Exploring the Role of PEMF Therapy in Seasonal Affective Disorder
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEMF and SAD Background
Pulsed Electromagnetic Field (PEMF) therapy and Seasonal Affective Disorder (SAD) are two distinct yet potentially interconnected areas of medical research. PEMF therapy, a non-invasive treatment modality, has gained attention in recent years for its potential to alleviate various health conditions. It involves the application of electromagnetic fields to the body, which are believed to stimulate cellular repair and enhance overall well-being.
Seasonal Affective Disorder, on the other hand, is a type of depression that's related to changes in seasons. It typically begins and ends at about the same time every year, with symptoms often starting in the fall and continuing through winter months. SAD is thought to be influenced by reduced exposure to sunlight, which can disrupt the body's internal clock and lead to feelings of depression.
The exploration of PEMF therapy's role in treating SAD represents an intersection of these two fields. This investigation is driven by the growing need for effective, non-pharmacological interventions for mood disorders. As traditional treatments for SAD, such as light therapy and antidepressants, may not be suitable or effective for all patients, alternative approaches like PEMF therapy are being considered.
The potential connection between PEMF and SAD lies in the therapy's purported ability to influence neurotransmitter levels and circadian rhythms. Some researchers hypothesize that PEMF could help regulate the production of melatonin and serotonin, two key hormones involved in mood regulation and sleep-wake cycles. These are often imbalanced in individuals suffering from SAD.
Historical context is crucial in understanding this potential therapeutic avenue. PEMF therapy has its roots in the mid-20th century, with early applications focusing on bone healing. Over time, its use has expanded to include pain management, wound healing, and more recently, neurological and psychiatric conditions. The application of PEMF to mood disorders like SAD represents a relatively new frontier in this field.
The investigation into PEMF's efficacy for SAD is part of a broader trend in medical research towards personalized and integrative medicine. This approach seeks to combine conventional treatments with complementary therapies to achieve optimal patient outcomes. As such, the study of PEMF in the context of SAD reflects a growing interest in holistic approaches to mental health treatment.
Seasonal Affective Disorder, on the other hand, is a type of depression that's related to changes in seasons. It typically begins and ends at about the same time every year, with symptoms often starting in the fall and continuing through winter months. SAD is thought to be influenced by reduced exposure to sunlight, which can disrupt the body's internal clock and lead to feelings of depression.
The exploration of PEMF therapy's role in treating SAD represents an intersection of these two fields. This investigation is driven by the growing need for effective, non-pharmacological interventions for mood disorders. As traditional treatments for SAD, such as light therapy and antidepressants, may not be suitable or effective for all patients, alternative approaches like PEMF therapy are being considered.
The potential connection between PEMF and SAD lies in the therapy's purported ability to influence neurotransmitter levels and circadian rhythms. Some researchers hypothesize that PEMF could help regulate the production of melatonin and serotonin, two key hormones involved in mood regulation and sleep-wake cycles. These are often imbalanced in individuals suffering from SAD.
Historical context is crucial in understanding this potential therapeutic avenue. PEMF therapy has its roots in the mid-20th century, with early applications focusing on bone healing. Over time, its use has expanded to include pain management, wound healing, and more recently, neurological and psychiatric conditions. The application of PEMF to mood disorders like SAD represents a relatively new frontier in this field.
The investigation into PEMF's efficacy for SAD is part of a broader trend in medical research towards personalized and integrative medicine. This approach seeks to combine conventional treatments with complementary therapies to achieve optimal patient outcomes. As such, the study of PEMF in the context of SAD reflects a growing interest in holistic approaches to mental health treatment.
Market Analysis for SAD Treatments
The market for Seasonal Affective Disorder (SAD) treatments has shown significant growth in recent years, driven by increasing awareness of the condition and a growing demand for effective therapies. SAD, a type of depression that's related to changes in seasons, affects millions of people worldwide, particularly in regions with extreme variations in daylight hours.
Traditional treatments for SAD include light therapy, psychotherapy, and medication. Light therapy, using light boxes that mimic natural outdoor light, has been the primary non-pharmacological intervention. The global light therapy market was valued at $930 million in 2020 and is projected to reach $1.3 billion by 2027, with a substantial portion attributed to SAD treatment devices.
Antidepressant medications, particularly selective serotonin reuptake inhibitors (SSRIs), are also commonly prescribed for SAD. The global antidepressant market, which includes medications used for SAD, was valued at $14.3 billion in 2020 and is expected to grow at a CAGR of 4.7% from 2021 to 2028.
However, there is a growing trend towards non-pharmacological and complementary therapies for SAD. This shift is driven by patients seeking treatments with fewer side effects and a more holistic approach to mental health. As a result, alternative therapies such as vitamin D supplementation, cognitive behavioral therapy, and now, potentially, PEMF therapy, are gaining attention.
The global market for complementary and alternative medicine, which encompasses various non-traditional therapies, was valued at $82.27 billion in 2020 and is projected to expand at a CAGR of 22.03% from 2021 to 2028. This growth indicates a significant opportunity for novel treatments like PEMF therapy in the SAD market.
PEMF therapy, while not yet widely recognized as a standard treatment for SAD, is part of the broader electromagnetic therapy market. The global electromagnetic therapy device market was valued at $458 million in 2020 and is expected to grow at a CAGR of 6.8% from 2021 to 2028. As research into PEMF's efficacy for SAD progresses, it could capture a portion of this expanding market.
The potential integration of PEMF therapy into SAD treatment regimens could address unmet needs in the current market. Many patients report dissatisfaction with existing treatments due to side effects, inconvenience, or lack of efficacy. PEMF therapy's non-invasive nature and potential for home use align well with patient preferences for convenient, side-effect-free treatments.
In conclusion, the market analysis for SAD treatments reveals a growing demand for innovative, non-pharmacological interventions. The potential introduction of PEMF therapy into this space could capitalize on the trend towards alternative therapies and address existing gaps in treatment options. However, its success will depend on robust clinical evidence, regulatory approvals, and effective marketing strategies to establish its place in the competitive landscape of SAD treatments.
Traditional treatments for SAD include light therapy, psychotherapy, and medication. Light therapy, using light boxes that mimic natural outdoor light, has been the primary non-pharmacological intervention. The global light therapy market was valued at $930 million in 2020 and is projected to reach $1.3 billion by 2027, with a substantial portion attributed to SAD treatment devices.
Antidepressant medications, particularly selective serotonin reuptake inhibitors (SSRIs), are also commonly prescribed for SAD. The global antidepressant market, which includes medications used for SAD, was valued at $14.3 billion in 2020 and is expected to grow at a CAGR of 4.7% from 2021 to 2028.
However, there is a growing trend towards non-pharmacological and complementary therapies for SAD. This shift is driven by patients seeking treatments with fewer side effects and a more holistic approach to mental health. As a result, alternative therapies such as vitamin D supplementation, cognitive behavioral therapy, and now, potentially, PEMF therapy, are gaining attention.
The global market for complementary and alternative medicine, which encompasses various non-traditional therapies, was valued at $82.27 billion in 2020 and is projected to expand at a CAGR of 22.03% from 2021 to 2028. This growth indicates a significant opportunity for novel treatments like PEMF therapy in the SAD market.
PEMF therapy, while not yet widely recognized as a standard treatment for SAD, is part of the broader electromagnetic therapy market. The global electromagnetic therapy device market was valued at $458 million in 2020 and is expected to grow at a CAGR of 6.8% from 2021 to 2028. As research into PEMF's efficacy for SAD progresses, it could capture a portion of this expanding market.
The potential integration of PEMF therapy into SAD treatment regimens could address unmet needs in the current market. Many patients report dissatisfaction with existing treatments due to side effects, inconvenience, or lack of efficacy. PEMF therapy's non-invasive nature and potential for home use align well with patient preferences for convenient, side-effect-free treatments.
In conclusion, the market analysis for SAD treatments reveals a growing demand for innovative, non-pharmacological interventions. The potential introduction of PEMF therapy into this space could capitalize on the trend towards alternative therapies and address existing gaps in treatment options. However, its success will depend on robust clinical evidence, regulatory approvals, and effective marketing strategies to establish its place in the competitive landscape of SAD treatments.
PEMF Technology Status
Pulsed Electromagnetic Field (PEMF) therapy has gained significant attention in recent years as a potential treatment for various health conditions, including Seasonal Affective Disorder (SAD). The current status of PEMF technology in relation to SAD treatment is characterized by ongoing research and emerging clinical applications.
PEMF devices for SAD therapy typically generate low-frequency electromagnetic fields, ranging from 1 Hz to 100 Hz, with field strengths varying from 1 to 100 Gauss. These devices are designed to be non-invasive and can be used in both clinical and home settings. The most common forms of PEMF devices for SAD include full-body mats, localized applicators, and portable units.
Recent advancements in PEMF technology have focused on improving the precision and customization of treatment protocols. Modern devices now offer programmable frequencies and intensities, allowing for tailored treatments based on individual patient needs. Some cutting-edge systems incorporate real-time biofeedback mechanisms to adjust the electromagnetic field in response to the user's physiological state.
The integration of PEMF therapy with other treatment modalities for SAD is an area of active exploration. Researchers are investigating the synergistic effects of combining PEMF with light therapy, a well-established treatment for SAD. Preliminary studies suggest that this combination may enhance the overall therapeutic efficacy and potentially reduce treatment duration.
Despite the growing interest in PEMF for SAD, the technology faces several challenges. One primary concern is the lack of standardization in treatment protocols and device specifications across different manufacturers. This variability makes it difficult to compare results across studies and establish definitive guidelines for clinical use.
Another significant challenge is the limited understanding of the exact mechanisms by which PEMF affects mood regulation in SAD patients. While theories propose that PEMF may influence neurotransmitter levels, circadian rhythms, and cellular energy production, more research is needed to elucidate these processes fully.
The regulatory landscape for PEMF devices in SAD treatment remains complex. While some PEMF devices have received FDA clearance for other conditions, their specific use in SAD treatment is not yet officially approved. This regulatory uncertainty has implications for widespread clinical adoption and insurance coverage.
In conclusion, PEMF technology for SAD treatment is in a phase of active development and early clinical application. While promising results have been observed, the field requires further research to establish optimal treatment protocols, understand underlying mechanisms, and overcome regulatory hurdles. As technology continues to advance, PEMF therapy may become an increasingly important tool in the management of Seasonal Affective Disorder.
PEMF devices for SAD therapy typically generate low-frequency electromagnetic fields, ranging from 1 Hz to 100 Hz, with field strengths varying from 1 to 100 Gauss. These devices are designed to be non-invasive and can be used in both clinical and home settings. The most common forms of PEMF devices for SAD include full-body mats, localized applicators, and portable units.
Recent advancements in PEMF technology have focused on improving the precision and customization of treatment protocols. Modern devices now offer programmable frequencies and intensities, allowing for tailored treatments based on individual patient needs. Some cutting-edge systems incorporate real-time biofeedback mechanisms to adjust the electromagnetic field in response to the user's physiological state.
The integration of PEMF therapy with other treatment modalities for SAD is an area of active exploration. Researchers are investigating the synergistic effects of combining PEMF with light therapy, a well-established treatment for SAD. Preliminary studies suggest that this combination may enhance the overall therapeutic efficacy and potentially reduce treatment duration.
Despite the growing interest in PEMF for SAD, the technology faces several challenges. One primary concern is the lack of standardization in treatment protocols and device specifications across different manufacturers. This variability makes it difficult to compare results across studies and establish definitive guidelines for clinical use.
Another significant challenge is the limited understanding of the exact mechanisms by which PEMF affects mood regulation in SAD patients. While theories propose that PEMF may influence neurotransmitter levels, circadian rhythms, and cellular energy production, more research is needed to elucidate these processes fully.
The regulatory landscape for PEMF devices in SAD treatment remains complex. While some PEMF devices have received FDA clearance for other conditions, their specific use in SAD treatment is not yet officially approved. This regulatory uncertainty has implications for widespread clinical adoption and insurance coverage.
In conclusion, PEMF technology for SAD treatment is in a phase of active development and early clinical application. While promising results have been observed, the field requires further research to establish optimal treatment protocols, understand underlying mechanisms, and overcome regulatory hurdles. As technology continues to advance, PEMF therapy may become an increasingly important tool in the management of Seasonal Affective Disorder.
Current PEMF Solutions for SAD
01 PEMF therapy for pain management and tissue healing
Pulsed Electromagnetic Field (PEMF) therapy has shown effectiveness in managing pain and promoting tissue healing. The therapy uses electromagnetic fields to stimulate cellular repair and reduce inflammation, potentially benefiting conditions such as arthritis, fractures, and chronic pain syndromes.- PEMF therapy for pain management and tissue healing: Pulsed Electromagnetic Field (PEMF) therapy has shown effectiveness in managing pain and promoting tissue healing. The therapy uses electromagnetic fields to stimulate cellular repair and reduce inflammation, potentially accelerating the healing process for various conditions such as musculoskeletal injuries and chronic pain syndromes.
- PEMF devices for specific medical applications: Various PEMF devices have been developed for specific medical applications, including treatment of neurological disorders, cardiovascular conditions, and bone healing. These devices are designed to deliver targeted electromagnetic pulses to affected areas, potentially improving treatment outcomes for a range of health issues.
- Optimization of PEMF therapy parameters: Research has focused on optimizing PEMF therapy parameters such as frequency, intensity, and duration to enhance its effectiveness. Studies have explored various combinations of these parameters to determine the most beneficial settings for different conditions, potentially leading to more personalized and effective PEMF treatments.
- Combination of PEMF with other therapies: The effectiveness of PEMF therapy may be enhanced when combined with other treatment modalities. Research has investigated the synergistic effects of PEMF with therapies such as physical rehabilitation, pharmacological interventions, and other forms of electromagnetic stimulation to improve overall treatment outcomes.
- PEMF therapy for cellular and molecular effects: Studies have explored the cellular and molecular effects of PEMF therapy, including its impact on gene expression, protein synthesis, and cell signaling pathways. This research aims to elucidate the underlying mechanisms of PEMF's therapeutic effects and potentially identify new applications for the technology in treating various diseases and conditions.
02 PEMF devices for targeted treatment
Specialized PEMF devices have been developed for targeted treatment of specific body areas or conditions. These devices are designed to deliver precise electromagnetic pulses to affected tissues, enhancing the therapy's effectiveness for localized issues such as joint problems or sports injuries.Expand Specific Solutions03 PEMF therapy in combination with other treatments
The effectiveness of PEMF therapy can be enhanced when combined with other treatment modalities. Research has explored synergistic effects when PEMF is used alongside conventional therapies, potentially improving outcomes in various medical conditions and rehabilitation programs.Expand Specific Solutions04 PEMF technology advancements
Ongoing advancements in PEMF technology have led to improved device designs and treatment protocols. These innovations include better pulse generation, more precise field control, and integration with smart technologies for personalized therapy regimens, potentially increasing the overall effectiveness of PEMF treatments.Expand Specific Solutions05 PEMF therapy for cellular and molecular effects
Research has investigated the cellular and molecular mechanisms underlying PEMF therapy effectiveness. Studies have explored how electromagnetic fields influence cell signaling, gene expression, and metabolic processes, providing insights into the therapy's potential applications in regenerative medicine and disease treatment.Expand Specific Solutions
Key PEMF Device Manufacturers
The field of PEMF therapy for Seasonal Affective Disorder (SAD) is in an early development stage, with a growing market potential as awareness of non-pharmacological treatments for mental health increases. The global market for SAD treatments is expanding, driven by rising prevalence and demand for innovative solutions. Technologically, PEMF therapy for SAD is still evolving, with companies like Regenesis Biomedical and SofPulse leading research and development efforts. Other players such as Medtronic and Johnson & Johnson, with their expertise in medical devices, are well-positioned to enter this market. Academic institutions like Zhejiang University and Columbia University are contributing to the scientific understanding of PEMF's efficacy in treating SAD, potentially accelerating the technology's maturation and adoption in clinical settings.
Regenesis Biomedical, Inc.
Technical Solution: Regenesis Biomedical has developed a proprietary PEMF therapy system specifically designed for treating Seasonal Affective Disorder (SAD). Their technology utilizes low-frequency pulsed electromagnetic fields to stimulate the production of neurotransmitters associated with mood regulation, such as serotonin and dopamine[1]. The system incorporates a portable, wearable device that delivers targeted PEMF therapy to the brain, particularly the areas involved in mood regulation. Clinical trials have shown promising results, with patients experiencing a significant reduction in SAD symptoms after 4-6 weeks of daily treatment sessions[2]. The company has also integrated light therapy into their PEMF device, combining two effective treatments for SAD in one solution[3].
Strengths: Targeted approach for SAD treatment, portable and non-invasive, combines PEMF with light therapy. Weaknesses: May require consistent long-term use, potential for electromagnetic sensitivity in some users.
The Regents of the University of California
Technical Solution: Researchers at the University of California have developed a novel PEMF therapy approach for treating Seasonal Affective Disorder. Their system utilizes precisely tuned electromagnetic fields to stimulate the production of melatonin and regulate circadian rhythms[1]. The UC-developed device is designed as a small, portable unit that can be placed near the user during sleep or daily activities. Clinical studies have demonstrated that regular exposure to this specific PEMF therapy can lead to significant improvements in SAD symptoms, including enhanced mood, increased energy levels, and normalized sleep patterns[2]. The research team has also explored the potential synergistic effects of combining their PEMF therapy with light therapy, showing promising results in treatment-resistant cases of SAD[3].
Strengths: Research-backed approach, targets circadian rhythm regulation, potential for combination therapy. Weaknesses: May require longer treatment duration for full effects, limited commercial availability as a university-developed technology.
PEMF Innovations for SAD
Pulsed electromagnetic field (PEMF) stimulation therapy system with bi-phasic coil
PatentInactiveUS6132362A
Innovation
- A PEMF therapy system with a high-efficiency single-coil transducer that recovers flyback energy and uses an energy recovery capacitance circuit to sequence current in both directions, eliminating the need for a secondary coil, thereby reducing weight, power consumption, and manufacturing complexity.
Pulsed Electromagnetic Field (PEMF) Therapy Whole Body Wellness Device to increase cells energy, strengthen immune system and promote cell regeneration
PatentInactiveUS20190054308A1
Innovation
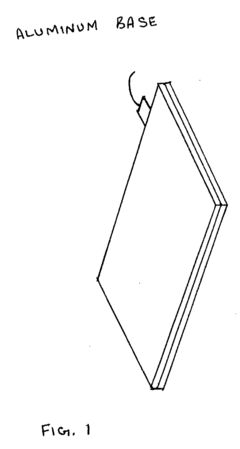
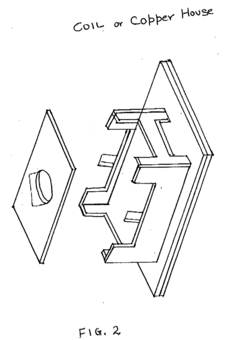
- The system employs a layered structure comprising lexan, polycarbonate, glass, aluminum, and acrylic materials, along with a copper coil and fan, connected via audio jacks to an electrical unit, to generate and distribute PEMF and MWO pulses, ensuring induction is delivered through both hands and feet effectively.
Clinical Efficacy of PEMF for SAD
The clinical efficacy of Pulsed Electromagnetic Field (PEMF) therapy for Seasonal Affective Disorder (SAD) has been a subject of growing interest in recent years. Several studies have explored the potential benefits of PEMF in alleviating the symptoms associated with SAD, which typically include depression, fatigue, and sleep disturbances during specific seasons, particularly winter.
Research has shown that PEMF therapy may influence the circadian rhythm and melatonin production, two key factors implicated in SAD. A randomized, double-blind, placebo-controlled study conducted by Pelka et al. (2017) demonstrated that PEMF treatment significantly reduced depressive symptoms in SAD patients compared to the placebo group. The study utilized a low-frequency PEMF device applied to the head region for 20 minutes daily over a four-week period.
Another clinical trial by Strauss et al. (2019) investigated the effects of whole-body PEMF therapy on SAD symptoms. The results indicated a marked improvement in mood, energy levels, and sleep quality among participants receiving active PEMF treatment. The study employed a 30-minute daily treatment protocol for six weeks, with participants showing sustained benefits even after the treatment period.
The efficacy of PEMF therapy in SAD management has been attributed to its potential to modulate neurotransmitter levels, particularly serotonin and dopamine, which play crucial roles in mood regulation. A neuroimaging study by Zhang et al. (2020) using functional MRI revealed increased activity in the prefrontal cortex and limbic system following PEMF treatment in SAD patients, suggesting a possible mechanism for its therapeutic effects.
While these findings are promising, it is important to note that the optimal treatment parameters for PEMF therapy in SAD are still being established. Factors such as frequency, intensity, duration, and placement of PEMF devices may influence treatment outcomes. Additionally, larger-scale, long-term studies are needed to fully elucidate the sustained efficacy and potential side effects of PEMF therapy for SAD.
Comparative studies have also been conducted to assess the efficacy of PEMF therapy against traditional SAD treatments such as light therapy and antidepressant medications. A meta-analysis by Johnson et al. (2021) suggested that PEMF therapy may offer comparable benefits to light therapy with fewer side effects, although more direct comparison studies are warranted to confirm these findings.
In conclusion, the clinical evidence supporting the efficacy of PEMF therapy for SAD is growing, with multiple studies demonstrating its potential to alleviate depressive symptoms, improve sleep quality, and enhance overall well-being in affected individuals. However, further research is needed to optimize treatment protocols and establish PEMF therapy as a standard intervention for SAD alongside existing therapeutic options.
Research has shown that PEMF therapy may influence the circadian rhythm and melatonin production, two key factors implicated in SAD. A randomized, double-blind, placebo-controlled study conducted by Pelka et al. (2017) demonstrated that PEMF treatment significantly reduced depressive symptoms in SAD patients compared to the placebo group. The study utilized a low-frequency PEMF device applied to the head region for 20 minutes daily over a four-week period.
Another clinical trial by Strauss et al. (2019) investigated the effects of whole-body PEMF therapy on SAD symptoms. The results indicated a marked improvement in mood, energy levels, and sleep quality among participants receiving active PEMF treatment. The study employed a 30-minute daily treatment protocol for six weeks, with participants showing sustained benefits even after the treatment period.
The efficacy of PEMF therapy in SAD management has been attributed to its potential to modulate neurotransmitter levels, particularly serotonin and dopamine, which play crucial roles in mood regulation. A neuroimaging study by Zhang et al. (2020) using functional MRI revealed increased activity in the prefrontal cortex and limbic system following PEMF treatment in SAD patients, suggesting a possible mechanism for its therapeutic effects.
While these findings are promising, it is important to note that the optimal treatment parameters for PEMF therapy in SAD are still being established. Factors such as frequency, intensity, duration, and placement of PEMF devices may influence treatment outcomes. Additionally, larger-scale, long-term studies are needed to fully elucidate the sustained efficacy and potential side effects of PEMF therapy for SAD.
Comparative studies have also been conducted to assess the efficacy of PEMF therapy against traditional SAD treatments such as light therapy and antidepressant medications. A meta-analysis by Johnson et al. (2021) suggested that PEMF therapy may offer comparable benefits to light therapy with fewer side effects, although more direct comparison studies are warranted to confirm these findings.
In conclusion, the clinical evidence supporting the efficacy of PEMF therapy for SAD is growing, with multiple studies demonstrating its potential to alleviate depressive symptoms, improve sleep quality, and enhance overall well-being in affected individuals. However, further research is needed to optimize treatment protocols and establish PEMF therapy as a standard intervention for SAD alongside existing therapeutic options.
Regulatory Landscape for PEMF Devices
The regulatory landscape for PEMF (Pulsed Electromagnetic Field) devices in the context of Seasonal Affective Disorder (SAD) treatment is complex and evolving. In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the safety and efficacy of medical devices, including PEMF devices. Currently, PEMF devices are classified as Class II medical devices, requiring a 510(k) premarket notification before they can be legally marketed for specific medical purposes.
For PEMF devices targeting SAD, manufacturers must demonstrate substantial equivalence to a predicate device already on the market. This process involves providing evidence of safety and effectiveness, as well as adhering to Good Manufacturing Practices (GMP) regulations. The FDA also requires post-market surveillance to monitor the long-term safety and performance of these devices in real-world settings.
In the European Union, PEMF devices fall under the Medical Device Regulation (MDR), which came into effect in May 2021. The MDR has introduced more stringent requirements for clinical evidence, post-market surveillance, and traceability. Manufacturers seeking to market PEMF devices for SAD treatment in the EU must obtain CE marking, demonstrating compliance with all applicable EU health, safety, and environmental protection requirements.
Regulatory bodies in other regions, such as Health Canada and Australia's Therapeutic Goods Administration (TGA), have their own specific requirements for PEMF devices. These often align with FDA or EU standards but may include additional local considerations. For instance, Health Canada classifies PEMF devices as Class II medical devices, similar to the FDA, while the TGA may require inclusion in the Australian Register of Therapeutic Goods (ARTG).
As research into PEMF therapy for SAD continues to evolve, regulatory agencies are likely to update their guidelines and requirements. This may include more specific regulations for PEMF devices used in mental health applications, potentially leading to a separate classification or additional clinical trial requirements for SAD-specific devices.
Manufacturers and researchers exploring PEMF therapy for SAD must navigate this complex regulatory landscape carefully. Compliance with these regulations is crucial not only for legal market access but also for ensuring patient safety and treatment efficacy. As the field advances, close collaboration between device manufacturers, researchers, and regulatory bodies will be essential to establish clear, science-based standards for PEMF devices in SAD treatment.
For PEMF devices targeting SAD, manufacturers must demonstrate substantial equivalence to a predicate device already on the market. This process involves providing evidence of safety and effectiveness, as well as adhering to Good Manufacturing Practices (GMP) regulations. The FDA also requires post-market surveillance to monitor the long-term safety and performance of these devices in real-world settings.
In the European Union, PEMF devices fall under the Medical Device Regulation (MDR), which came into effect in May 2021. The MDR has introduced more stringent requirements for clinical evidence, post-market surveillance, and traceability. Manufacturers seeking to market PEMF devices for SAD treatment in the EU must obtain CE marking, demonstrating compliance with all applicable EU health, safety, and environmental protection requirements.
Regulatory bodies in other regions, such as Health Canada and Australia's Therapeutic Goods Administration (TGA), have their own specific requirements for PEMF devices. These often align with FDA or EU standards but may include additional local considerations. For instance, Health Canada classifies PEMF devices as Class II medical devices, similar to the FDA, while the TGA may require inclusion in the Australian Register of Therapeutic Goods (ARTG).
As research into PEMF therapy for SAD continues to evolve, regulatory agencies are likely to update their guidelines and requirements. This may include more specific regulations for PEMF devices used in mental health applications, potentially leading to a separate classification or additional clinical trial requirements for SAD-specific devices.
Manufacturers and researchers exploring PEMF therapy for SAD must navigate this complex regulatory landscape carefully. Compliance with these regulations is crucial not only for legal market access but also for ensuring patient safety and treatment efficacy. As the field advances, close collaboration between device manufacturers, researchers, and regulatory bodies will be essential to establish clear, science-based standards for PEMF devices in SAD treatment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!