.
Chemical substitution strategies further enhance environmental sustainability. Replacing traditional acid digestion procedures with milder reagents or green chemistry approaches can reduce the toxicity of nebulizer waste. Several research groups have demonstrated successful implementation of citric acid or dilute organic acid mixtures as alternatives to concentrated nitric or hydrochloric acids for certain sample types.
Laboratory-scale waste treatment systems specifically designed for ICP-MS waste streams represent an emerging technology. These systems can neutralize acidic waste, precipitate heavy metals, and reduce the hazard classification of waste materials, potentially converting hazardous waste to non-hazardous categories and significantly reducing disposal costs and environmental liability.
Implementing waste reduction strategies in ICP-MS nebulization processes offers multiple environmental and economic benefits. Micro-flow and nano-flow nebulizers represent a significant advancement, reducing sample consumption from traditional 1-2 mL/min to as low as 20-50 μL/min. This 20-50 fold reduction in sample volume directly correlates to proportional decreases in waste generation, minimizing environmental impact and reducing disposal costs.
Recycling systems for nebulizer waste present another viable approach. Advanced systems can collect, filter, and recirculate unused sample solution, particularly valuable for precious or limited samples. Some laboratories have reported up to 80% reduction in waste volume through implementation of closed-loop recycling systems, though these require careful monitoring to prevent cross-contamination.
The environmental footprint extends beyond waste volume to include energy consumption. Optimized nebulizer designs that achieve consistent spray patterns at lower gas pressures can reduce argon consumption by 15-30%. Given that argon production is energy-intensive, this reduction translates to significant decreases in the carbon footprint of analytical operations.
Chemical substitution strategies further enhance environmental sustainability. Replacing traditional acid digestion procedures with milder reagents or green chemistry approaches can reduce the toxicity of nebulizer waste. Several research groups have demonstrated successful implementation of citric acid or dilute organic acid mixtures as alternatives to concentrated nitric or hydrochloric acids for certain sample types.
Laboratory-scale waste treatment systems specifically designed for ICP-MS waste streams represent an emerging technology. These systems can neutralize acidic waste, precipitate heavy metals, and reduce the hazard classification of waste materials, potentially converting hazardous waste to non-hazardous categories and significantly reducing disposal costs and environmental liability.
Quality Control Standards for Analytical Instrumentation
Quality control standards for analytical instrumentation in ICP-MS nebulizer systems represent a critical framework for ensuring reliable and reproducible results in elemental analysis. These standards encompass comprehensive protocols for performance verification, calibration procedures, and operational parameters that directly impact nebulizer efficiency and spray consistency.
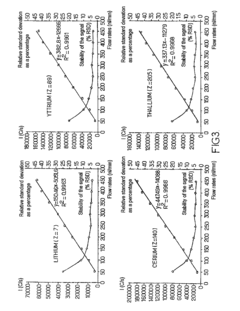
The establishment of robust quality control standards begins with defining acceptable performance metrics for nebulizer systems, including droplet size distribution, aerosol density, and transport efficiency. These parameters must be quantifiable and reproducible across different analytical sessions to maintain data integrity. Industry standards typically require nebulizer performance to demonstrate less than 2% RSD (Relative Standard Deviation) in signal intensity during continuous operation, with droplet size distributions maintaining consistency within predetermined ranges specific to the application.
Calibration verification protocols constitute another essential component of quality control standards, requiring regular assessment of nebulizer performance using certified reference materials. These protocols typically mandate daily verification using multi-element standards that span the mass range of analytical interest, with acceptance criteria for sensitivity, precision, and accuracy clearly defined. Modern quality control frameworks increasingly incorporate automated system suitability tests that evaluate nebulizer performance before analytical sequences commence.
Documentation requirements represent a fundamental aspect of quality control standards, necessitating comprehensive records of nebulizer performance, maintenance history, and operational parameters. These records must include detailed information about gas flow rates, sample uptake rates, and spray chamber temperatures, all of which significantly influence nebulizer efficiency. Regulatory frameworks in pharmaceutical and environmental testing laboratories often require traceability of all nebulizer components and performance verification data.
Preventive maintenance schedules form an integral part of quality control standards, specifying regular inspection and cleaning procedures to prevent performance degradation. These standards typically mandate periodic evaluation of critical components such as capillary tubes, gas connectors, and sample introduction systems, with clear criteria for replacement or reconditioning when performance metrics indicate deterioration.
Proficiency testing programs provide external validation of nebulizer performance and analytical capability, with participation in these programs increasingly becoming a requirement for laboratory accreditation. These programs evaluate a laboratory's ability to maintain consistent nebulizer performance across diverse sample matrices and concentration ranges, offering valuable benchmarking against peer laboratories using similar instrumentation.
The establishment of robust quality control standards begins with defining acceptable performance metrics for nebulizer systems, including droplet size distribution, aerosol density, and transport efficiency. These parameters must be quantifiable and reproducible across different analytical sessions to maintain data integrity. Industry standards typically require nebulizer performance to demonstrate less than 2% RSD (Relative Standard Deviation) in signal intensity during continuous operation, with droplet size distributions maintaining consistency within predetermined ranges specific to the application.
Calibration verification protocols constitute another essential component of quality control standards, requiring regular assessment of nebulizer performance using certified reference materials. These protocols typically mandate daily verification using multi-element standards that span the mass range of analytical interest, with acceptance criteria for sensitivity, precision, and accuracy clearly defined. Modern quality control frameworks increasingly incorporate automated system suitability tests that evaluate nebulizer performance before analytical sequences commence.
Documentation requirements represent a fundamental aspect of quality control standards, necessitating comprehensive records of nebulizer performance, maintenance history, and operational parameters. These records must include detailed information about gas flow rates, sample uptake rates, and spray chamber temperatures, all of which significantly influence nebulizer efficiency. Regulatory frameworks in pharmaceutical and environmental testing laboratories often require traceability of all nebulizer components and performance verification data.
Preventive maintenance schedules form an integral part of quality control standards, specifying regular inspection and cleaning procedures to prevent performance degradation. These standards typically mandate periodic evaluation of critical components such as capillary tubes, gas connectors, and sample introduction systems, with clear criteria for replacement or reconditioning when performance metrics indicate deterioration.
Proficiency testing programs provide external validation of nebulizer performance and analytical capability, with participation in these programs increasingly becoming a requirement for laboratory accreditation. These programs evaluate a laboratory's ability to maintain consistent nebulizer performance across diverse sample matrices and concentration ranges, offering valuable benchmarking against peer laboratories using similar instrumentation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!