How to Ensure Quality Control in PEMF Therapy Equipment Production?
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEMF Therapy Background and Objectives
Pulsed Electromagnetic Field (PEMF) therapy has emerged as a promising non-invasive treatment modality in recent decades. This technology harnesses the power of electromagnetic fields to stimulate cellular repair and regeneration, offering potential benefits for various health conditions. The evolution of PEMF therapy can be traced back to the mid-20th century, with significant advancements in understanding and application occurring in the past 30 years.
The primary objective of PEMF therapy is to promote healing and alleviate pain by influencing cellular behavior and enhancing the body's natural recovery processes. As research in this field progresses, the technology has found applications in treating musculoskeletal disorders, chronic pain, and even neurological conditions. The growing interest in non-pharmacological interventions has further propelled the development and adoption of PEMF therapy.
In recent years, the PEMF therapy market has witnessed substantial growth, driven by increasing awareness of its potential benefits and a shift towards preventive healthcare. This growth trajectory is expected to continue, with projections indicating a compound annual growth rate of over 5% in the coming years. The expanding applications of PEMF therapy across various medical specialties contribute to its rising prominence in the healthcare landscape.
As the demand for PEMF therapy equipment increases, ensuring quality control in production becomes paramount. The efficacy and safety of PEMF therapy heavily rely on the precision and reliability of the equipment used. Manufacturers face the challenge of consistently producing devices that meet stringent regulatory standards while delivering the intended therapeutic effects.
The technological landscape of PEMF therapy is characterized by ongoing innovations in device design, signal generation, and treatment protocols. These advancements aim to enhance the effectiveness of therapy, improve user experience, and expand the range of treatable conditions. Consequently, quality control measures must evolve in tandem with these technological developments to maintain the highest standards of safety and efficacy.
The objectives of quality control in PEMF therapy equipment production are multifaceted. They encompass ensuring consistent electromagnetic field generation, maintaining precise frequency and intensity control, and guaranteeing the durability and reliability of devices. Additionally, quality control measures must address the unique challenges posed by the integration of electronic components, power management systems, and user interfaces in PEMF devices.
As the field of PEMF therapy continues to evolve, quality control strategies must adapt to new scientific findings and regulatory requirements. This dynamic environment necessitates a comprehensive approach to quality assurance, encompassing all stages of product development and manufacturing. The ultimate goal is to deliver PEMF therapy equipment that not only meets current standards but also anticipates future advancements in the field, ensuring optimal patient outcomes and maintaining trust in this promising therapeutic modality.
The primary objective of PEMF therapy is to promote healing and alleviate pain by influencing cellular behavior and enhancing the body's natural recovery processes. As research in this field progresses, the technology has found applications in treating musculoskeletal disorders, chronic pain, and even neurological conditions. The growing interest in non-pharmacological interventions has further propelled the development and adoption of PEMF therapy.
In recent years, the PEMF therapy market has witnessed substantial growth, driven by increasing awareness of its potential benefits and a shift towards preventive healthcare. This growth trajectory is expected to continue, with projections indicating a compound annual growth rate of over 5% in the coming years. The expanding applications of PEMF therapy across various medical specialties contribute to its rising prominence in the healthcare landscape.
As the demand for PEMF therapy equipment increases, ensuring quality control in production becomes paramount. The efficacy and safety of PEMF therapy heavily rely on the precision and reliability of the equipment used. Manufacturers face the challenge of consistently producing devices that meet stringent regulatory standards while delivering the intended therapeutic effects.
The technological landscape of PEMF therapy is characterized by ongoing innovations in device design, signal generation, and treatment protocols. These advancements aim to enhance the effectiveness of therapy, improve user experience, and expand the range of treatable conditions. Consequently, quality control measures must evolve in tandem with these technological developments to maintain the highest standards of safety and efficacy.
The objectives of quality control in PEMF therapy equipment production are multifaceted. They encompass ensuring consistent electromagnetic field generation, maintaining precise frequency and intensity control, and guaranteeing the durability and reliability of devices. Additionally, quality control measures must address the unique challenges posed by the integration of electronic components, power management systems, and user interfaces in PEMF devices.
As the field of PEMF therapy continues to evolve, quality control strategies must adapt to new scientific findings and regulatory requirements. This dynamic environment necessitates a comprehensive approach to quality assurance, encompassing all stages of product development and manufacturing. The ultimate goal is to deliver PEMF therapy equipment that not only meets current standards but also anticipates future advancements in the field, ensuring optimal patient outcomes and maintaining trust in this promising therapeutic modality.
Market Analysis for PEMF Devices
The PEMF (Pulsed Electromagnetic Field) therapy equipment market has been experiencing significant growth in recent years, driven by increasing awareness of non-invasive treatment options and the rising prevalence of chronic diseases. The global PEMF devices market is expected to continue its upward trajectory, with a compound annual growth rate (CAGR) projected to be in the double digits over the next five years.
The market for PEMF devices can be segmented based on application areas, including orthopedic conditions, pain management, neurological disorders, and wound healing. Orthopedic applications currently dominate the market share, owing to the effectiveness of PEMF therapy in treating conditions such as osteoarthritis, fractures, and osteoporosis. However, the pain management segment is anticipated to witness the fastest growth due to the increasing adoption of PEMF devices for chronic pain relief.
Geographically, North America holds the largest market share, attributed to the high prevalence of chronic diseases, well-established healthcare infrastructure, and favorable reimbursement policies. Europe follows closely, with Germany and the UK being key markets. The Asia-Pacific region is expected to exhibit the highest growth rate, driven by improving healthcare access, rising disposable incomes, and growing awareness of alternative therapies.
The PEMF devices market is characterized by the presence of both established players and new entrants. Key market players are focusing on product innovations, such as developing portable and user-friendly devices for home use. This trend is expected to continue, potentially expanding the market reach to a broader consumer base.
Consumer demand for PEMF devices is influenced by factors such as the aging population, increasing sports injuries, and the growing preference for non-pharmacological pain management solutions. The COVID-19 pandemic has also contributed to market growth, as patients seek alternative therapies that can be administered at home.
Challenges in the PEMF devices market include regulatory hurdles, particularly in obtaining FDA approval for new devices, and the need for more extensive clinical evidence to support efficacy claims. Additionally, the high cost of advanced PEMF equipment may limit adoption in emerging markets.
Looking ahead, technological advancements are expected to play a crucial role in shaping the market landscape. Integration of PEMF devices with smart technologies, development of wearable PEMF solutions, and customization of treatment protocols are likely to drive market growth and improve patient outcomes.
The market for PEMF devices can be segmented based on application areas, including orthopedic conditions, pain management, neurological disorders, and wound healing. Orthopedic applications currently dominate the market share, owing to the effectiveness of PEMF therapy in treating conditions such as osteoarthritis, fractures, and osteoporosis. However, the pain management segment is anticipated to witness the fastest growth due to the increasing adoption of PEMF devices for chronic pain relief.
Geographically, North America holds the largest market share, attributed to the high prevalence of chronic diseases, well-established healthcare infrastructure, and favorable reimbursement policies. Europe follows closely, with Germany and the UK being key markets. The Asia-Pacific region is expected to exhibit the highest growth rate, driven by improving healthcare access, rising disposable incomes, and growing awareness of alternative therapies.
The PEMF devices market is characterized by the presence of both established players and new entrants. Key market players are focusing on product innovations, such as developing portable and user-friendly devices for home use. This trend is expected to continue, potentially expanding the market reach to a broader consumer base.
Consumer demand for PEMF devices is influenced by factors such as the aging population, increasing sports injuries, and the growing preference for non-pharmacological pain management solutions. The COVID-19 pandemic has also contributed to market growth, as patients seek alternative therapies that can be administered at home.
Challenges in the PEMF devices market include regulatory hurdles, particularly in obtaining FDA approval for new devices, and the need for more extensive clinical evidence to support efficacy claims. Additionally, the high cost of advanced PEMF equipment may limit adoption in emerging markets.
Looking ahead, technological advancements are expected to play a crucial role in shaping the market landscape. Integration of PEMF devices with smart technologies, development of wearable PEMF solutions, and customization of treatment protocols are likely to drive market growth and improve patient outcomes.
PEMF Equipment Manufacturing Challenges
The production of Pulsed Electromagnetic Field (PEMF) therapy equipment presents several significant challenges that manufacturers must address to ensure quality control and regulatory compliance. One of the primary difficulties lies in maintaining consistent electromagnetic field generation across different devices. This requires precise calibration of components and rigorous testing procedures to verify that each unit produces the intended field strength and frequency patterns.
Another major challenge is the integration of complex electronic systems within compact, user-friendly designs. PEMF devices often incorporate microprocessors, power management circuits, and user interfaces, all of which must function reliably in a small form factor. Ensuring proper heat dissipation and electromagnetic shielding within these confined spaces adds another layer of complexity to the manufacturing process.
Material selection poses additional challenges, as PEMF devices must be constructed from non-ferromagnetic materials to prevent interference with the generated fields. This limits the range of materials available for structural components and necessitates careful sourcing and quality control of raw materials. Furthermore, the biocompatibility of materials in contact with the user's skin must be guaranteed, requiring extensive testing and documentation.
The durability and longevity of PEMF equipment are critical factors that manufacturers must address. These devices are often subjected to frequent use and transportation, necessitating robust construction that can withstand mechanical stress and environmental factors. Developing effective sealing methods to protect internal components from moisture and dust ingress is essential for maintaining long-term performance and safety.
Regulatory compliance presents a significant hurdle in PEMF equipment production. Manufacturers must navigate a complex landscape of international standards and certifications, including electromagnetic compatibility (EMC) regulations, electrical safety standards, and medical device directives. Meeting these requirements often involves extensive documentation, rigorous testing protocols, and potentially costly design modifications.
Lastly, the variability in treatment protocols and user requirements across different medical applications complicates the design and manufacturing process. PEMF devices may need to offer a range of field strengths, frequencies, and waveforms to accommodate diverse therapeutic needs. This versatility must be built into the equipment without compromising reliability or ease of use, requiring sophisticated software development and user interface design.
Addressing these manufacturing challenges demands a multidisciplinary approach, combining expertise in electronics, materials science, regulatory affairs, and medical device engineering. Manufacturers must implement robust quality management systems, invest in advanced testing equipment, and maintain close collaboration with healthcare professionals to ensure that PEMF therapy equipment meets the highest standards of safety, efficacy, and reliability.
Another major challenge is the integration of complex electronic systems within compact, user-friendly designs. PEMF devices often incorporate microprocessors, power management circuits, and user interfaces, all of which must function reliably in a small form factor. Ensuring proper heat dissipation and electromagnetic shielding within these confined spaces adds another layer of complexity to the manufacturing process.
Material selection poses additional challenges, as PEMF devices must be constructed from non-ferromagnetic materials to prevent interference with the generated fields. This limits the range of materials available for structural components and necessitates careful sourcing and quality control of raw materials. Furthermore, the biocompatibility of materials in contact with the user's skin must be guaranteed, requiring extensive testing and documentation.
The durability and longevity of PEMF equipment are critical factors that manufacturers must address. These devices are often subjected to frequent use and transportation, necessitating robust construction that can withstand mechanical stress and environmental factors. Developing effective sealing methods to protect internal components from moisture and dust ingress is essential for maintaining long-term performance and safety.
Regulatory compliance presents a significant hurdle in PEMF equipment production. Manufacturers must navigate a complex landscape of international standards and certifications, including electromagnetic compatibility (EMC) regulations, electrical safety standards, and medical device directives. Meeting these requirements often involves extensive documentation, rigorous testing protocols, and potentially costly design modifications.
Lastly, the variability in treatment protocols and user requirements across different medical applications complicates the design and manufacturing process. PEMF devices may need to offer a range of field strengths, frequencies, and waveforms to accommodate diverse therapeutic needs. This versatility must be built into the equipment without compromising reliability or ease of use, requiring sophisticated software development and user interface design.
Addressing these manufacturing challenges demands a multidisciplinary approach, combining expertise in electronics, materials science, regulatory affairs, and medical device engineering. Manufacturers must implement robust quality management systems, invest in advanced testing equipment, and maintain close collaboration with healthcare professionals to ensure that PEMF therapy equipment meets the highest standards of safety, efficacy, and reliability.
Current QC Methods in PEMF Production
01 Electromagnetic field generation and control
PEMF therapy equipment quality control involves precise generation and control of electromagnetic fields. This includes mechanisms for adjusting field strength, frequency, and waveform to ensure consistent and accurate therapy delivery. Advanced control systems may incorporate microprocessors or digital signal processors to maintain field parameters within specified tolerances.- Electromagnetic field generation and control: PEMF therapy equipment quality control involves precise generation and control of electromagnetic fields. This includes mechanisms for adjusting field strength, frequency, and waveform to ensure consistent and accurate therapy delivery. Advanced control systems may incorporate microprocessors or digital signal processors to maintain field parameters within specified tolerances.
- Calibration and measurement techniques: Quality control measures for PEMF devices include regular calibration and measurement of output parameters. This may involve the use of specialized sensors or probes to verify field strength and uniformity. Automated self-testing routines can be implemented to ensure the equipment maintains accuracy over time and alerts users to any deviations from expected performance.
- Safety features and compliance monitoring: PEMF therapy equipment incorporates various safety features as part of quality control. This includes overload protection, automatic shut-off mechanisms, and real-time monitoring of device performance. Compliance with regulatory standards is ensured through built-in checks and documentation of operating parameters during therapy sessions.
- User interface and feedback systems: Quality control in PEMF devices extends to user interfaces and feedback systems. This includes clear displays of treatment parameters, intuitive controls for adjusting settings, and real-time feedback on therapy progress. Advanced systems may incorporate error detection and troubleshooting guides to assist users in maintaining optimal device performance.
- Manufacturing and testing protocols: Ensuring quality control in PEMF therapy equipment involves rigorous manufacturing and testing protocols. This includes standardized production processes, component selection criteria, and comprehensive quality assurance testing at various stages of assembly. Final product testing may involve simulated use scenarios and stress testing to verify durability and consistent performance over the device's expected lifespan.
02 Calibration and measurement techniques
Quality control measures for PEMF devices include regular calibration and measurement of output parameters. This may involve using specialized sensors or probes to verify field strength and uniformity across the treatment area. Automated self-testing routines can be implemented to ensure the equipment maintains accuracy over time and alerts users to any deviations from expected performance.Expand Specific Solutions03 Safety features and compliance monitoring
PEMF therapy equipment must incorporate safety features to prevent overexposure or unintended field emissions. This can include automatic shut-off mechanisms, real-time monitoring of treatment duration and intensity, and fail-safe systems. Quality control processes ensure compliance with relevant medical device regulations and standards for electromagnetic compatibility and electrical safety.Expand Specific Solutions04 User interface and treatment protocol management
Quality control in PEMF therapy equipment extends to the user interface and treatment protocol management. This includes designing intuitive controls, clear displays of treatment parameters, and systems for storing and recalling preset protocols. Ensuring accurate execution of prescribed treatments and providing detailed session logs contribute to overall quality assurance.Expand Specific Solutions05 Component reliability and durability testing
Maintaining high quality in PEMF therapy equipment involves rigorous testing of individual components and the assembled device. This includes accelerated life testing, environmental stress screening, and long-term reliability assessments. Quality control measures focus on ensuring consistent performance of critical components such as coils, power supplies, and control circuitry over the expected lifetime of the device.Expand Specific Solutions
Key PEMF Equipment Manufacturers
The PEMF therapy equipment production market is in a growth phase, driven by increasing awareness of non-invasive pain management solutions. The global market size is projected to expand significantly in the coming years, with a compound annual growth rate expected to be in the double digits. Technologically, the field is advancing rapidly, with companies like Regenesis Biomedical and SofPulse leading innovation in PEMF devices. However, the technology is still evolving, with ongoing research at institutions such as Huazhong University of Science & Technology and the National University of Singapore contributing to its development. Quality control remains a critical challenge, as manufacturers like Biomagnetic Sciences and Yourui Biomedical Technology strive to meet stringent regulatory standards while scaling production to meet growing demand.
Regenesis Biomedical, Inc.
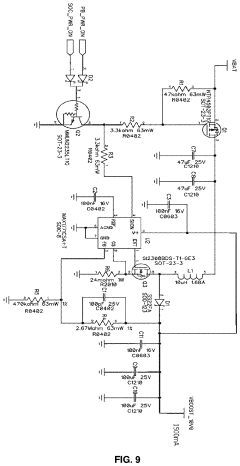
Technical Solution: Regenesis Biomedical, Inc. has implemented a robust quality control system for their PEMF therapy equipment production. They utilize a combination of in-house developed software and hardware solutions to ensure consistent performance of their devices[1]. Their quality control process includes automated calibration systems that verify the accuracy of PEMF output parameters such as frequency, intensity, and waveform[2]. The company also employs advanced electromagnetic compatibility (EMC) testing to ensure their devices meet regulatory standards and do not interfere with other medical equipment[3]. Regenesis has integrated IoT-enabled sensors in their production line to monitor environmental conditions and equipment performance in real-time, allowing for immediate corrective actions when deviations are detected[4].
Strengths: Proprietary software and hardware solutions, advanced EMC testing, and IoT-enabled real-time monitoring. Weaknesses: Reliance on custom solutions may lead to longer development cycles for new products and potential difficulties in scaling production.
SofPulse, Inc.
Technical Solution: SofPulse, Inc. has developed a comprehensive quality control strategy for their PEMF therapy equipment production. They have implemented a Six Sigma methodology to minimize defects and variations in their manufacturing process[1]. The company utilizes advanced spectral analysis techniques to verify the electromagnetic field characteristics of each device, ensuring precise therapeutic dosage[2]. SofPulse has also developed a proprietary automated testing system that simulates various usage scenarios to assess device durability and long-term performance[3]. Additionally, they have implemented a blockchain-based traceability system to track components and finished products throughout the supply chain, enhancing quality assurance and facilitating rapid recall procedures if necessary[4].
Strengths: Six Sigma methodology implementation, advanced spectral analysis, and blockchain-based traceability. Weaknesses: The complex quality control system may result in longer production times and potentially higher costs.
Critical PEMF QC Patents and Literature
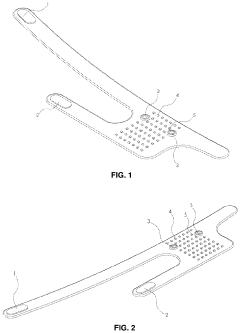
Flexible Photobiomodulation and Pulsed Electromagnetic Field Therapy Device
PatentPendingUS20230001222A1
Innovation
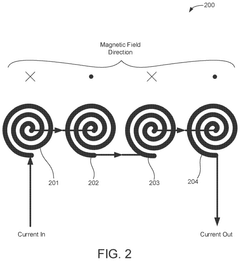
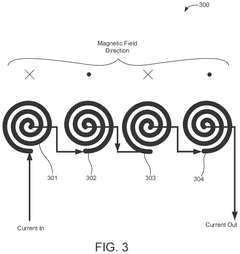
- A flexible wearable device that combines PEMF and PBM therapies, featuring a flexible substrate with electromagnetic coils and light-emitting diodes, controlled by a single module that can switch between pre-set frequency sequences, and is wirelessly enabled for remote control.
Method and apparatus for providing pulsed electromagnetic field therapy
PatentPendingUS20240278031A1
Innovation
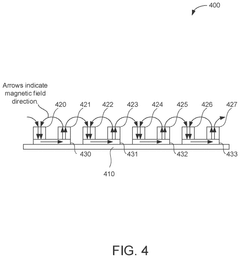
- The development of PEMF therapy applicators that include multiple PEMF emitter coils arranged to direct magnetic fields uniformly and additional therapy pads for providing heating, cooling, or transcutaneous electrical nerve stimulation (TENS) therapies, with a ferromagnetic field director to enhance magnetic field strength and a substrate that can conform to body parts, allowing for directed and intensified magnetic field application.
Regulatory Standards for PEMF Devices
Regulatory standards for PEMF (Pulsed Electromagnetic Field) devices play a crucial role in ensuring the safety and efficacy of these therapeutic instruments. In the United States, the Food and Drug Administration (FDA) oversees the regulation of PEMF devices, classifying them as Class II medical devices. This classification requires manufacturers to adhere to strict quality control measures and obtain 510(k) clearance before marketing their products.
The International Electrotechnical Commission (IEC) has established specific standards for electromagnetic compatibility and electrical safety of medical devices, including PEMF equipment. IEC 60601-1 serves as the primary standard for medical electrical equipment, outlining general requirements for basic safety and essential performance. Additionally, IEC 60601-1-2 addresses electromagnetic compatibility, ensuring that PEMF devices do not interfere with other electronic equipment and are not susceptible to external electromagnetic interference.
In the European Union, PEMF devices must comply with the Medical Device Regulation (MDR) 2017/745, which replaced the previous Medical Device Directive (MDD) 93/42/EEC. The MDR imposes more stringent requirements on manufacturers, including enhanced clinical evaluation, post-market surveillance, and unique device identification (UDI) systems.
Quality management systems are integral to maintaining regulatory compliance. ISO 13485 is the internationally recognized standard for quality management systems in the medical device industry. Manufacturers of PEMF devices are expected to implement and maintain a robust quality management system that aligns with ISO 13485 requirements, ensuring consistent production of safe and effective devices.
Specific to electromagnetic field exposure, the International Commission on Non-Ionizing Radiation Protection (ICNIRP) provides guidelines for limiting exposure to electromagnetic fields. These guidelines are widely recognized and adopted by regulatory bodies worldwide, influencing the design and output parameters of PEMF devices.
To ensure compliance with these regulatory standards, manufacturers must implement comprehensive quality control processes throughout the production lifecycle. This includes rigorous testing of components, assembly verification, and final product validation. Documentation of these processes, including design history files, risk management reports, and technical files, is essential for regulatory submissions and audits.
Continuous monitoring of regulatory changes and updates is crucial for maintaining compliance. As the field of PEMF therapy evolves, regulatory bodies may introduce new requirements or modify existing standards. Manufacturers must stay informed and adapt their quality control processes accordingly to ensure ongoing compliance and market access for their PEMF therapy equipment.
The International Electrotechnical Commission (IEC) has established specific standards for electromagnetic compatibility and electrical safety of medical devices, including PEMF equipment. IEC 60601-1 serves as the primary standard for medical electrical equipment, outlining general requirements for basic safety and essential performance. Additionally, IEC 60601-1-2 addresses electromagnetic compatibility, ensuring that PEMF devices do not interfere with other electronic equipment and are not susceptible to external electromagnetic interference.
In the European Union, PEMF devices must comply with the Medical Device Regulation (MDR) 2017/745, which replaced the previous Medical Device Directive (MDD) 93/42/EEC. The MDR imposes more stringent requirements on manufacturers, including enhanced clinical evaluation, post-market surveillance, and unique device identification (UDI) systems.
Quality management systems are integral to maintaining regulatory compliance. ISO 13485 is the internationally recognized standard for quality management systems in the medical device industry. Manufacturers of PEMF devices are expected to implement and maintain a robust quality management system that aligns with ISO 13485 requirements, ensuring consistent production of safe and effective devices.
Specific to electromagnetic field exposure, the International Commission on Non-Ionizing Radiation Protection (ICNIRP) provides guidelines for limiting exposure to electromagnetic fields. These guidelines are widely recognized and adopted by regulatory bodies worldwide, influencing the design and output parameters of PEMF devices.
To ensure compliance with these regulatory standards, manufacturers must implement comprehensive quality control processes throughout the production lifecycle. This includes rigorous testing of components, assembly verification, and final product validation. Documentation of these processes, including design history files, risk management reports, and technical files, is essential for regulatory submissions and audits.
Continuous monitoring of regulatory changes and updates is crucial for maintaining compliance. As the field of PEMF therapy evolves, regulatory bodies may introduce new requirements or modify existing standards. Manufacturers must stay informed and adapt their quality control processes accordingly to ensure ongoing compliance and market access for their PEMF therapy equipment.
PEMF Safety and Efficacy Testing
Ensuring the safety and efficacy of Pulsed Electromagnetic Field (PEMF) therapy equipment is crucial for maintaining quality control in production. Rigorous testing protocols must be implemented to verify that the devices meet regulatory standards and deliver the intended therapeutic benefits. The testing process typically begins with component-level evaluations, where individual parts are assessed for quality and performance. This includes testing the electromagnetic coils, power supplies, and control circuits to ensure they meet specifications.
Once the components are validated, the assembled PEMF devices undergo comprehensive safety testing. Electrical safety tests are conducted to verify insulation integrity, ground continuity, and leakage current levels. These tests are essential to prevent potential electrical hazards to users. Additionally, electromagnetic compatibility (EMC) testing is performed to ensure the device does not interfere with other electronic equipment and is not susceptible to external electromagnetic interference.
Efficacy testing is equally important in the quality control process. This involves measuring the output parameters of the PEMF device, such as magnetic field strength, frequency, and waveform characteristics. Specialized equipment, including gaussmeters and oscilloscopes, is used to verify that the device produces the intended electromagnetic fields within specified tolerances. The uniformity of the field across the treatment area is also assessed to ensure consistent therapy delivery.
Thermal testing is another critical aspect of PEMF device evaluation. Devices are operated for extended periods to monitor heat generation and ensure that surface temperatures remain within safe limits. This is particularly important for devices intended for long-term or overnight use. Durability and reliability testing involve subjecting the equipment to simulated long-term use conditions, including mechanical stress and environmental factors, to verify the device's longevity and consistent performance over time.
Software validation is essential for PEMF devices with programmable features or digital interfaces. This includes testing user interface functionality, treatment protocol accuracy, and data logging capabilities. Cybersecurity measures are also evaluated to protect patient data and prevent unauthorized access or manipulation of device settings.
Finally, clinical testing may be conducted to validate the therapeutic efficacy of the PEMF device. This involves controlled studies to assess the device's effectiveness in treating specific conditions, such as pain relief or bone healing. The results of these clinical trials not only contribute to quality control but also provide valuable data for regulatory submissions and marketing claims.
Throughout the testing process, meticulous documentation is maintained, including test protocols, results, and any corrective actions taken. This documentation forms a crucial part of the quality management system and is essential for regulatory compliance and continuous improvement of the production process.
Once the components are validated, the assembled PEMF devices undergo comprehensive safety testing. Electrical safety tests are conducted to verify insulation integrity, ground continuity, and leakage current levels. These tests are essential to prevent potential electrical hazards to users. Additionally, electromagnetic compatibility (EMC) testing is performed to ensure the device does not interfere with other electronic equipment and is not susceptible to external electromagnetic interference.
Efficacy testing is equally important in the quality control process. This involves measuring the output parameters of the PEMF device, such as magnetic field strength, frequency, and waveform characteristics. Specialized equipment, including gaussmeters and oscilloscopes, is used to verify that the device produces the intended electromagnetic fields within specified tolerances. The uniformity of the field across the treatment area is also assessed to ensure consistent therapy delivery.
Thermal testing is another critical aspect of PEMF device evaluation. Devices are operated for extended periods to monitor heat generation and ensure that surface temperatures remain within safe limits. This is particularly important for devices intended for long-term or overnight use. Durability and reliability testing involve subjecting the equipment to simulated long-term use conditions, including mechanical stress and environmental factors, to verify the device's longevity and consistent performance over time.
Software validation is essential for PEMF devices with programmable features or digital interfaces. This includes testing user interface functionality, treatment protocol accuracy, and data logging capabilities. Cybersecurity measures are also evaluated to protect patient data and prevent unauthorized access or manipulation of device settings.
Finally, clinical testing may be conducted to validate the therapeutic efficacy of the PEMF device. This involves controlled studies to assess the device's effectiveness in treating specific conditions, such as pain relief or bone healing. The results of these clinical trials not only contribute to quality control but also provide valuable data for regulatory submissions and marketing claims.
Throughout the testing process, meticulous documentation is maintained, including test protocols, results, and any corrective actions taken. This documentation forms a crucial part of the quality management system and is essential for regulatory compliance and continuous improvement of the production process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!