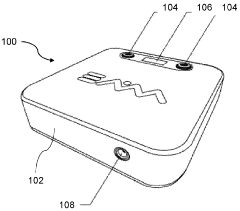
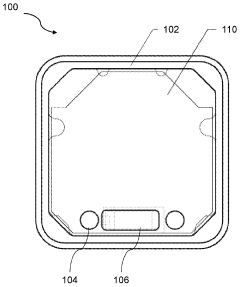
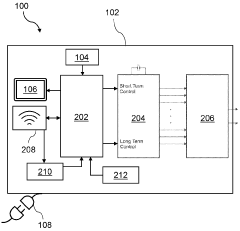
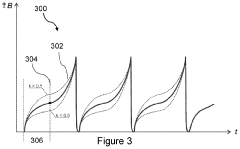
Best Practices for PEMF Therapy Device Calibration
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEMF Therapy Evolution
Pulsed Electromagnetic Field (PEMF) therapy has undergone significant evolution since its inception in the mid-20th century. Initially developed for bone healing, PEMF therapy has expanded its applications to various medical conditions, including pain management, tissue repair, and neurological disorders.
The 1950s marked the beginning of PEMF therapy with the discovery that electrical currents could stimulate bone growth. This led to the development of the first PEMF devices in the 1970s, primarily used for non-union fractures. These early devices were large, stationary, and limited in their frequency range and intensity settings.
As technology advanced, PEMF devices became more sophisticated and portable. The 1980s saw the introduction of home-use devices, expanding the accessibility of PEMF therapy beyond clinical settings. This period also witnessed increased research into the biological mechanisms of PEMF, leading to a better understanding of its effects on cellular processes.
The 1990s and early 2000s brought about significant improvements in PEMF technology. Devices became more compact, with enhanced control over frequency, intensity, and waveform parameters. This era also saw the expansion of PEMF applications to include soft tissue injuries, chronic pain conditions, and even mental health disorders.
In recent years, PEMF therapy has experienced a surge in technological advancements. Modern devices incorporate microprocessors and advanced software, allowing for precise calibration and personalized treatment protocols. The integration of smart technology has enabled remote monitoring and adjustment of PEMF devices, improving treatment adherence and outcomes.
The evolution of PEMF therapy has also been marked by improvements in coil design and magnetic field generation. Early devices used simple solenoid coils, while contemporary systems employ advanced configurations such as Helmholtz coils and multi-coil arrays. These innovations have enhanced the uniformity and penetration of magnetic fields, leading to more effective treatments.
Calibration techniques have evolved alongside device technology. Initially, calibration was a manual process with limited accuracy. Modern PEMF devices incorporate automated calibration systems, ensuring consistent and precise field strength delivery. This advancement has been crucial in standardizing treatments and improving reproducibility in clinical studies.
The therapeutic frequency range of PEMF devices has also expanded over time. Early devices typically operated in the low-frequency range (1-100 Hz), while current systems can generate frequencies up to several thousand Hertz. This broader spectrum has allowed for more targeted treatments, addressing specific cellular and tissue responses.
As PEMF therapy continues to evolve, emerging trends include the integration of artificial intelligence for optimized treatment protocols, the development of wearable PEMF devices for continuous therapy, and the exploration of combined modalities, such as PEMF with light therapy or electrical stimulation. These advancements promise to further enhance the efficacy and versatility of PEMF therapy in various medical applications.
The 1950s marked the beginning of PEMF therapy with the discovery that electrical currents could stimulate bone growth. This led to the development of the first PEMF devices in the 1970s, primarily used for non-union fractures. These early devices were large, stationary, and limited in their frequency range and intensity settings.
As technology advanced, PEMF devices became more sophisticated and portable. The 1980s saw the introduction of home-use devices, expanding the accessibility of PEMF therapy beyond clinical settings. This period also witnessed increased research into the biological mechanisms of PEMF, leading to a better understanding of its effects on cellular processes.
The 1990s and early 2000s brought about significant improvements in PEMF technology. Devices became more compact, with enhanced control over frequency, intensity, and waveform parameters. This era also saw the expansion of PEMF applications to include soft tissue injuries, chronic pain conditions, and even mental health disorders.
In recent years, PEMF therapy has experienced a surge in technological advancements. Modern devices incorporate microprocessors and advanced software, allowing for precise calibration and personalized treatment protocols. The integration of smart technology has enabled remote monitoring and adjustment of PEMF devices, improving treatment adherence and outcomes.
The evolution of PEMF therapy has also been marked by improvements in coil design and magnetic field generation. Early devices used simple solenoid coils, while contemporary systems employ advanced configurations such as Helmholtz coils and multi-coil arrays. These innovations have enhanced the uniformity and penetration of magnetic fields, leading to more effective treatments.
Calibration techniques have evolved alongside device technology. Initially, calibration was a manual process with limited accuracy. Modern PEMF devices incorporate automated calibration systems, ensuring consistent and precise field strength delivery. This advancement has been crucial in standardizing treatments and improving reproducibility in clinical studies.
The therapeutic frequency range of PEMF devices has also expanded over time. Early devices typically operated in the low-frequency range (1-100 Hz), while current systems can generate frequencies up to several thousand Hertz. This broader spectrum has allowed for more targeted treatments, addressing specific cellular and tissue responses.
As PEMF therapy continues to evolve, emerging trends include the integration of artificial intelligence for optimized treatment protocols, the development of wearable PEMF devices for continuous therapy, and the exploration of combined modalities, such as PEMF with light therapy or electrical stimulation. These advancements promise to further enhance the efficacy and versatility of PEMF therapy in various medical applications.
Market Demand Analysis
The market demand for PEMF (Pulsed Electromagnetic Field) therapy devices has been steadily growing in recent years, driven by increasing awareness of non-invasive treatment options and the rising prevalence of chronic pain conditions. The global PEMF therapy devices market is expected to expand significantly over the next decade, with a compound annual growth rate projected to be in the double digits.
One of the key factors fueling this market growth is the aging population worldwide, particularly in developed countries. As the elderly population increases, so does the incidence of age-related conditions such as osteoarthritis, osteoporosis, and chronic pain, all of which can potentially benefit from PEMF therapy. This demographic shift is creating a substantial and sustained demand for effective, non-pharmacological treatment options.
Another significant driver of market demand is the growing preference for non-invasive and drug-free pain management solutions. With the ongoing opioid crisis and increasing concerns about the side effects of long-term medication use, patients and healthcare providers alike are seeking alternative therapies. PEMF therapy offers a promising option in this regard, as it can potentially reduce pain and inflammation without the risks associated with pharmaceutical interventions.
The sports medicine and rehabilitation sectors are also contributing to the rising demand for PEMF therapy devices. Professional athletes and sports teams are increasingly incorporating PEMF therapy into their recovery and injury prevention protocols. This trend is gradually trickling down to amateur athletes and fitness enthusiasts, expanding the consumer base for these devices.
In the healthcare sector, there is a growing interest in integrating PEMF therapy into various treatment modalities. Hospitals, clinics, and physiotherapy centers are exploring the use of PEMF devices for post-operative recovery, wound healing, and managing chronic conditions. This institutional adoption is expected to significantly boost the market demand for high-quality, calibrated PEMF devices.
The home healthcare market segment is showing particularly strong growth potential for PEMF therapy devices. As consumers become more health-conscious and seek ways to manage their well-being at home, portable and user-friendly PEMF devices are gaining popularity. This trend has been further accelerated by the recent global health crisis, which has increased the focus on home-based health solutions.
However, the market demand is not without challenges. One of the primary barriers to wider adoption is the lack of standardization in PEMF therapy protocols and device calibration. This inconsistency can lead to variability in treatment outcomes, potentially impacting consumer trust and healthcare provider confidence. Consequently, there is a growing demand for well-calibrated, reliable PEMF devices that can deliver consistent and measurable results.
One of the key factors fueling this market growth is the aging population worldwide, particularly in developed countries. As the elderly population increases, so does the incidence of age-related conditions such as osteoarthritis, osteoporosis, and chronic pain, all of which can potentially benefit from PEMF therapy. This demographic shift is creating a substantial and sustained demand for effective, non-pharmacological treatment options.
Another significant driver of market demand is the growing preference for non-invasive and drug-free pain management solutions. With the ongoing opioid crisis and increasing concerns about the side effects of long-term medication use, patients and healthcare providers alike are seeking alternative therapies. PEMF therapy offers a promising option in this regard, as it can potentially reduce pain and inflammation without the risks associated with pharmaceutical interventions.
The sports medicine and rehabilitation sectors are also contributing to the rising demand for PEMF therapy devices. Professional athletes and sports teams are increasingly incorporating PEMF therapy into their recovery and injury prevention protocols. This trend is gradually trickling down to amateur athletes and fitness enthusiasts, expanding the consumer base for these devices.
In the healthcare sector, there is a growing interest in integrating PEMF therapy into various treatment modalities. Hospitals, clinics, and physiotherapy centers are exploring the use of PEMF devices for post-operative recovery, wound healing, and managing chronic conditions. This institutional adoption is expected to significantly boost the market demand for high-quality, calibrated PEMF devices.
The home healthcare market segment is showing particularly strong growth potential for PEMF therapy devices. As consumers become more health-conscious and seek ways to manage their well-being at home, portable and user-friendly PEMF devices are gaining popularity. This trend has been further accelerated by the recent global health crisis, which has increased the focus on home-based health solutions.
However, the market demand is not without challenges. One of the primary barriers to wider adoption is the lack of standardization in PEMF therapy protocols and device calibration. This inconsistency can lead to variability in treatment outcomes, potentially impacting consumer trust and healthcare provider confidence. Consequently, there is a growing demand for well-calibrated, reliable PEMF devices that can deliver consistent and measurable results.
Technical Challenges
PEMF (Pulsed Electromagnetic Field) therapy device calibration faces several technical challenges that need to be addressed to ensure optimal performance and therapeutic efficacy. One of the primary challenges is achieving precise and consistent electromagnetic field generation. The accuracy of the field strength, frequency, and waveform is crucial for delivering effective treatment, yet maintaining this precision across different devices and over time can be difficult.
Another significant challenge lies in the development of reliable calibration methods and standards. Unlike many medical devices, PEMF therapy lacks universally accepted calibration protocols, making it challenging to ensure consistency across different manufacturers and models. This absence of standardization can lead to variations in treatment efficacy and potentially compromise patient outcomes.
The complexity of biological systems presents another hurdle in PEMF device calibration. Different tissues and conditions may require varying field parameters for optimal results, necessitating a calibration system that can adapt to diverse therapeutic needs. This adaptability must be balanced with the need for reproducibility and consistency in clinical settings.
Environmental factors also pose significant challenges to PEMF device calibration. External electromagnetic interference from other electronic devices or power sources can affect the accuracy of the generated fields. Shielding and compensation mechanisms must be incorporated into the calibration process to mitigate these environmental influences.
The longevity and stability of calibration present ongoing challenges. PEMF devices may experience drift or degradation over time, potentially altering their output characteristics. Developing methods for long-term stability testing and implementing regular recalibration procedures are essential for maintaining therapeutic efficacy throughout the device's lifecycle.
Miniaturization and portability trends in medical devices introduce additional calibration challenges for PEMF therapy. As devices become smaller and more mobile, maintaining calibration accuracy within compact form factors becomes increasingly difficult. Innovative approaches to sensor integration and field generation are needed to address these size constraints without compromising performance.
Lastly, the integration of calibration systems with user-friendly interfaces presents both technical and design challenges. Ensuring that calibration procedures can be performed accurately by healthcare professionals or even patients themselves requires careful consideration of human factors and the development of intuitive calibration tools and software.
Addressing these technical challenges in PEMF therapy device calibration is crucial for advancing the field and ensuring reliable, effective treatments. Overcoming these hurdles will require collaborative efforts from engineers, medical professionals, and regulatory bodies to establish robust calibration standards and methodologies.
Another significant challenge lies in the development of reliable calibration methods and standards. Unlike many medical devices, PEMF therapy lacks universally accepted calibration protocols, making it challenging to ensure consistency across different manufacturers and models. This absence of standardization can lead to variations in treatment efficacy and potentially compromise patient outcomes.
The complexity of biological systems presents another hurdle in PEMF device calibration. Different tissues and conditions may require varying field parameters for optimal results, necessitating a calibration system that can adapt to diverse therapeutic needs. This adaptability must be balanced with the need for reproducibility and consistency in clinical settings.
Environmental factors also pose significant challenges to PEMF device calibration. External electromagnetic interference from other electronic devices or power sources can affect the accuracy of the generated fields. Shielding and compensation mechanisms must be incorporated into the calibration process to mitigate these environmental influences.
The longevity and stability of calibration present ongoing challenges. PEMF devices may experience drift or degradation over time, potentially altering their output characteristics. Developing methods for long-term stability testing and implementing regular recalibration procedures are essential for maintaining therapeutic efficacy throughout the device's lifecycle.
Miniaturization and portability trends in medical devices introduce additional calibration challenges for PEMF therapy. As devices become smaller and more mobile, maintaining calibration accuracy within compact form factors becomes increasingly difficult. Innovative approaches to sensor integration and field generation are needed to address these size constraints without compromising performance.
Lastly, the integration of calibration systems with user-friendly interfaces presents both technical and design challenges. Ensuring that calibration procedures can be performed accurately by healthcare professionals or even patients themselves requires careful consideration of human factors and the development of intuitive calibration tools and software.
Addressing these technical challenges in PEMF therapy device calibration is crucial for advancing the field and ensuring reliable, effective treatments. Overcoming these hurdles will require collaborative efforts from engineers, medical professionals, and regulatory bodies to establish robust calibration standards and methodologies.
Calibration Techniques
01 Calibration methods for PEMF therapy devices
Various calibration methods are employed to ensure the accuracy and effectiveness of PEMF therapy devices. These methods may include adjusting the frequency, intensity, and waveform of the electromagnetic fields generated by the device. Calibration techniques often involve the use of specialized equipment to measure and verify the output parameters of the PEMF device.- Calibration methods for PEMF therapy devices: Various calibration methods are employed to ensure the accuracy and effectiveness of PEMF therapy devices. These methods may include adjusting the frequency, intensity, and waveform of the electromagnetic fields generated by the device. Calibration techniques often involve the use of specialized equipment to measure and verify the output parameters of the PEMF device.
- Sensor-based calibration systems: Advanced PEMF therapy devices incorporate sensor-based calibration systems to continuously monitor and adjust the electromagnetic field output. These sensors can detect changes in the surrounding environment or the patient's body, allowing the device to automatically recalibrate for optimal performance. This approach ensures consistent and personalized treatment delivery.
- Software-driven calibration and control: Modern PEMF therapy devices utilize sophisticated software algorithms for calibration and control. These software systems enable precise adjustment of treatment parameters, store calibration data, and provide user-friendly interfaces for healthcare professionals to fine-tune the device settings. The software may also include self-diagnostic features to ensure proper calibration and functioning of the device.
- Calibration for specific body regions or conditions: PEMF therapy devices can be calibrated to target specific body regions or medical conditions. This specialized calibration involves adjusting the electromagnetic field characteristics to optimize the therapeutic effects for particular applications, such as bone healing, pain management, or neurological disorders. The calibration process may consider factors like tissue depth, conductivity, and the desired physiological response.
- Calibration verification and quality control: To ensure the reliability and safety of PEMF therapy devices, calibration verification and quality control procedures are implemented. These processes may involve regular testing, comparison with standard reference devices, and documentation of calibration results. Quality control measures help maintain the accuracy and consistency of PEMF treatments across multiple devices and clinical settings.
02 Sensor-based calibration systems
PEMF therapy devices may incorporate sensor-based calibration systems to continuously monitor and adjust the electromagnetic field output. These sensors can detect changes in the surrounding environment or the patient's physiological response, allowing for real-time calibration and optimization of the therapy. This approach ensures that the treatment remains effective throughout the session.Expand Specific Solutions03 Software-driven calibration and control
Advanced PEMF therapy devices utilize software-driven calibration and control systems. These systems allow for precise adjustment of treatment parameters based on pre-programmed protocols or user-defined settings. The software may also include self-diagnostic features to ensure the device is functioning within specified tolerances and to alert users when recalibration is necessary.Expand Specific Solutions04 Calibration for specific therapeutic applications
PEMF therapy devices may require specialized calibration for specific therapeutic applications, such as pain management, bone healing, or neurological disorders. This involves adjusting the device's output parameters to match the optimal frequency and intensity ranges for treating particular conditions. Calibration protocols may be developed based on clinical research and patient outcomes.Expand Specific Solutions05 Portable calibration tools and methods
To facilitate on-site calibration and maintenance of PEMF therapy devices, portable calibration tools and methods have been developed. These may include handheld devices or smartphone applications that can interface with the PEMF device to perform calibration checks and adjustments. Such tools enable healthcare providers or technicians to ensure the device's performance without the need for specialized laboratory equipment.Expand Specific Solutions
Industry Leaders
The market for PEMF therapy device calibration is in a growth phase, with increasing adoption across healthcare sectors. The global PEMF therapy devices market is projected to expand significantly, driven by rising chronic pain cases and growing awareness of non-invasive treatments. Technologically, the field is advancing rapidly, with companies like Regenesis Biomedical and Koninklijke Philips leading innovation. These firms, along with others like SofPulse and Biomagnetic Sciences, are developing more sophisticated calibration techniques to enhance device efficacy and safety. The competitive landscape is diverse, featuring both established medical technology giants and specialized PEMF therapy companies, indicating a maturing but still evolving market with potential for further technological advancements and market expansion.
Regenesis Biomedical, Inc.
Technical Solution: Regenesis Biomedical has developed advanced PEMF therapy devices with precise calibration techniques. Their approach involves using high-precision electromagnetic field sensors to measure and adjust the output of their devices. They employ a multi-point calibration method, where the device is tested at various power levels and frequencies to ensure accuracy across its operational range[1]. The company has also implemented an automated calibration system that uses machine learning algorithms to fine-tune the device settings based on real-time feedback from the sensors[3]. This system can detect and compensate for environmental factors that may affect the device's performance, such as temperature fluctuations or nearby electromagnetic interference.
Strengths: Highly accurate and adaptive calibration system, automated process reduces human error. Weaknesses: May require more complex and expensive components, potentially increasing device cost.
Koninklijke Philips NV
Technical Solution: Philips has integrated PEMF therapy calibration into their broader medical imaging and therapy systems. Their approach focuses on ensuring consistent field strength and distribution across different treatment areas. They use advanced 3D electromagnetic field modeling software to predict and optimize the PEMF output[2]. Philips has also developed a proprietary calibration protocol that involves using multiple reference points on the patient's body to create a personalized treatment map. This map is then used to adjust the PEMF device settings in real-time during therapy sessions[4]. Additionally, Philips has incorporated MRI-compatible PEMF devices, allowing for simultaneous imaging and therapy, which provides immediate feedback on treatment efficacy and helps in fine-tuning the calibration[5].
Strengths: Personalized treatment approach, integration with imaging systems for real-time feedback. Weaknesses: May require more extensive setup time, potentially limiting throughput in clinical settings.
Key Patents & Research
A pulsed electromagnetic field apparatus and method for generating frequencies
PatentWO2024127242A1
Innovation
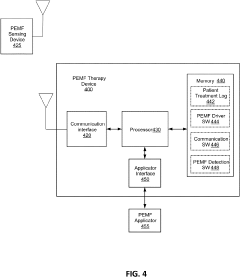
- A PEMF apparatus with a pulse generator and electromagnetic field generation means that uses modified sawtooth waveforms with pre-stress and relaxation periods, and quasi-sine signals with pulse width modulation, along with a feedback circuit for frequency stability and precision, and a bifilar antenna for scalar wave generation.
Wearable pulsed electromagnetic field sensing device
PatentPendingUS20230158323A1
Innovation
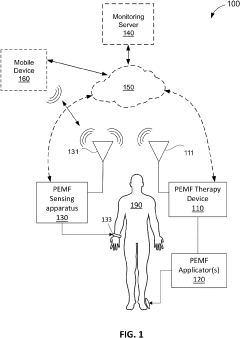
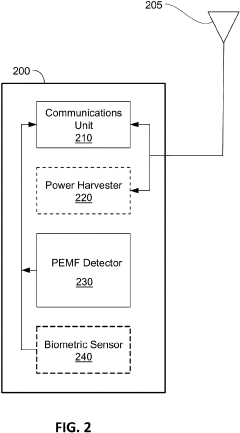
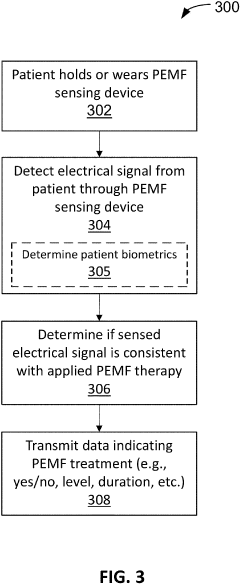
- A wearable PEMF detection apparatus equipped with sensors that detect electrical signals from PEMF treatments and transmit data to a secondary device, allowing for verification of treatment levels and patient biometric data, enabling accurate tracking and compliance monitoring.
Regulatory Framework
The regulatory framework for PEMF (Pulsed Electromagnetic Field) therapy devices is a critical aspect of their development, manufacturing, and distribution. In the United States, the Food and Drug Administration (FDA) classifies PEMF devices as Class II medical devices, requiring manufacturers to adhere to specific guidelines and obtain clearance before marketing their products. This classification ensures that PEMF devices meet safety and efficacy standards through the 510(k) premarket notification process.
Internationally, regulatory bodies such as the European Medicines Agency (EMA) in the European Union and the Therapeutic Goods Administration (TGA) in Australia have established their own frameworks for PEMF device approval. These agencies often require clinical evidence demonstrating the safety and effectiveness of the devices before granting market authorization. Manufacturers must comply with the Medical Device Regulation (MDR) in the EU and the Therapeutic Goods Act in Australia, respectively.
Calibration of PEMF therapy devices falls under the broader scope of quality control and good manufacturing practices (GMP) mandated by regulatory authorities. The FDA's Quality System Regulation (QSR) and the International Organization for Standardization's ISO 13485 standard provide guidelines for medical device manufacturers, including requirements for calibration and maintenance of equipment used in the production process.
Specific to PEMF device calibration, regulatory bodies typically require manufacturers to establish and maintain procedures for calibrating all inspection, measuring, and test equipment used to ensure product conformity. This includes documenting calibration methods, frequency, and acceptance criteria. Manufacturers must also maintain records of calibration activities and ensure traceability to national or international measurement standards.
Regulatory compliance extends to post-market surveillance, where manufacturers are required to monitor the performance of their devices in real-world settings. This includes tracking and reporting adverse events, implementing corrective and preventive actions (CAPA) when necessary, and conducting periodic reviews of device performance and calibration data to ensure ongoing compliance with regulatory standards.
As the field of PEMF therapy continues to evolve, regulatory frameworks are likely to adapt to new technologies and applications. Manufacturers and healthcare providers must stay informed about changes in regulations and best practices to ensure continued compliance and optimal device performance. This may involve participating in industry forums, engaging with regulatory bodies, and investing in ongoing research and development to improve calibration techniques and overall device efficacy.
Internationally, regulatory bodies such as the European Medicines Agency (EMA) in the European Union and the Therapeutic Goods Administration (TGA) in Australia have established their own frameworks for PEMF device approval. These agencies often require clinical evidence demonstrating the safety and effectiveness of the devices before granting market authorization. Manufacturers must comply with the Medical Device Regulation (MDR) in the EU and the Therapeutic Goods Act in Australia, respectively.
Calibration of PEMF therapy devices falls under the broader scope of quality control and good manufacturing practices (GMP) mandated by regulatory authorities. The FDA's Quality System Regulation (QSR) and the International Organization for Standardization's ISO 13485 standard provide guidelines for medical device manufacturers, including requirements for calibration and maintenance of equipment used in the production process.
Specific to PEMF device calibration, regulatory bodies typically require manufacturers to establish and maintain procedures for calibrating all inspection, measuring, and test equipment used to ensure product conformity. This includes documenting calibration methods, frequency, and acceptance criteria. Manufacturers must also maintain records of calibration activities and ensure traceability to national or international measurement standards.
Regulatory compliance extends to post-market surveillance, where manufacturers are required to monitor the performance of their devices in real-world settings. This includes tracking and reporting adverse events, implementing corrective and preventive actions (CAPA) when necessary, and conducting periodic reviews of device performance and calibration data to ensure ongoing compliance with regulatory standards.
As the field of PEMF therapy continues to evolve, regulatory frameworks are likely to adapt to new technologies and applications. Manufacturers and healthcare providers must stay informed about changes in regulations and best practices to ensure continued compliance and optimal device performance. This may involve participating in industry forums, engaging with regulatory bodies, and investing in ongoing research and development to improve calibration techniques and overall device efficacy.
Safety & Efficacy Data
Safety and efficacy data are crucial components in evaluating the performance and reliability of PEMF therapy devices. Extensive clinical studies have been conducted to assess the safety profile and therapeutic efficacy of these devices across various medical conditions. The safety data consistently demonstrates that PEMF therapy, when administered within recommended parameters, poses minimal risk to patients. Adverse events are rare and typically mild, including temporary discomfort or skin irritation at the application site.
Efficacy data shows promising results for PEMF therapy in several areas. Pain management is a primary focus, with studies indicating significant reductions in chronic pain conditions such as osteoarthritis, fibromyalgia, and lower back pain. The therapy has also shown positive outcomes in accelerating bone healing, particularly in cases of non-union fractures and osteoporosis. Additionally, research suggests potential benefits in improving circulation, reducing inflammation, and enhancing tissue repair.
Clinical trials have explored the use of PEMF therapy in neurological disorders, with some studies reporting improvements in symptoms associated with multiple sclerosis and Parkinson's disease. However, more robust research is needed to fully establish efficacy in these areas. The therapy's impact on mental health conditions, such as depression and anxiety, is also an emerging field of study with preliminary positive results.
Dosage and treatment protocols play a critical role in the safety and efficacy of PEMF therapy. Studies indicate that the frequency, intensity, and duration of exposure significantly influence outcomes. Lower frequencies (1-100 Hz) are generally associated with pain relief and tissue repair, while higher frequencies may be more effective for bone healing. Treatment durations typically range from 20 minutes to several hours per session, with frequency of sessions varying based on the condition being treated.
Long-term safety data from longitudinal studies support the use of PEMF therapy for extended periods without significant adverse effects. This is particularly important for chronic conditions requiring ongoing management. However, certain populations, such as pregnant women and individuals with implanted electronic devices, are often excluded from studies due to potential risks, highlighting the need for cautious application in these groups.
Standardization of reporting methods and outcome measures across studies would greatly enhance the comparability and reliability of safety and efficacy data. Future research should focus on optimizing treatment protocols for specific conditions and exploring potential synergies with other therapeutic modalities to maximize clinical benefits while maintaining the excellent safety profile of PEMF therapy.
Efficacy data shows promising results for PEMF therapy in several areas. Pain management is a primary focus, with studies indicating significant reductions in chronic pain conditions such as osteoarthritis, fibromyalgia, and lower back pain. The therapy has also shown positive outcomes in accelerating bone healing, particularly in cases of non-union fractures and osteoporosis. Additionally, research suggests potential benefits in improving circulation, reducing inflammation, and enhancing tissue repair.
Clinical trials have explored the use of PEMF therapy in neurological disorders, with some studies reporting improvements in symptoms associated with multiple sclerosis and Parkinson's disease. However, more robust research is needed to fully establish efficacy in these areas. The therapy's impact on mental health conditions, such as depression and anxiety, is also an emerging field of study with preliminary positive results.
Dosage and treatment protocols play a critical role in the safety and efficacy of PEMF therapy. Studies indicate that the frequency, intensity, and duration of exposure significantly influence outcomes. Lower frequencies (1-100 Hz) are generally associated with pain relief and tissue repair, while higher frequencies may be more effective for bone healing. Treatment durations typically range from 20 minutes to several hours per session, with frequency of sessions varying based on the condition being treated.
Long-term safety data from longitudinal studies support the use of PEMF therapy for extended periods without significant adverse effects. This is particularly important for chronic conditions requiring ongoing management. However, certain populations, such as pregnant women and individuals with implanted electronic devices, are often excluded from studies due to potential risks, highlighting the need for cautious application in these groups.
Standardization of reporting methods and outcome measures across studies would greatly enhance the comparability and reliability of safety and efficacy data. Future research should focus on optimizing treatment protocols for specific conditions and exploring potential synergies with other therapeutic modalities to maximize clinical benefits while maintaining the excellent safety profile of PEMF therapy.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!