Advancing ICP-MS Techniques Through Technological Integration
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ICP-MS Evolution and Objectives
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has evolved significantly since its commercial introduction in the early 1980s. This analytical technique combines a high-temperature inductively coupled plasma source with a mass spectrometer to detect and quantify trace elements in samples with remarkable precision. The evolution of ICP-MS technology has been characterized by continuous improvements in sensitivity, accuracy, and applicability across various scientific disciplines.
The initial development of ICP-MS stemmed from the limitations of existing analytical methods such as Atomic Absorption Spectroscopy (AAS) and Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES). While these techniques provided valuable analytical capabilities, they lacked the combination of multi-element analysis capability and ultra-low detection limits that modern scientific applications demanded.
Throughout the 1990s and early 2000s, significant advancements were made in addressing the primary challenges of ICP-MS, particularly in reducing polyatomic interferences and improving ion transmission efficiency. The introduction of collision/reaction cell technology represented a pivotal moment in ICP-MS development, enabling more accurate analysis of complex matrices by minimizing spectral interferences.
Recent technological trends have focused on enhancing sample introduction systems, improving plasma stability, and developing more sophisticated mass analyzers. The integration of ICP-MS with other analytical techniques, such as chromatography systems, has expanded its application scope considerably, allowing for speciation analysis and enhanced selectivity.
The primary objective of advancing ICP-MS through technological integration is to overcome current limitations while expanding analytical capabilities. Specifically, goals include improving detection limits for challenging elements, enhancing throughput without sacrificing accuracy, developing more robust systems for complex sample matrices, and creating more user-friendly interfaces that require less specialized expertise to operate effectively.
Future development aims to establish ICP-MS as an even more versatile analytical platform through integration with emerging technologies such as artificial intelligence for automated data interpretation, miniaturization for field deployment, and novel sample introduction techniques for previously challenging sample types. These advancements would position ICP-MS to address emerging analytical challenges in environmental monitoring, pharmaceutical development, food safety, and advanced materials characterization.
The trajectory of ICP-MS evolution points toward systems that offer greater analytical power while simultaneously becoming more accessible, reliable, and adaptable to diverse scientific applications, ultimately supporting innovation across multiple industries and research domains.
The initial development of ICP-MS stemmed from the limitations of existing analytical methods such as Atomic Absorption Spectroscopy (AAS) and Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES). While these techniques provided valuable analytical capabilities, they lacked the combination of multi-element analysis capability and ultra-low detection limits that modern scientific applications demanded.
Throughout the 1990s and early 2000s, significant advancements were made in addressing the primary challenges of ICP-MS, particularly in reducing polyatomic interferences and improving ion transmission efficiency. The introduction of collision/reaction cell technology represented a pivotal moment in ICP-MS development, enabling more accurate analysis of complex matrices by minimizing spectral interferences.
Recent technological trends have focused on enhancing sample introduction systems, improving plasma stability, and developing more sophisticated mass analyzers. The integration of ICP-MS with other analytical techniques, such as chromatography systems, has expanded its application scope considerably, allowing for speciation analysis and enhanced selectivity.
The primary objective of advancing ICP-MS through technological integration is to overcome current limitations while expanding analytical capabilities. Specifically, goals include improving detection limits for challenging elements, enhancing throughput without sacrificing accuracy, developing more robust systems for complex sample matrices, and creating more user-friendly interfaces that require less specialized expertise to operate effectively.
Future development aims to establish ICP-MS as an even more versatile analytical platform through integration with emerging technologies such as artificial intelligence for automated data interpretation, miniaturization for field deployment, and novel sample introduction techniques for previously challenging sample types. These advancements would position ICP-MS to address emerging analytical challenges in environmental monitoring, pharmaceutical development, food safety, and advanced materials characterization.
The trajectory of ICP-MS evolution points toward systems that offer greater analytical power while simultaneously becoming more accessible, reliable, and adaptable to diverse scientific applications, ultimately supporting innovation across multiple industries and research domains.
Market Applications and Analytical Demands
The ICP-MS (Inductively Coupled Plasma Mass Spectrometry) market continues to expand rapidly across diverse sectors, driven by increasing demands for precise elemental analysis. The global ICP-MS market, valued at approximately 1.2 billion USD in 2022, is projected to grow at a compound annual growth rate of 7.8% through 2028, reflecting the technology's critical importance in analytical chemistry.
Environmental monitoring represents a significant application domain, with regulatory bodies worldwide implementing stricter guidelines for heavy metal detection in soil, water, and air samples. The demand for lower detection limits, particularly for toxic elements like mercury, arsenic, and lead, has pushed manufacturers to develop systems capable of parts-per-trillion sensitivity with minimal matrix interference.
The pharmaceutical and biomedical sectors have emerged as major growth drivers for ICP-MS technology. Drug development processes require thorough elemental impurity testing to comply with ICH Q3D guidelines, while clinical laboratories increasingly utilize ICP-MS for trace element analysis in biological samples. The rising prevalence of metabolic disorders and heavy metal toxicity testing has created substantial demand for high-throughput systems capable of processing large sample batches with minimal operator intervention.
Food safety represents another critical application area, with regulatory frameworks becoming increasingly stringent regarding permissible levels of contaminants. Manufacturers and regulatory laboratories require systems capable of multi-elemental analysis across diverse food matrices, from beverages to solid foodstuffs, driving demand for versatile sample introduction systems and robust interference management.
The semiconductor industry presents unique analytical challenges, requiring ultra-trace detection capabilities for manufacturing process control. As chip architectures continue to shrink, the tolerance for elemental contaminants approaches zero, necessitating ICP-MS systems with enhanced sensitivity and specialized sample introduction techniques for analyzing small volumes of high-purity chemicals.
Emerging applications in nanomaterial characterization, isotope ratio analysis for authentication purposes, and single-cell analysis are creating new market opportunities. These specialized applications demand technological innovations such as hyphenated techniques (coupling ICP-MS with chromatography or laser ablation) and advanced data processing algorithms to extract meaningful information from complex samples.
The analytical demands across these markets converge on several key requirements: improved sensitivity, enhanced throughput, simplified operation, reduced maintenance, and more sophisticated data analysis capabilities. Meeting these demands requires technological integration across multiple disciplines, from plasma physics to software development, creating opportunities for significant innovation in next-generation ICP-MS systems.
Environmental monitoring represents a significant application domain, with regulatory bodies worldwide implementing stricter guidelines for heavy metal detection in soil, water, and air samples. The demand for lower detection limits, particularly for toxic elements like mercury, arsenic, and lead, has pushed manufacturers to develop systems capable of parts-per-trillion sensitivity with minimal matrix interference.
The pharmaceutical and biomedical sectors have emerged as major growth drivers for ICP-MS technology. Drug development processes require thorough elemental impurity testing to comply with ICH Q3D guidelines, while clinical laboratories increasingly utilize ICP-MS for trace element analysis in biological samples. The rising prevalence of metabolic disorders and heavy metal toxicity testing has created substantial demand for high-throughput systems capable of processing large sample batches with minimal operator intervention.
Food safety represents another critical application area, with regulatory frameworks becoming increasingly stringent regarding permissible levels of contaminants. Manufacturers and regulatory laboratories require systems capable of multi-elemental analysis across diverse food matrices, from beverages to solid foodstuffs, driving demand for versatile sample introduction systems and robust interference management.
The semiconductor industry presents unique analytical challenges, requiring ultra-trace detection capabilities for manufacturing process control. As chip architectures continue to shrink, the tolerance for elemental contaminants approaches zero, necessitating ICP-MS systems with enhanced sensitivity and specialized sample introduction techniques for analyzing small volumes of high-purity chemicals.
Emerging applications in nanomaterial characterization, isotope ratio analysis for authentication purposes, and single-cell analysis are creating new market opportunities. These specialized applications demand technological innovations such as hyphenated techniques (coupling ICP-MS with chromatography or laser ablation) and advanced data processing algorithms to extract meaningful information from complex samples.
The analytical demands across these markets converge on several key requirements: improved sensitivity, enhanced throughput, simplified operation, reduced maintenance, and more sophisticated data analysis capabilities. Meeting these demands requires technological integration across multiple disciplines, from plasma physics to software development, creating opportunities for significant innovation in next-generation ICP-MS systems.
Current ICP-MS Limitations and Technical Barriers
Despite significant advancements in ICP-MS technology over recent decades, several persistent limitations and technical barriers continue to challenge researchers and analytical chemists. One of the most significant challenges remains spectral interferences, where polyatomic ions formed in the plasma or interface regions overlap with analyte signals, particularly affecting transition metals and metalloids. Current collision/reaction cell technologies, while helpful, cannot completely eliminate all interference patterns, especially in complex matrices.
Sample introduction inefficiency represents another major limitation, with conventional nebulizer systems typically delivering only 1-3% of the sample to the plasma. This inefficiency not only increases detection limits but also necessitates larger sample volumes, which is problematic when sample availability is restricted, as in clinical or forensic applications.
Matrix effects continue to pose significant challenges, particularly when analyzing samples with high dissolved solid content. These effects can suppress or enhance analyte signals, leading to inaccurate quantification. Current calibration strategies, including internal standardization and standard addition, provide only partial solutions and often require time-consuming method development for each matrix type.
The plasma-sample interface region remains a critical bottleneck in ICP-MS systems. Ion transmission efficiency through the sampling cone, skimmer cone, and ion optics typically ranges from 0.1-1%, resulting in substantial sensitivity loss. Space-charge effects in this region disproportionately affect lighter elements, creating mass-dependent transmission biases that complicate accurate multi-element analysis.
Instrument drift presents ongoing challenges for long analytical sequences, necessitating frequent recalibration and limiting throughput. Current drift correction methods using internal standards provide incomplete compensation, particularly for extended analytical runs or when analyzing unstable samples.
From a practical perspective, conventional ICP-MS systems demand significant expertise for operation and maintenance. The complexity of method development, particularly for challenging matrices, requires specialized knowledge that limits broader adoption in routine laboratories. Additionally, the substantial infrastructure requirements—including high-purity gases, chilled water systems, and specialized laboratory facilities—restrict deployment in field settings or resource-limited environments.
The integration of ICP-MS with separation techniques like chromatography and field-flow fractionation introduces additional complications related to flow rate compatibility, signal stability during gradients, and data processing challenges for transient signals. These integration issues limit the full potential of hyphenated techniques for speciation analysis and nanoparticle characterization.
Sample introduction inefficiency represents another major limitation, with conventional nebulizer systems typically delivering only 1-3% of the sample to the plasma. This inefficiency not only increases detection limits but also necessitates larger sample volumes, which is problematic when sample availability is restricted, as in clinical or forensic applications.
Matrix effects continue to pose significant challenges, particularly when analyzing samples with high dissolved solid content. These effects can suppress or enhance analyte signals, leading to inaccurate quantification. Current calibration strategies, including internal standardization and standard addition, provide only partial solutions and often require time-consuming method development for each matrix type.
The plasma-sample interface region remains a critical bottleneck in ICP-MS systems. Ion transmission efficiency through the sampling cone, skimmer cone, and ion optics typically ranges from 0.1-1%, resulting in substantial sensitivity loss. Space-charge effects in this region disproportionately affect lighter elements, creating mass-dependent transmission biases that complicate accurate multi-element analysis.
Instrument drift presents ongoing challenges for long analytical sequences, necessitating frequent recalibration and limiting throughput. Current drift correction methods using internal standards provide incomplete compensation, particularly for extended analytical runs or when analyzing unstable samples.
From a practical perspective, conventional ICP-MS systems demand significant expertise for operation and maintenance. The complexity of method development, particularly for challenging matrices, requires specialized knowledge that limits broader adoption in routine laboratories. Additionally, the substantial infrastructure requirements—including high-purity gases, chilled water systems, and specialized laboratory facilities—restrict deployment in field settings or resource-limited environments.
The integration of ICP-MS with separation techniques like chromatography and field-flow fractionation introduces additional complications related to flow rate compatibility, signal stability during gradients, and data processing challenges for transient signals. These integration issues limit the full potential of hyphenated techniques for speciation analysis and nanoparticle characterization.
Contemporary ICP-MS Integration Solutions
01 ICP-MS system integration with sample preparation
Integration of ICP-MS with automated sample preparation systems enhances analytical efficiency and accuracy. These integrated systems combine sample introduction, digestion, and pretreatment processes with the mass spectrometry analysis, reducing contamination risks and human error. The automation allows for precise handling of multiple samples, improving throughput and reproducibility in elemental analysis applications.- ICP-MS system integration with sample preparation: Integration of ICP-MS with automated sample preparation systems enhances analytical efficiency and accuracy. These integrated systems combine sample introduction, digestion, and pretreatment processes with the mass spectrometry analysis, reducing contamination risks and human error. The automation allows for precise handling of multiple samples, improving throughput and reproducibility in elemental analysis applications.
- Coupling ICP-MS with chromatographic techniques: Combining ICP-MS with various chromatographic separation techniques creates powerful hyphenated analytical systems. These integrations include HPLC-ICP-MS, GC-ICP-MS, and IC-ICP-MS configurations that enable simultaneous separation and detection of complex elemental species. Such technological integration significantly enhances speciation analysis capabilities, allowing for differentiation between various chemical forms of elements with improved sensitivity and selectivity.
- Miniaturization and portable ICP-MS systems: Advancements in miniaturization technology have led to the development of portable and field-deployable ICP-MS systems. These compact instruments integrate plasma generation, ion optics, and detection systems in reduced form factors while maintaining analytical performance. Miniaturized ICP-MS systems enable on-site analysis in environmental monitoring, geological surveys, and industrial quality control applications where traditional laboratory analysis is impractical.
- Multi-element detection and data processing innovations: Enhanced multi-element detection capabilities in ICP-MS are achieved through innovations in ion optics, detector technology, and data processing algorithms. These systems integrate advanced collision/reaction cell technologies to minimize interferences while simultaneously analyzing numerous elements. Sophisticated software platforms process complex spectral data, apply interference corrections, and provide comprehensive analytical reports with improved accuracy for trace element quantification.
- ICP-MS integration with industrial and environmental monitoring systems: ICP-MS technology is increasingly integrated into continuous monitoring systems for industrial processes and environmental applications. These integrated systems feature automated sampling interfaces, real-time data acquisition, and feedback control mechanisms. The integration enables continuous elemental analysis in industrial effluents, water quality monitoring, and manufacturing quality control, providing immediate detection of contaminants and process deviations.
02 Coupling ICP-MS with chromatographic techniques
The combination of ICP-MS with various chromatographic separation techniques creates powerful hyphenated analytical systems. These integrations include HPLC-ICP-MS, GC-ICP-MS, and IC-ICP-MS configurations that enable simultaneous separation and detection of complex elemental species. Such technological integration significantly improves speciation analysis capabilities, allowing for differentiation between various chemical forms of elements with enhanced sensitivity and selectivity.Expand Specific Solutions03 Miniaturization and portable ICP-MS systems
Advancements in miniaturization technology have led to the development of compact and portable ICP-MS systems. These innovations integrate traditional ICP-MS capabilities into smaller footprints through redesigned plasma sources, ion optics, and detection systems. Portable systems enable on-site analysis in environmental monitoring, geological surveys, and industrial quality control applications, bringing laboratory-grade analytical capabilities to field settings.Expand Specific Solutions04 Multi-element detection and data processing improvements
Enhanced multi-element detection capabilities in ICP-MS systems integrate advanced data processing algorithms and improved detector technologies. These systems feature simultaneous detection of multiple isotopes across the periodic table with expanded dynamic range and reduced interferences. Integration of machine learning and automated data interpretation tools enables real-time analysis of complex elemental patterns and improves detection limits for trace element analysis.Expand Specific Solutions05 Interface optimization for enhanced sensitivity
Technological innovations in ICP-MS interface design focus on optimizing the transition between the plasma source and the mass analyzer. These developments include improved ion extraction systems, collision/reaction cell technologies, and vacuum interface configurations that enhance transmission efficiency. Such interface optimizations significantly reduce polyatomic interferences and matrix effects, resulting in improved sensitivity, precision, and accuracy for challenging sample types.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The ICP-MS technology market is currently in a growth phase, characterized by increasing integration with advanced analytical techniques. The global market size is expanding rapidly, driven by applications in environmental monitoring, pharmaceuticals, and materials science. Technologically, the field shows varying maturity levels, with established players like Agilent Technologies and PerkinElmer (Revvity) leading innovation in high-end systems, while specialized companies such as Elemental Scientific focus on sample introduction advancements. Chinese companies including Jiangsu Skyray and Lihe Technology are emerging as significant competitors, particularly in cost-effective solutions. European research institutions like ETH Zurich and TITK contribute cutting-edge research, pushing technological boundaries through cross-disciplinary approaches that combine ICP-MS with other analytical methods.
Elemental Scientific, Inc.
Technical Solution: Elemental Scientific has revolutionized ICP-MS sample introduction and automation systems with their prepFAST technology. Their integrated approach focuses on enhancing traditional ICP-MS instruments through sophisticated sample delivery systems that dramatically improve precision, accuracy, and throughput. The prepFAST platform incorporates inline dilution capabilities that automatically adjust sample concentrations to fall within calibration ranges, eliminating manual dilution steps and reducing analysis time by up to 70%. Their dual-valve technology enables rapid switching between samples with minimal carryover (<0.1%), even for challenging elements like mercury and boron. Elemental Scientific has also developed specialized microflow nebulizers that reduce sample consumption to as little as 20 μL/min while maintaining sensitivity, making their systems ideal for limited-volume precious samples. Their latest innovation integrates automated internal standard addition with real-time flow monitoring to compensate for matrix effects and instrument drift, significantly improving long-term measurement stability.
Strengths: Exceptional sample handling efficiency; dramatic reduction in sample preparation time; superior precision for trace element analysis; compatibility with virtually all ICP-MS manufacturers. Weaknesses: Requires integration with existing ICP-MS systems rather than being a standalone solution; higher complexity in method development; additional training requirements for operators.
Revvity Health Sciences, Inc.
Technical Solution: Revvity (formerly PerkinElmer) has developed innovative ICP-MS solutions focused on high-throughput clinical and pharmaceutical applications. Their NexION series integrates patented Triple Cone Interface technology with Quadrupole Ion Deflector systems to maximize ion transmission while minimizing background noise. This architecture delivers exceptional sensitivity even in complex biological matrices. Revvity's Universal Cell Technology (UCT) provides flexible reaction/collision capabilities that can be optimized for specific analytical challenges. Their recent technological integration includes advanced sample introduction systems with specialized nebulizers and spray chambers designed to handle high-salt and organic-rich samples without compromising stability. Revvity has also pioneered the integration of automated sample preparation platforms directly with their ICP-MS systems, creating end-to-end workflows that minimize contamination risks and analyst intervention. Their Syngistix software platform incorporates intelligent quality control protocols and automated troubleshooting algorithms to ensure data integrity.
Strengths: Superior performance in biological and pharmaceutical matrices; highly automated workflows; excellent long-term stability; comprehensive software validation packages for regulated environments. Weaknesses: Less flexibility for non-standard applications compared to some competitors; higher consumable costs for specialized applications; more complex maintenance requirements.
Breakthrough Patents in ICP-MS Enhancement
Inductively coupled plasma mass spectrometry
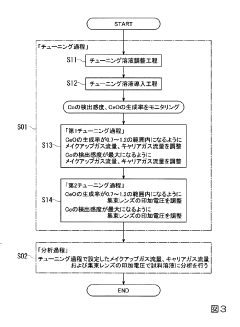
PatentInactiveJP2020027038A
Innovation
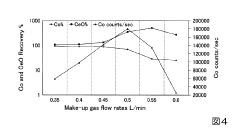
- The method involves tuning the ICP-MS system using a sample solution with a high-concentration acid matrix as a tuning liquid, adjusting carrier gas flow rates and focusing lens settings to control the production rate of coexisting element oxides within a specific range, thereby maximizing detection sensitivity.
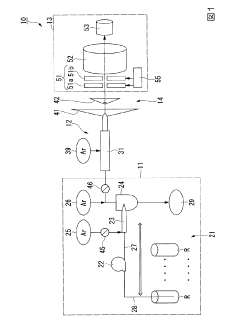
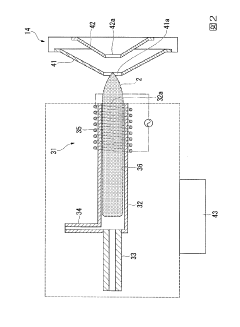
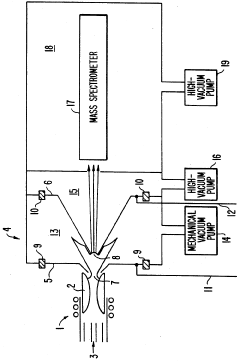
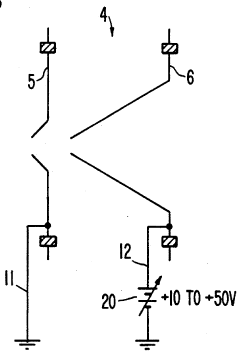
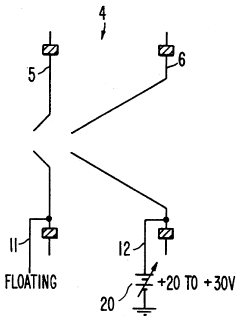
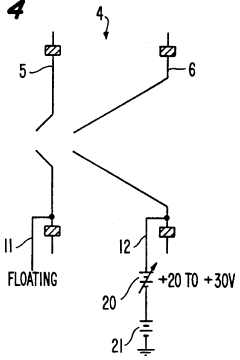
Plasma sampling interface for inductively coupled plasma-mass spectrometry (ICP-MS)
PatentInactiveUS5218204A
Innovation
- A plasma sampling interface with insulating spacers and an adjustable DC bias voltage source applying a DC bias voltage of 10 to 50 V to the skimmer, allowing the sampler to float or grounding it, enhances ion transmission by using a DC offset voltage for mass spectrometers requiring higher initial ion energy.
Environmental and Safety Considerations
The integration of ICP-MS technology with advanced analytical systems brings significant environmental and safety considerations that must be addressed throughout implementation and operation. Laboratory environments utilizing ICP-MS systems generate various waste streams including acidic solutions, heavy metal residues, and argon gas emissions that require proper management protocols. Modern integrated systems have incorporated closed-loop waste handling mechanisms that significantly reduce environmental impact compared to earlier generations of equipment.
Regulatory compliance represents a critical aspect of ICP-MS implementation, with facilities needing to adhere to increasingly stringent international standards for hazardous waste disposal, air quality management, and worker safety. The European Union's REACH regulations and the United States EPA guidelines have established comprehensive frameworks that directly impact ICP-MS operations, particularly in sample preparation and waste handling processes.
Energy consumption presents another environmental consideration, as traditional ICP-MS systems require substantial power for plasma generation and maintenance. Technological integration has enabled significant improvements in this area, with newer systems incorporating energy-efficient components and intelligent power management algorithms that can reduce consumption by up to 40% compared to systems from a decade ago.
Worker safety concerns in ICP-MS environments include exposure to radiofrequency radiation, UV emissions from plasma, and potential contact with hazardous chemicals. Integrated safety systems now incorporate automated sample handling, sealed plasma chambers, and real-time monitoring technologies that minimize operator exposure. Advanced ventilation systems with specialized filtration capabilities have become standard in modern laboratory designs to address aerosol and vapor management.
The miniaturization trend in ICP-MS technology has yielded additional environmental benefits through reduced reagent consumption and waste generation. Microfluidic sample introduction systems can decrease sample volume requirements by orders of magnitude, while maintaining or even improving analytical performance. This development aligns with green chemistry principles and supports sustainable laboratory practices.
Lifecycle assessment of integrated ICP-MS systems has become an important consideration for manufacturers and end-users alike. From raw material sourcing to end-of-life disposal, modern systems are increasingly designed with recyclability and reduced environmental footprint in mind. Several manufacturers have implemented take-back programs for obsolete equipment components, particularly those containing rare earth elements or potentially hazardous materials.
Regulatory compliance represents a critical aspect of ICP-MS implementation, with facilities needing to adhere to increasingly stringent international standards for hazardous waste disposal, air quality management, and worker safety. The European Union's REACH regulations and the United States EPA guidelines have established comprehensive frameworks that directly impact ICP-MS operations, particularly in sample preparation and waste handling processes.
Energy consumption presents another environmental consideration, as traditional ICP-MS systems require substantial power for plasma generation and maintenance. Technological integration has enabled significant improvements in this area, with newer systems incorporating energy-efficient components and intelligent power management algorithms that can reduce consumption by up to 40% compared to systems from a decade ago.
Worker safety concerns in ICP-MS environments include exposure to radiofrequency radiation, UV emissions from plasma, and potential contact with hazardous chemicals. Integrated safety systems now incorporate automated sample handling, sealed plasma chambers, and real-time monitoring technologies that minimize operator exposure. Advanced ventilation systems with specialized filtration capabilities have become standard in modern laboratory designs to address aerosol and vapor management.
The miniaturization trend in ICP-MS technology has yielded additional environmental benefits through reduced reagent consumption and waste generation. Microfluidic sample introduction systems can decrease sample volume requirements by orders of magnitude, while maintaining or even improving analytical performance. This development aligns with green chemistry principles and supports sustainable laboratory practices.
Lifecycle assessment of integrated ICP-MS systems has become an important consideration for manufacturers and end-users alike. From raw material sourcing to end-of-life disposal, modern systems are increasingly designed with recyclability and reduced environmental footprint in mind. Several manufacturers have implemented take-back programs for obsolete equipment components, particularly those containing rare earth elements or potentially hazardous materials.
Standardization and Quality Control Protocols
The standardization and quality control protocols for ICP-MS techniques represent a critical foundation for ensuring reliable, reproducible, and comparable analytical results across different laboratories and applications. As technological integration advances ICP-MS capabilities, establishing robust standardization frameworks becomes increasingly important to maintain analytical integrity.
Current standardization efforts in ICP-MS focus on several key areas, including sample preparation protocols, instrument calibration procedures, and data processing methodologies. International organizations such as ISO, ASTM, and IUPAC have developed comprehensive guidelines that address these aspects, though implementation varies significantly across different sectors and regions.
Quality control measures for integrated ICP-MS systems typically involve multi-level approaches, including system suitability tests, regular performance verification, and continuous monitoring of critical parameters. The integration of automated quality control features in modern ICP-MS platforms has significantly improved consistency, with real-time monitoring systems capable of detecting deviations and triggering corrective actions without operator intervention.
Reference materials play a pivotal role in standardization efforts, serving as benchmarks for method validation and instrument performance verification. The development of certified reference materials specifically designed for complex matrices and emerging applications represents an ongoing challenge in the field, particularly for novel integrated systems that combine ICP-MS with other analytical techniques.
Interlaboratory comparison programs have emerged as valuable tools for assessing method performance and identifying systematic biases across different laboratories. These programs provide essential feedback mechanisms for refining standardization protocols and improving analytical practices, especially when implementing new technological integrations in ICP-MS workflows.
The harmonization of data reporting formats and uncertainty estimation procedures remains a significant challenge in the standardization landscape. As ICP-MS systems generate increasingly complex datasets through technological integration with complementary techniques, establishing standardized approaches for data processing, interpretation, and reporting becomes essential for ensuring comparability of results.
Regulatory compliance frameworks vary considerably across different application domains, creating challenges for laboratories operating in multiple sectors. The development of flexible yet robust standardization protocols that can accommodate diverse regulatory requirements while maintaining scientific rigor represents a key priority for advancing ICP-MS applications in regulated environments.
Current standardization efforts in ICP-MS focus on several key areas, including sample preparation protocols, instrument calibration procedures, and data processing methodologies. International organizations such as ISO, ASTM, and IUPAC have developed comprehensive guidelines that address these aspects, though implementation varies significantly across different sectors and regions.
Quality control measures for integrated ICP-MS systems typically involve multi-level approaches, including system suitability tests, regular performance verification, and continuous monitoring of critical parameters. The integration of automated quality control features in modern ICP-MS platforms has significantly improved consistency, with real-time monitoring systems capable of detecting deviations and triggering corrective actions without operator intervention.
Reference materials play a pivotal role in standardization efforts, serving as benchmarks for method validation and instrument performance verification. The development of certified reference materials specifically designed for complex matrices and emerging applications represents an ongoing challenge in the field, particularly for novel integrated systems that combine ICP-MS with other analytical techniques.
Interlaboratory comparison programs have emerged as valuable tools for assessing method performance and identifying systematic biases across different laboratories. These programs provide essential feedback mechanisms for refining standardization protocols and improving analytical practices, especially when implementing new technological integrations in ICP-MS workflows.
The harmonization of data reporting formats and uncertainty estimation procedures remains a significant challenge in the standardization landscape. As ICP-MS systems generate increasingly complex datasets through technological integration with complementary techniques, establishing standardized approaches for data processing, interpretation, and reporting becomes essential for ensuring comparability of results.
Regulatory compliance frameworks vary considerably across different application domains, creating challenges for laboratories operating in multiple sectors. The development of flexible yet robust standardization protocols that can accommodate diverse regulatory requirements while maintaining scientific rigor represents a key priority for advancing ICP-MS applications in regulated environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!