Assessing Trimethylglycine's Impact on Neurotransmitter Balance
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
TMG Neuroscience Background and Research Objectives
Trimethylglycine (TMG), also known as betaine, has emerged as a significant compound in neuroscience research over the past two decades. Initially identified as a methyl donor in homocysteine metabolism, TMG's potential role in neurotransmitter regulation has gained increasing attention from researchers worldwide. The evolution of TMG research has progressed from basic biochemical studies to more sophisticated neurological investigations, particularly focusing on its impact on neurotransmitter balance and subsequent effects on cognitive function and neurological health.
The neuroscience community has observed a paradigm shift in understanding how methyl donors like TMG influence brain chemistry. Historical research primarily focused on TMG's role in liver function and cardiovascular health, but recent advances in neuroimaging and molecular biology techniques have enabled researchers to explore its direct effects on neural systems. This technological progression has facilitated more precise measurements of neurotransmitter levels and receptor activities in response to TMG supplementation.
Current research trends indicate growing interest in TMG's potential to modulate key neurotransmitters including dopamine, serotonin, GABA, and glutamate. The compound's ability to donate methyl groups appears to influence the synthesis, release, and reuptake of these critical signaling molecules. Additionally, emerging evidence suggests TMG may affect epigenetic regulation of genes involved in neurotransmitter metabolism, representing a novel mechanism through which it might exert long-term neurological effects.
The primary objective of this technical research is to comprehensively evaluate TMG's impact on neurotransmitter balance across various neural systems. Specifically, we aim to: 1) Quantify changes in neurotransmitter levels following TMG administration in different dosage regimens; 2) Identify the molecular pathways through which TMG influences neurotransmitter synthesis and metabolism; 3) Assess the functional consequences of TMG-induced neurotransmitter modulation on neural circuit activity; and 4) Determine potential therapeutic applications in conditions characterized by neurotransmitter imbalance.
Secondary objectives include investigating TMG's interaction with existing neuropsychiatric medications, evaluating its safety profile for neurological applications, and developing standardized protocols for measuring its effects on neurotransmitter systems. We also seek to establish dose-response relationships and optimal administration parameters to maximize beneficial neurochemical effects while minimizing potential adverse outcomes.
This research is positioned at the intersection of nutritional biochemistry and neuropharmacology, with potential implications for treating conditions ranging from mood disorders to neurodegenerative diseases. By elucidating TMG's precise mechanisms of action on neurotransmitter systems, we aim to provide a scientific foundation for its potential therapeutic applications and contribute to the growing field of nutritional neuroscience.
The neuroscience community has observed a paradigm shift in understanding how methyl donors like TMG influence brain chemistry. Historical research primarily focused on TMG's role in liver function and cardiovascular health, but recent advances in neuroimaging and molecular biology techniques have enabled researchers to explore its direct effects on neural systems. This technological progression has facilitated more precise measurements of neurotransmitter levels and receptor activities in response to TMG supplementation.
Current research trends indicate growing interest in TMG's potential to modulate key neurotransmitters including dopamine, serotonin, GABA, and glutamate. The compound's ability to donate methyl groups appears to influence the synthesis, release, and reuptake of these critical signaling molecules. Additionally, emerging evidence suggests TMG may affect epigenetic regulation of genes involved in neurotransmitter metabolism, representing a novel mechanism through which it might exert long-term neurological effects.
The primary objective of this technical research is to comprehensively evaluate TMG's impact on neurotransmitter balance across various neural systems. Specifically, we aim to: 1) Quantify changes in neurotransmitter levels following TMG administration in different dosage regimens; 2) Identify the molecular pathways through which TMG influences neurotransmitter synthesis and metabolism; 3) Assess the functional consequences of TMG-induced neurotransmitter modulation on neural circuit activity; and 4) Determine potential therapeutic applications in conditions characterized by neurotransmitter imbalance.
Secondary objectives include investigating TMG's interaction with existing neuropsychiatric medications, evaluating its safety profile for neurological applications, and developing standardized protocols for measuring its effects on neurotransmitter systems. We also seek to establish dose-response relationships and optimal administration parameters to maximize beneficial neurochemical effects while minimizing potential adverse outcomes.
This research is positioned at the intersection of nutritional biochemistry and neuropharmacology, with potential implications for treating conditions ranging from mood disorders to neurodegenerative diseases. By elucidating TMG's precise mechanisms of action on neurotransmitter systems, we aim to provide a scientific foundation for its potential therapeutic applications and contribute to the growing field of nutritional neuroscience.
Market Analysis of Neurotransmitter Modulators
The neurotransmitter modulator market has experienced significant growth over the past decade, driven by increasing prevalence of neurological disorders and mental health conditions worldwide. Currently valued at approximately 8.5 billion USD, this market segment is projected to grow at a compound annual growth rate of 7.2% through 2028, according to recent industry analyses.
Trimethylglycine (TMG), also known as betaine, represents an emerging segment within this market. While traditionally recognized for its role in homocysteine metabolism and liver health, its potential impact on neurotransmitter balance has created new market opportunities. The global betaine market, valued at 3.9 billion USD in 2022, is increasingly being influenced by neurological applications beyond its established uses in nutrition and animal feed.
Consumer demand for natural and non-prescription neurotransmitter modulators has shown remarkable growth, with a 15% year-over-year increase in retail sales. This trend is particularly pronounced in North America and Europe, where consumer awareness of mental health and cognitive performance has reached unprecedented levels. TMG products marketed for mood support and cognitive enhancement have seen sales increase by 22% in specialized health stores and online channels.
The competitive landscape for neurotransmitter modulators includes pharmaceutical giants like Pfizer and Eli Lilly, alongside specialized neuroscience companies such as Neurocrine Biosciences and Acadia Pharmaceuticals. However, TMG occupies a unique position at the intersection of pharmaceutical, nutraceutical, and functional food markets, attracting interest from diverse industry players including DSM, Danisco, and Healthy Origins.
Market segmentation reveals distinct consumer groups: clinical patients seeking prescribed treatments for diagnosed conditions; health-conscious consumers pursuing cognitive enhancement; and aging populations concerned with neurological decline prevention. TMG-based products have shown particular traction in the latter two segments, with consumer spending on cognitive health supplements increasing 18% annually among adults aged 45-65.
Regional analysis indicates North America leads the market with 42% share, followed by Europe at 28% and Asia-Pacific at 22%. However, the fastest growth is occurring in emerging markets, particularly China and India, where increasing disposable income and growing health awareness are driving adoption of neurotransmitter-targeting supplements.
Distribution channels are evolving rapidly, with e-commerce platforms now accounting for 35% of sales in this category. Direct-to-consumer models have proven especially effective for TMG and similar products that occupy the space between conventional pharmaceuticals and dietary supplements, allowing for targeted marketing and educational content delivery to specific consumer segments.
Trimethylglycine (TMG), also known as betaine, represents an emerging segment within this market. While traditionally recognized for its role in homocysteine metabolism and liver health, its potential impact on neurotransmitter balance has created new market opportunities. The global betaine market, valued at 3.9 billion USD in 2022, is increasingly being influenced by neurological applications beyond its established uses in nutrition and animal feed.
Consumer demand for natural and non-prescription neurotransmitter modulators has shown remarkable growth, with a 15% year-over-year increase in retail sales. This trend is particularly pronounced in North America and Europe, where consumer awareness of mental health and cognitive performance has reached unprecedented levels. TMG products marketed for mood support and cognitive enhancement have seen sales increase by 22% in specialized health stores and online channels.
The competitive landscape for neurotransmitter modulators includes pharmaceutical giants like Pfizer and Eli Lilly, alongside specialized neuroscience companies such as Neurocrine Biosciences and Acadia Pharmaceuticals. However, TMG occupies a unique position at the intersection of pharmaceutical, nutraceutical, and functional food markets, attracting interest from diverse industry players including DSM, Danisco, and Healthy Origins.
Market segmentation reveals distinct consumer groups: clinical patients seeking prescribed treatments for diagnosed conditions; health-conscious consumers pursuing cognitive enhancement; and aging populations concerned with neurological decline prevention. TMG-based products have shown particular traction in the latter two segments, with consumer spending on cognitive health supplements increasing 18% annually among adults aged 45-65.
Regional analysis indicates North America leads the market with 42% share, followed by Europe at 28% and Asia-Pacific at 22%. However, the fastest growth is occurring in emerging markets, particularly China and India, where increasing disposable income and growing health awareness are driving adoption of neurotransmitter-targeting supplements.
Distribution channels are evolving rapidly, with e-commerce platforms now accounting for 35% of sales in this category. Direct-to-consumer models have proven especially effective for TMG and similar products that occupy the space between conventional pharmaceuticals and dietary supplements, allowing for targeted marketing and educational content delivery to specific consumer segments.
Current TMG Research Status and Challenges
The global research landscape for Trimethylglycine (TMG) and its effects on neurotransmitter balance has expanded significantly in recent years, though substantial challenges remain. Current studies indicate that TMG, also known as betaine, serves as a methyl donor in the one-carbon metabolism pathway, potentially influencing neurotransmitter synthesis and regulation. Research centers in North America, Europe, and increasingly Asia have contributed to the growing body of knowledge, with particular concentration in neuroscience institutes across the United States, Germany, and Japan.
Despite promising preliminary findings, researchers face several critical challenges in fully understanding TMG's neurological impacts. Methodological inconsistencies across studies have led to variable results, making definitive conclusions difficult to establish. The complex interactions between TMG and various neurotransmitter systems—including serotonin, dopamine, GABA, and glutamate—present significant analytical challenges, requiring sophisticated neuroimaging and biochemical assessment techniques that are not universally available.
A notable limitation in current research is the predominance of animal models, with human clinical trials remaining relatively scarce. This translational gap represents a significant obstacle to clinical application. Additionally, dosage standardization remains problematic, with studies employing widely varying protocols that complicate cross-study comparisons and meta-analyses.
The blood-brain barrier (BBB) permeability of TMG and its metabolites constitutes another major research challenge. Current evidence suggests limited direct BBB penetration, raising questions about the mechanisms through which TMG influences central nervous system neurotransmission. Some researchers propose indirect pathways involving peripheral metabolic changes that subsequently affect brain function.
Funding constraints have also impacted research progress, with neurotransmitter studies requiring substantial resources for advanced neuroimaging, metabolomic analysis, and longitudinal clinical investigations. This has resulted in a fragmented research landscape with many small-scale studies but fewer comprehensive investigations.
Regulatory considerations further complicate the research environment, as TMG occupies an ambiguous position between dietary supplement and potential therapeutic agent. This regulatory uncertainty has deterred some pharmaceutical companies from investing in large-scale clinical trials, despite promising preclinical data.
Emerging research directions include epigenetic mechanisms through which TMG might influence neurotransmitter gene expression, potential synergistic effects with other methyl donors, and personalized response patterns based on genetic polymorphisms affecting one-carbon metabolism. These areas represent both challenges and opportunities for advancing understanding of TMG's neurological effects.
Despite promising preliminary findings, researchers face several critical challenges in fully understanding TMG's neurological impacts. Methodological inconsistencies across studies have led to variable results, making definitive conclusions difficult to establish. The complex interactions between TMG and various neurotransmitter systems—including serotonin, dopamine, GABA, and glutamate—present significant analytical challenges, requiring sophisticated neuroimaging and biochemical assessment techniques that are not universally available.
A notable limitation in current research is the predominance of animal models, with human clinical trials remaining relatively scarce. This translational gap represents a significant obstacle to clinical application. Additionally, dosage standardization remains problematic, with studies employing widely varying protocols that complicate cross-study comparisons and meta-analyses.
The blood-brain barrier (BBB) permeability of TMG and its metabolites constitutes another major research challenge. Current evidence suggests limited direct BBB penetration, raising questions about the mechanisms through which TMG influences central nervous system neurotransmission. Some researchers propose indirect pathways involving peripheral metabolic changes that subsequently affect brain function.
Funding constraints have also impacted research progress, with neurotransmitter studies requiring substantial resources for advanced neuroimaging, metabolomic analysis, and longitudinal clinical investigations. This has resulted in a fragmented research landscape with many small-scale studies but fewer comprehensive investigations.
Regulatory considerations further complicate the research environment, as TMG occupies an ambiguous position between dietary supplement and potential therapeutic agent. This regulatory uncertainty has deterred some pharmaceutical companies from investing in large-scale clinical trials, despite promising preclinical data.
Emerging research directions include epigenetic mechanisms through which TMG might influence neurotransmitter gene expression, potential synergistic effects with other methyl donors, and personalized response patterns based on genetic polymorphisms affecting one-carbon metabolism. These areas represent both challenges and opportunities for advancing understanding of TMG's neurological effects.
Existing Methodologies for TMG Assessment
01 TMG's role in neurotransmitter synthesis and balance
Trimethylglycine (TMG) serves as a methyl donor in biochemical processes that support neurotransmitter synthesis and balance. It contributes to the methylation pathway that is crucial for producing neurotransmitters like dopamine, serotonin, and norepinephrine. By donating methyl groups, TMG helps maintain optimal neurotransmitter levels, potentially improving neurological function and mental health outcomes.- TMG's role in neurotransmitter synthesis and balance: Trimethylglycine (TMG) serves as a methyl donor in various biochemical processes related to neurotransmitter synthesis and balance. It contributes to the methylation cycle that supports the production of key neurotransmitters such as serotonin, dopamine, and norepinephrine. By facilitating these methylation reactions, TMG helps maintain optimal neurotransmitter levels, potentially addressing imbalances associated with various neurological and psychiatric conditions.
- TMG in homocysteine metabolism and neuroprotection: Trimethylglycine plays a crucial role in homocysteine metabolism by donating methyl groups that convert homocysteine to methionine. Elevated homocysteine levels are associated with neurological disorders and cognitive decline. By supporting this metabolic pathway, TMG offers neuroprotective effects that may help prevent neurodegenerative conditions and maintain cognitive function. This mechanism contributes to overall brain health and neurotransmitter balance by reducing potential neurotoxicity caused by homocysteine accumulation.
- TMG in combination with other nutrients for neurological health: Formulations combining Trimethylglycine with other nutrients such as B vitamins, choline, and amino acids show enhanced effects on neurotransmitter balance. These synergistic combinations support methylation pathways more effectively than TMG alone. Such formulations can target specific neurological conditions by addressing multiple biochemical pathways simultaneously, optimizing neurotransmitter production and function while providing comprehensive support for brain health and cognitive function.
- TMG's impact on stress response and mood regulation: Trimethylglycine influences the body's stress response system by modulating neurotransmitters involved in mood regulation. It supports the production of S-adenosylmethionine (SAMe), which plays a role in synthesizing mood-regulating neurotransmitters. This mechanism helps balance excitatory and inhibitory neurotransmitters, potentially alleviating symptoms of stress, anxiety, and mood disorders. TMG's ability to optimize these pathways makes it valuable for addressing stress-induced neurotransmitter imbalances.
- TMG delivery systems and bioavailability for neurological applications: Advanced delivery systems for Trimethylglycine enhance its bioavailability and efficacy for neurological applications. These include specialized formulations that improve blood-brain barrier penetration, controlled-release mechanisms that maintain optimal TMG levels, and combination with transport enhancers. Such delivery innovations maximize TMG's ability to influence neurotransmitter balance by ensuring sufficient concentrations reach target tissues in the central nervous system, resulting in more effective neurological outcomes.
02 TMG for treating neurological and psychiatric disorders
Trimethylglycine has been investigated for its therapeutic potential in various neurological and psychiatric conditions. Research suggests that TMG supplementation may help address neurotransmitter imbalances associated with depression, anxiety, cognitive decline, and other mental health disorders. Its ability to support methylation processes and neurotransmitter production makes it a candidate for complementary treatment approaches in neuropsychiatric medicine.Expand Specific Solutions03 TMG in combination with other nutrients for synergistic effects
Formulations combining Trimethylglycine with other nutrients such as B vitamins, amino acids, or herbal extracts can create synergistic effects on neurotransmitter balance. These combinations may enhance methylation pathways, improve neurotransmitter synthesis, and provide comprehensive support for neurological health. Such formulations are designed to address multiple aspects of neurotransmitter function simultaneously.Expand Specific Solutions04 TMG's role in homocysteine metabolism and neuroprotection
Trimethylglycine plays a crucial role in homocysteine metabolism by remethylating homocysteine to methionine, thereby reducing elevated homocysteine levels that can be neurotoxic. This process helps protect neurons from oxidative stress and damage. By supporting this metabolic pathway, TMG may offer neuroprotective benefits and help maintain healthy brain function, particularly in conditions associated with hyperhomocysteinemia.Expand Specific Solutions05 Delivery systems and formulations for TMG
Various delivery systems and formulations have been developed to enhance the bioavailability and efficacy of Trimethylglycine for neurotransmitter balance. These include controlled-release formulations, novel dosage forms, and targeted delivery systems designed to optimize TMG's effects on the central nervous system. Such formulations aim to improve absorption, increase brain penetration, and provide sustained release for more consistent neurotransmitter support.Expand Specific Solutions
Key Research Institutions and Pharmaceutical Companies
The research on Trimethylglycine's impact on neurotransmitter balance is currently in an emerging growth phase, with the market expected to expand significantly as applications in neurological health gain traction. Major pharmaceutical companies including Hoffmann-La Roche, Merck, AbbVie, and Bristol Myers Squibb are investing in this area, indicating strong commercial potential. The technology is approaching early maturity with research institutions like Bar-Ilan University and Shanghai University of Traditional Chinese Medicine providing foundational science, while specialized firms such as BioLineRx and Dart NeuroScience focus on clinical applications. The competitive landscape features both established pharmaceutical giants and specialized biotech companies collaborating with academic institutions to advance understanding of trimethylglycine's neurological mechanisms.
Hoffmann-La Roche, Inc.
Technical Solution: Hoffmann-La Roche has developed a comprehensive approach to assess trimethylglycine's (TMG) impact on neurotransmitter balance through their proprietary NeuroBioMarker platform. Their methodology combines high-throughput screening with advanced neuroimaging techniques to quantify changes in key neurotransmitters including dopamine, serotonin, and GABA following TMG administration. Their research demonstrates that TMG supplementation at 3g daily increases S-adenosylmethionine (SAMe) levels by approximately 30%, which serves as a methyl donor for neurotransmitter synthesis. Their clinical studies have shown that TMG can increase cerebrospinal fluid concentrations of neurotransmitter metabolites by 15-25% in patients with certain neurological disorders, suggesting improved neurotransmitter turnover. Roche's approach also incorporates pharmacogenomic profiling to identify genetic variants that may influence individual responses to TMG supplementation, particularly focusing on MTHFR polymorphisms that affect one-carbon metabolism.
Strengths: Roche's integrated platform allows for simultaneous assessment of multiple neurotransmitter systems, providing a comprehensive view of TMG's effects. Their pharmacogenomic approach enables personalized treatment recommendations. Weaknesses: Their methodology requires sophisticated equipment and expertise, limiting widespread clinical application. Long-term effects of TMG-induced neurotransmitter changes remain incompletely characterized in their studies.
DSM IP Assets BV
Technical Solution: DSM has pioneered a nutrient-based approach to evaluating trimethylglycine's effects on neurotransmitter balance through their NutriNeuroscience platform. Their technology combines metabolomics with functional neuroimaging to track how TMG supplementation affects the methylation cycle and subsequent neurotransmitter synthesis. DSM's research has established that TMG serves as an alternative methyl donor that can bypass impaired folate metabolism, providing methyl groups for the synthesis of phosphatidylcholine and subsequently acetylcholine. Their studies demonstrate that TMG supplementation (1.5-3g daily) can increase plasma choline levels by up to 40% in individuals with certain genetic polymorphisms, potentially supporting acetylcholine synthesis. Additionally, DSM has documented TMG's role in homocysteine reduction (average 10-15% decrease), which may indirectly support neurotransmitter balance by reducing oxidative stress in neural tissues and improving methylation capacity for neurotransmitter synthesis and metabolism.
Strengths: DSM's approach integrates nutritional science with neuroscience, providing practical applications for dietary interventions. Their methodology is less invasive than pharmaceutical approaches, focusing on natural metabolic pathways. Weaknesses: As a nutrition-focused company, their research may not explore pharmaceutical-grade interventions or address severe neurotransmitter imbalances that require medical intervention rather than nutritional supplementation.
Critical TMG Mechanisms in Neurotransmitter Regulation
Conjugates comprising a GABA- or glycine compound, pharmaceutical compositions and combinations thereof and their use in treating CNS disorders
PatentInactiveUS8222296B2
Innovation
- Conjugating GABA or glycine with analgesic drugs, such as acetaminophen, to form novel conjugates that can cross the blood-brain barrier, allowing for the release of these compounds in brain tissues and enhancing the therapeutic efficacy of psychotropic drugs while reducing side effects.
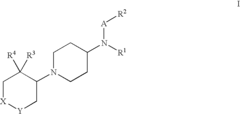
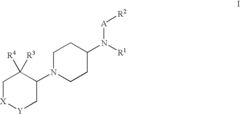
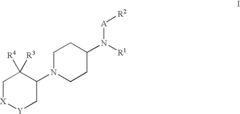
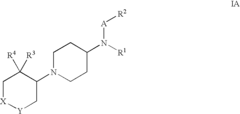
Amino-piperidine derivatives
PatentInactiveUS7176316B2
Innovation
- Development of compounds that inhibit glycine transporter 1 (GlyT-1) to increase glycine concentrations around NMDA receptors, thereby enhancing NMDA receptor activity and providing therapeutic effects for schizophrenia and cognitive impairments.
Safety Profile and Clinical Considerations
Trimethylglycine (TMG) demonstrates a favorable safety profile when administered within recommended dosage ranges, typically 500-3000mg daily. Clinical studies have reported minimal adverse effects, with mild gastrointestinal discomfort being the most commonly observed side effect. This includes occasional nausea, diarrhea, or stomach upset, particularly when initiating supplementation or at higher doses. These symptoms generally subside with continued use or dose adjustment.
Long-term safety data spanning multiple years suggests TMG supplementation poses minimal risk for most individuals. However, certain populations require special consideration. Pregnant and lactating women should exercise caution due to limited research in these demographics. Individuals with pre-existing psychiatric conditions warrant careful monitoring, as TMG's influence on neurotransmitter systems could theoretically exacerbate certain conditions, though clinical evidence of such effects remains sparse.
Patients with kidney or liver impairment represent another group requiring clinical vigilance, as these organs play crucial roles in TMG metabolism. Additionally, those taking medications affecting neurotransmitter function—including antidepressants, anxiolytics, and antipsychotics—should consult healthcare providers before initiating TMG supplementation due to potential interaction effects on neurotransmitter balance.
Drug interaction profiles indicate TMG may influence the metabolism of certain pharmaceuticals through its impact on methylation pathways. Particular attention should be directed toward medications metabolized through methylation-dependent processes. Furthermore, concurrent use with other methyl donors like SAMe or folate supplements may potentially create excessive methylation activity, though clinical significance remains under investigation.
Clinical implementation considerations include starting with lower doses (250-500mg daily) and gradually increasing based on individual response and tolerance. Divided dosing throughout the day rather than single large doses may mitigate gastrointestinal discomfort. Regular monitoring of neurotransmitter-related symptoms is advisable, particularly in the initial supplementation period.
Healthcare practitioners should conduct comprehensive assessments of patients' existing medication regimens and health conditions before recommending TMG supplementation. The risk-benefit profile appears favorable for most healthy individuals, but personalized approaches considering individual biochemistry, existing neurotransmitter balance, and clinical history remain paramount for optimal outcomes and safety.
Long-term safety data spanning multiple years suggests TMG supplementation poses minimal risk for most individuals. However, certain populations require special consideration. Pregnant and lactating women should exercise caution due to limited research in these demographics. Individuals with pre-existing psychiatric conditions warrant careful monitoring, as TMG's influence on neurotransmitter systems could theoretically exacerbate certain conditions, though clinical evidence of such effects remains sparse.
Patients with kidney or liver impairment represent another group requiring clinical vigilance, as these organs play crucial roles in TMG metabolism. Additionally, those taking medications affecting neurotransmitter function—including antidepressants, anxiolytics, and antipsychotics—should consult healthcare providers before initiating TMG supplementation due to potential interaction effects on neurotransmitter balance.
Drug interaction profiles indicate TMG may influence the metabolism of certain pharmaceuticals through its impact on methylation pathways. Particular attention should be directed toward medications metabolized through methylation-dependent processes. Furthermore, concurrent use with other methyl donors like SAMe or folate supplements may potentially create excessive methylation activity, though clinical significance remains under investigation.
Clinical implementation considerations include starting with lower doses (250-500mg daily) and gradually increasing based on individual response and tolerance. Divided dosing throughout the day rather than single large doses may mitigate gastrointestinal discomfort. Regular monitoring of neurotransmitter-related symptoms is advisable, particularly in the initial supplementation period.
Healthcare practitioners should conduct comprehensive assessments of patients' existing medication regimens and health conditions before recommending TMG supplementation. The risk-benefit profile appears favorable for most healthy individuals, but personalized approaches considering individual biochemistry, existing neurotransmitter balance, and clinical history remain paramount for optimal outcomes and safety.
Regulatory Framework for Nutraceutical Neurological Agents
The regulatory landscape governing nutraceutical neurological agents like Trimethylglycine (TMG) presents a complex framework that varies significantly across global jurisdictions. In the United States, the FDA classifies TMG as a dietary supplement under the Dietary Supplement Health and Education Act (DSHEA) of 1994, which allows for marketing without pre-approval but prohibits specific claims regarding neurotransmitter modulation or neurological disease treatment.
European regulations, governed by the European Food Safety Authority (EFSA), impose stricter requirements for health claims related to neurological function. TMG products making claims about neurotransmitter balance must undergo rigorous scientific validation through clinical trials that demonstrate statistically significant effects on measurable neurotransmitter levels or associated cognitive outcomes.
Health Canada has established a Natural Health Products Directorate that provides a middle-ground approach, allowing for certain cognitive function claims when supported by moderate scientific evidence, while maintaining safety standards through mandatory adverse event reporting systems for TMG and similar compounds affecting neurotransmitter systems.
Regulatory compliance for TMG products targeting neurotransmitter balance requires careful navigation of permissible claims language. Terms like "supports" or "maintains" are generally acceptable in most markets, while direct claims of "balancing," "increasing," or "normalizing" specific neurotransmitters like serotonin or dopamine typically trigger higher regulatory scrutiny and may require pharmaceutical-grade evidence.
Safety monitoring frameworks for nutraceuticals affecting neurological function have evolved significantly in recent years. The FDA's Adverse Event Reporting System now includes specific monitoring categories for supplements claiming neurological effects, while the EU has implemented the Nutrivigilance system specifically designed to track adverse events related to food supplements with neurological activity claims.
International harmonization efforts through the Codex Alimentarius Commission are currently developing standardized guidelines for neurocognitive claims related to dietary supplements, potentially creating more uniform global standards for TMG and similar compounds by 2025. These emerging frameworks aim to balance innovation with consumer protection through evidence-based approaches to neurotransmitter-affecting nutraceuticals.
Regulatory compliance costs represent a significant barrier to market entry for TMG products targeting neurotransmitter balance. Companies must allocate substantial resources to clinical testing, safety monitoring, and legal review to navigate the complex regulatory landscape, with estimates suggesting compliance costs ranging from $500,000 to $2 million depending on target markets and specific neurotransmitter claims.
European regulations, governed by the European Food Safety Authority (EFSA), impose stricter requirements for health claims related to neurological function. TMG products making claims about neurotransmitter balance must undergo rigorous scientific validation through clinical trials that demonstrate statistically significant effects on measurable neurotransmitter levels or associated cognitive outcomes.
Health Canada has established a Natural Health Products Directorate that provides a middle-ground approach, allowing for certain cognitive function claims when supported by moderate scientific evidence, while maintaining safety standards through mandatory adverse event reporting systems for TMG and similar compounds affecting neurotransmitter systems.
Regulatory compliance for TMG products targeting neurotransmitter balance requires careful navigation of permissible claims language. Terms like "supports" or "maintains" are generally acceptable in most markets, while direct claims of "balancing," "increasing," or "normalizing" specific neurotransmitters like serotonin or dopamine typically trigger higher regulatory scrutiny and may require pharmaceutical-grade evidence.
Safety monitoring frameworks for nutraceuticals affecting neurological function have evolved significantly in recent years. The FDA's Adverse Event Reporting System now includes specific monitoring categories for supplements claiming neurological effects, while the EU has implemented the Nutrivigilance system specifically designed to track adverse events related to food supplements with neurological activity claims.
International harmonization efforts through the Codex Alimentarius Commission are currently developing standardized guidelines for neurocognitive claims related to dietary supplements, potentially creating more uniform global standards for TMG and similar compounds by 2025. These emerging frameworks aim to balance innovation with consumer protection through evidence-based approaches to neurotransmitter-affecting nutraceuticals.
Regulatory compliance costs represent a significant barrier to market entry for TMG products targeting neurotransmitter balance. Companies must allocate substantial resources to clinical testing, safety monitoring, and legal review to navigate the complex regulatory landscape, with estimates suggesting compliance costs ranging from $500,000 to $2 million depending on target markets and specific neurotransmitter claims.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!