How to Bridge PEMF Therapy with Evidence-Based Practice?
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEMF Therapy Background and Objectives
Pulsed Electromagnetic Field (PEMF) therapy has emerged as a promising non-invasive treatment modality in recent years. This technology harnesses the power of electromagnetic fields to stimulate cellular repair and regeneration, offering potential benefits across a wide range of medical conditions. The evolution of PEMF therapy can be traced back to the mid-20th century, with significant advancements in understanding and application occurring over the past few decades.
The primary objective of PEMF therapy is to enhance the body's natural healing processes by influencing cellular function at a fundamental level. By generating pulsed electromagnetic fields, PEMF devices aim to improve cellular energy production, increase blood circulation, and promote tissue repair. These effects have been associated with various therapeutic outcomes, including pain reduction, improved bone healing, and enhanced wound repair.
As the field of PEMF therapy continues to develop, there is a growing need to bridge the gap between its promising potential and evidence-based practice. This integration is crucial for establishing PEMF as a credible and effective treatment option within mainstream healthcare. The current landscape of PEMF research shows a mix of encouraging results and areas requiring further investigation, highlighting the importance of rigorous scientific inquiry.
One of the key challenges in advancing PEMF therapy lies in standardizing treatment protocols and parameters. The variability in device specifications, treatment durations, and field intensities across different studies has made it difficult to draw definitive conclusions about optimal therapeutic approaches. Addressing this challenge is essential for developing evidence-based guidelines that can inform clinical practice.
The technological trajectory of PEMF therapy points towards more sophisticated and targeted applications. Recent innovations include the development of portable and wearable PEMF devices, allowing for more convenient and continuous treatment. Additionally, there is ongoing research into combining PEMF therapy with other treatment modalities to enhance overall therapeutic efficacy.
As we look towards the future of PEMF therapy, the primary goal is to establish a robust evidence base that can support its integration into standard medical practice. This involves conducting large-scale, well-designed clinical trials, exploring the underlying mechanisms of action, and refining treatment protocols based on empirical data. By bridging PEMF therapy with evidence-based practice, we aim to unlock its full potential as a valuable tool in modern healthcare, offering patients new options for managing a variety of health conditions.
The primary objective of PEMF therapy is to enhance the body's natural healing processes by influencing cellular function at a fundamental level. By generating pulsed electromagnetic fields, PEMF devices aim to improve cellular energy production, increase blood circulation, and promote tissue repair. These effects have been associated with various therapeutic outcomes, including pain reduction, improved bone healing, and enhanced wound repair.
As the field of PEMF therapy continues to develop, there is a growing need to bridge the gap between its promising potential and evidence-based practice. This integration is crucial for establishing PEMF as a credible and effective treatment option within mainstream healthcare. The current landscape of PEMF research shows a mix of encouraging results and areas requiring further investigation, highlighting the importance of rigorous scientific inquiry.
One of the key challenges in advancing PEMF therapy lies in standardizing treatment protocols and parameters. The variability in device specifications, treatment durations, and field intensities across different studies has made it difficult to draw definitive conclusions about optimal therapeutic approaches. Addressing this challenge is essential for developing evidence-based guidelines that can inform clinical practice.
The technological trajectory of PEMF therapy points towards more sophisticated and targeted applications. Recent innovations include the development of portable and wearable PEMF devices, allowing for more convenient and continuous treatment. Additionally, there is ongoing research into combining PEMF therapy with other treatment modalities to enhance overall therapeutic efficacy.
As we look towards the future of PEMF therapy, the primary goal is to establish a robust evidence base that can support its integration into standard medical practice. This involves conducting large-scale, well-designed clinical trials, exploring the underlying mechanisms of action, and refining treatment protocols based on empirical data. By bridging PEMF therapy with evidence-based practice, we aim to unlock its full potential as a valuable tool in modern healthcare, offering patients new options for managing a variety of health conditions.
Market Analysis for PEMF Devices
The global market for Pulsed Electromagnetic Field (PEMF) therapy devices has been experiencing significant growth in recent years, driven by increasing awareness of non-invasive treatment options and a growing emphasis on evidence-based medical practices. The PEMF device market is expected to continue its upward trajectory, with projections indicating substantial expansion over the next decade.
Several factors contribute to the rising demand for PEMF devices. Firstly, the aging population in many developed countries has led to a higher prevalence of chronic conditions such as osteoarthritis, osteoporosis, and chronic pain, for which PEMF therapy has shown promising results. Additionally, the growing interest in alternative and complementary medicine has opened new avenues for PEMF therapy applications.
The market for PEMF devices can be segmented based on application areas, including orthopedics, neurology, pain management, and sports medicine. Among these, orthopedics represents the largest segment, driven by the increasing incidence of bone and joint disorders. The pain management segment is also experiencing rapid growth, as patients and healthcare providers seek non-pharmacological alternatives for chronic pain treatment.
Geographically, North America currently dominates the PEMF device market, owing to advanced healthcare infrastructure, high healthcare expenditure, and early adoption of innovative medical technologies. Europe follows closely, with countries like Germany and the UK showing significant market potential. The Asia-Pacific region is emerging as a lucrative market, propelled by improving healthcare access, rising disposable incomes, and increasing awareness of alternative therapies.
The competitive landscape of the PEMF device market is characterized by a mix of established medical device manufacturers and specialized PEMF therapy companies. Key players are focusing on product innovation, clinical research collaborations, and strategic partnerships to strengthen their market position. The market is also witnessing the entry of new players, particularly in the consumer-grade PEMF device segment, catering to the growing demand for home-use devices.
Despite the positive market outlook, challenges remain in bridging PEMF therapy with evidence-based practice. The lack of standardized protocols and limited large-scale clinical trials have hindered widespread acceptance in mainstream medical practice. However, ongoing research efforts and increasing collaboration between industry and academia are addressing these gaps, potentially leading to broader adoption and market expansion.
As the demand for non-invasive and drug-free treatment options continues to rise, the PEMF device market is poised for sustained growth. The integration of advanced technologies such as AI-driven personalization and IoT connectivity in PEMF devices is expected to further drive market innovation and create new opportunities for market players.
Several factors contribute to the rising demand for PEMF devices. Firstly, the aging population in many developed countries has led to a higher prevalence of chronic conditions such as osteoarthritis, osteoporosis, and chronic pain, for which PEMF therapy has shown promising results. Additionally, the growing interest in alternative and complementary medicine has opened new avenues for PEMF therapy applications.
The market for PEMF devices can be segmented based on application areas, including orthopedics, neurology, pain management, and sports medicine. Among these, orthopedics represents the largest segment, driven by the increasing incidence of bone and joint disorders. The pain management segment is also experiencing rapid growth, as patients and healthcare providers seek non-pharmacological alternatives for chronic pain treatment.
Geographically, North America currently dominates the PEMF device market, owing to advanced healthcare infrastructure, high healthcare expenditure, and early adoption of innovative medical technologies. Europe follows closely, with countries like Germany and the UK showing significant market potential. The Asia-Pacific region is emerging as a lucrative market, propelled by improving healthcare access, rising disposable incomes, and increasing awareness of alternative therapies.
The competitive landscape of the PEMF device market is characterized by a mix of established medical device manufacturers and specialized PEMF therapy companies. Key players are focusing on product innovation, clinical research collaborations, and strategic partnerships to strengthen their market position. The market is also witnessing the entry of new players, particularly in the consumer-grade PEMF device segment, catering to the growing demand for home-use devices.
Despite the positive market outlook, challenges remain in bridging PEMF therapy with evidence-based practice. The lack of standardized protocols and limited large-scale clinical trials have hindered widespread acceptance in mainstream medical practice. However, ongoing research efforts and increasing collaboration between industry and academia are addressing these gaps, potentially leading to broader adoption and market expansion.
As the demand for non-invasive and drug-free treatment options continues to rise, the PEMF device market is poised for sustained growth. The integration of advanced technologies such as AI-driven personalization and IoT connectivity in PEMF devices is expected to further drive market innovation and create new opportunities for market players.
Current PEMF Technology Challenges
Despite the growing popularity of Pulsed Electromagnetic Field (PEMF) therapy, several significant challenges hinder its widespread adoption and integration into evidence-based practice. One of the primary obstacles is the lack of standardization in PEMF devices and treatment protocols. The wide variety of devices available in the market, each with different frequencies, intensities, and waveforms, makes it difficult to compare results across studies and establish consistent treatment guidelines.
Another major challenge is the limited understanding of the precise mechanisms of action through which PEMF therapy exerts its therapeutic effects. While some theories exist, such as the modulation of cellular ion channels and the stimulation of cellular repair processes, the exact pathways and their interactions with various physiological systems remain unclear. This knowledge gap hampers the development of targeted therapies and optimal treatment protocols.
The quality and quantity of clinical evidence supporting PEMF therapy also present a significant hurdle. While numerous studies have shown promising results, many suffer from small sample sizes, inadequate control groups, or inconsistent methodologies. This lack of robust, large-scale clinical trials makes it challenging to draw definitive conclusions about the efficacy of PEMF therapy for specific conditions and to gain widespread acceptance within the medical community.
Furthermore, the regulatory landscape surrounding PEMF devices varies significantly across different countries and regions. In some areas, PEMF devices are classified as medical devices and subject to stringent regulations, while in others, they may be considered wellness products with less oversight. This inconsistency in regulatory approaches complicates the process of conducting multinational clinical trials and obtaining approvals for widespread clinical use.
The integration of PEMF therapy into existing treatment paradigms also poses a challenge. Many healthcare providers are unfamiliar with PEMF technology and may be hesitant to incorporate it into their practice without clear guidelines and extensive training. Additionally, the lack of standardized reimbursement policies from insurance companies for PEMF treatments creates financial barriers for both patients and healthcare providers.
Lastly, the potential for electromagnetic interference with other medical devices and implants raises safety concerns that need to be thoroughly addressed. While PEMF therapy is generally considered safe, more research is needed to establish clear safety protocols and contraindications, particularly for patients with electronic implants or those undergoing concurrent treatments that may be affected by electromagnetic fields.
Another major challenge is the limited understanding of the precise mechanisms of action through which PEMF therapy exerts its therapeutic effects. While some theories exist, such as the modulation of cellular ion channels and the stimulation of cellular repair processes, the exact pathways and their interactions with various physiological systems remain unclear. This knowledge gap hampers the development of targeted therapies and optimal treatment protocols.
The quality and quantity of clinical evidence supporting PEMF therapy also present a significant hurdle. While numerous studies have shown promising results, many suffer from small sample sizes, inadequate control groups, or inconsistent methodologies. This lack of robust, large-scale clinical trials makes it challenging to draw definitive conclusions about the efficacy of PEMF therapy for specific conditions and to gain widespread acceptance within the medical community.
Furthermore, the regulatory landscape surrounding PEMF devices varies significantly across different countries and regions. In some areas, PEMF devices are classified as medical devices and subject to stringent regulations, while in others, they may be considered wellness products with less oversight. This inconsistency in regulatory approaches complicates the process of conducting multinational clinical trials and obtaining approvals for widespread clinical use.
The integration of PEMF therapy into existing treatment paradigms also poses a challenge. Many healthcare providers are unfamiliar with PEMF technology and may be hesitant to incorporate it into their practice without clear guidelines and extensive training. Additionally, the lack of standardized reimbursement policies from insurance companies for PEMF treatments creates financial barriers for both patients and healthcare providers.
Lastly, the potential for electromagnetic interference with other medical devices and implants raises safety concerns that need to be thoroughly addressed. While PEMF therapy is generally considered safe, more research is needed to establish clear safety protocols and contraindications, particularly for patients with electronic implants or those undergoing concurrent treatments that may be affected by electromagnetic fields.
Evidence-Based PEMF Protocols
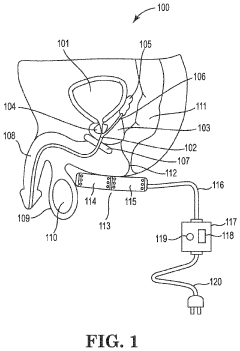
01 PEMF devices for therapeutic applications
Pulsed Electromagnetic Field (PEMF) therapy devices are designed for various therapeutic applications. These devices generate electromagnetic fields to stimulate cellular repair and regeneration, potentially treating conditions such as pain, inflammation, and bone healing. The devices can be configured for different body parts and treatment protocols.- PEMF devices for therapeutic applications: Pulsed Electromagnetic Field (PEMF) therapy devices are designed for various therapeutic applications. These devices generate electromagnetic fields to stimulate cellular activity and promote healing. They can be used for pain management, tissue repair, and improving overall well-being.
- PEMF therapy for specific medical conditions: PEMF therapy is applied to treat specific medical conditions such as musculoskeletal disorders, neurological issues, and cardiovascular problems. The therapy can be tailored to target particular areas of the body or specific health concerns, offering a non-invasive treatment option for various ailments.
- Advancements in PEMF technology: Recent advancements in PEMF technology have led to more sophisticated and efficient devices. These improvements include better control over field intensity, frequency modulation, and treatment protocols. New designs also focus on portability and ease of use for home-based treatments.
- Combination of PEMF with other therapies: PEMF therapy is often combined with other treatment modalities to enhance overall therapeutic effects. This may include integration with light therapy, heat therapy, or other forms of electromagnetic stimulation. The synergistic approach aims to provide more comprehensive and effective treatment outcomes.
- PEMF applications in veterinary and agricultural fields: PEMF therapy has expanded beyond human applications to include veterinary medicine and agriculture. In these fields, PEMF is used for animal health, plant growth stimulation, and improving crop yields. The technology is adapted to suit the specific needs of different species and agricultural practices.
02 PEMF therapy for pain management and tissue healing
PEMF therapy is utilized for pain management and tissue healing. The electromagnetic fields generated by PEMF devices can help reduce pain, improve circulation, and accelerate the healing process of various tissues, including bones, muscles, and soft tissues. This non-invasive treatment approach is applied in both clinical and home settings.Expand Specific Solutions03 Innovative PEMF delivery systems
Advancements in PEMF therapy include innovative delivery systems such as wearable devices, portable units, and integrated treatment platforms. These systems aim to improve the convenience and effectiveness of PEMF therapy by allowing for targeted treatment, continuous application, and integration with other therapeutic modalities.Expand Specific Solutions04 PEMF therapy in veterinary and agricultural applications
PEMF therapy is being explored for veterinary and agricultural applications. This includes the treatment of animals for various conditions and the potential use in enhancing plant growth and crop yield. The technology is adapted to suit different species and environmental conditions.Expand Specific Solutions05 Combination of PEMF with other therapeutic modalities
Research is ongoing into combining PEMF therapy with other therapeutic modalities to enhance overall treatment efficacy. This includes integration with light therapy, thermal therapy, and other forms of electromagnetic stimulation. The combined approach aims to provide synergistic effects and broader treatment options for various health conditions.Expand Specific Solutions
Key PEMF Industry Players
The field of PEMF (Pulsed Electromagnetic Field) therapy is in a growth phase, with increasing market size and technological advancements. The global PEMF therapy market is expanding rapidly, driven by growing awareness of non-invasive treatment options and increasing prevalence of chronic diseases. Companies like Regenesis Biomedical, SofPulse, and Galvanize Therapeutics are at the forefront, developing innovative PEMF devices for various medical applications. Academic institutions such as Hefei University of Technology and the National University of Singapore are contributing to the research base, enhancing the technology's credibility. While PEMF therapy is gaining traction, its integration into evidence-based practice remains a challenge, requiring more robust clinical trials and standardization of protocols to fully bridge the gap between emerging technology and mainstream medical acceptance.
Regenesis Biomedical, Inc.
Technical Solution: Regenesis Biomedical has developed a proprietary PEMF therapy system called Provant Therapy System. This device utilizes a specific pulsed electromagnetic field to stimulate cellular repair and reduce pain. The company has conducted several clinical trials to validate the efficacy of their technology in various medical conditions, including post-operative pain management and wound healing. Their approach involves using a precise frequency and waveform that has been shown to activate cellular signaling pathways associated with tissue repair[1][3]. The device is FDA-cleared and has been used in numerous healthcare settings, providing a non-invasive treatment option that aligns with evidence-based practice guidelines.
Strengths: FDA-cleared technology, extensive clinical trial data, non-invasive treatment. Weaknesses: Limited to specific frequency ranges, may require multiple treatment sessions for optimal results.
SofPulse, Inc.
Technical Solution: SofPulse has developed a targeted PEMF therapy device that focuses on reducing post-operative pain and inflammation. Their technology uses a specific electromagnetic field strength and pulse rate that has been shown to modulate the body's natural healing processes. The company has conducted several clinical studies demonstrating the efficacy of their device in reducing pain medication use and accelerating recovery after surgery[2][4]. SofPulse's approach to bridging PEMF therapy with evidence-based practice involves integrating their device into standard post-operative care protocols and collecting real-world data to support its use. The device is designed to be portable and easy to use, allowing for continuous treatment during the critical early stages of healing.
Strengths: Targeted therapy for post-operative care, portable design, reduction in pain medication use. Weaknesses: Limited to post-operative applications, may require patient compliance for home use.
PEMF Research Breakthroughs
Treatment of conditions susceptible to pulsed electromagnetic field therapy
PatentActiveUS20170354830A1
Innovation
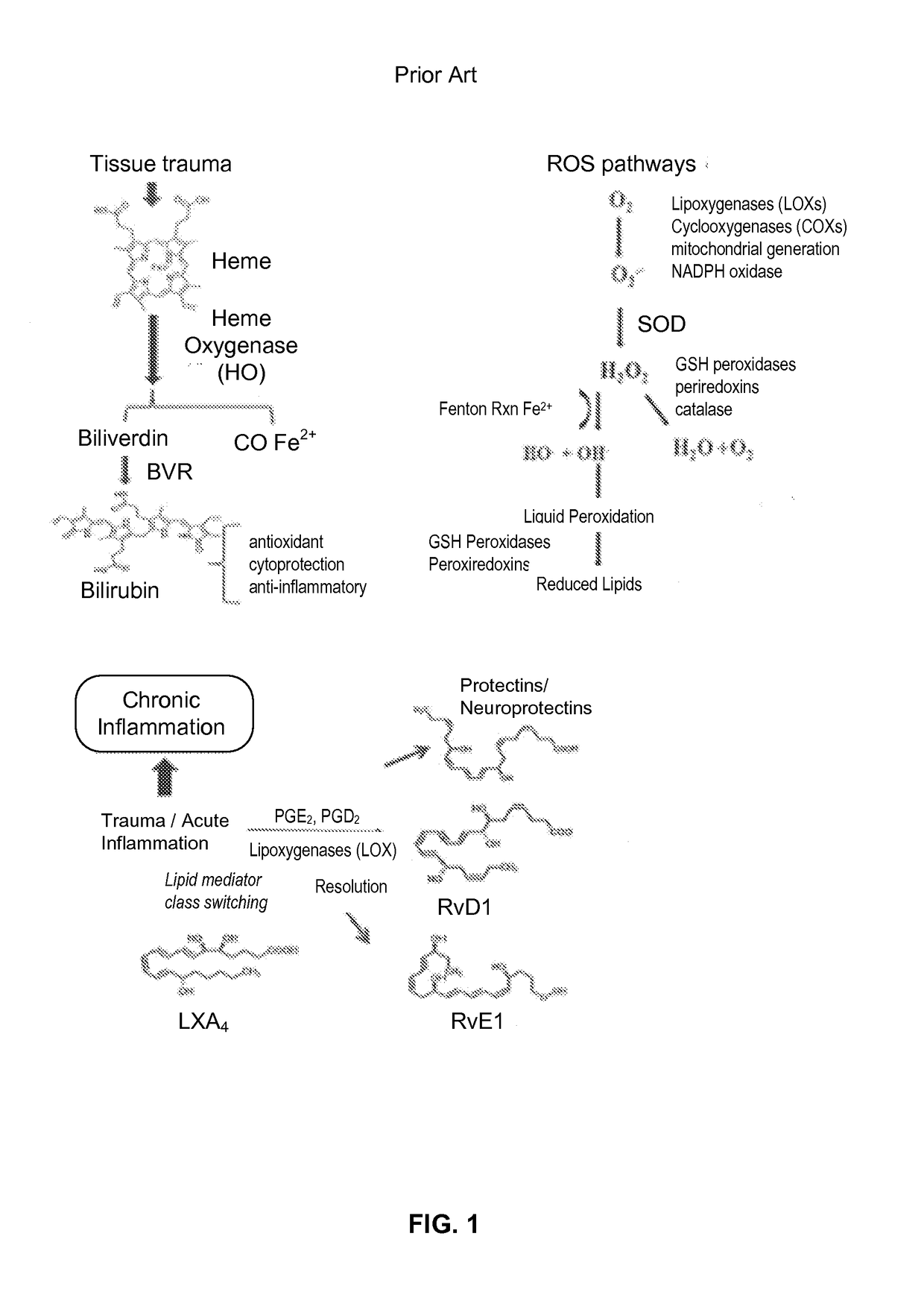
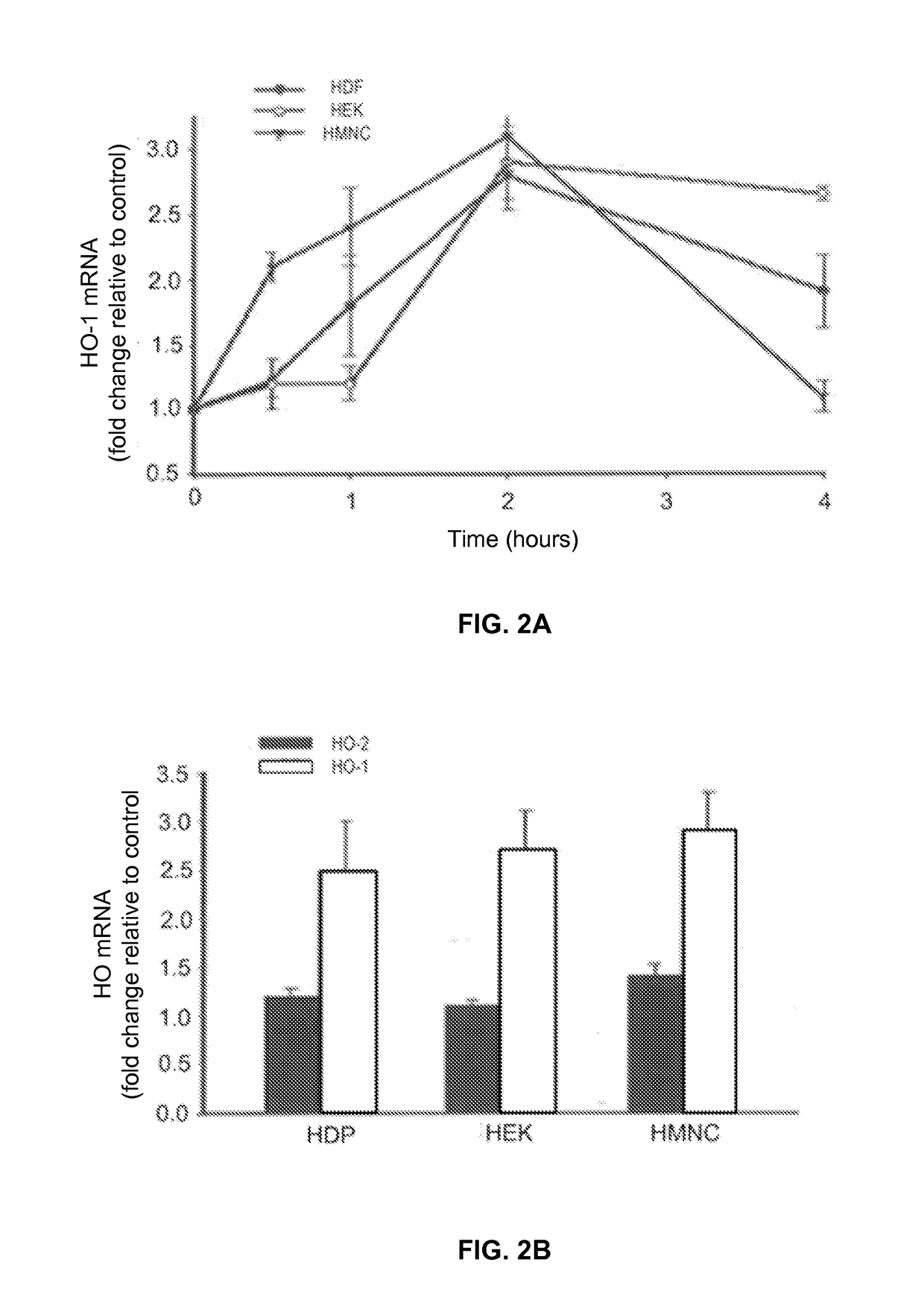
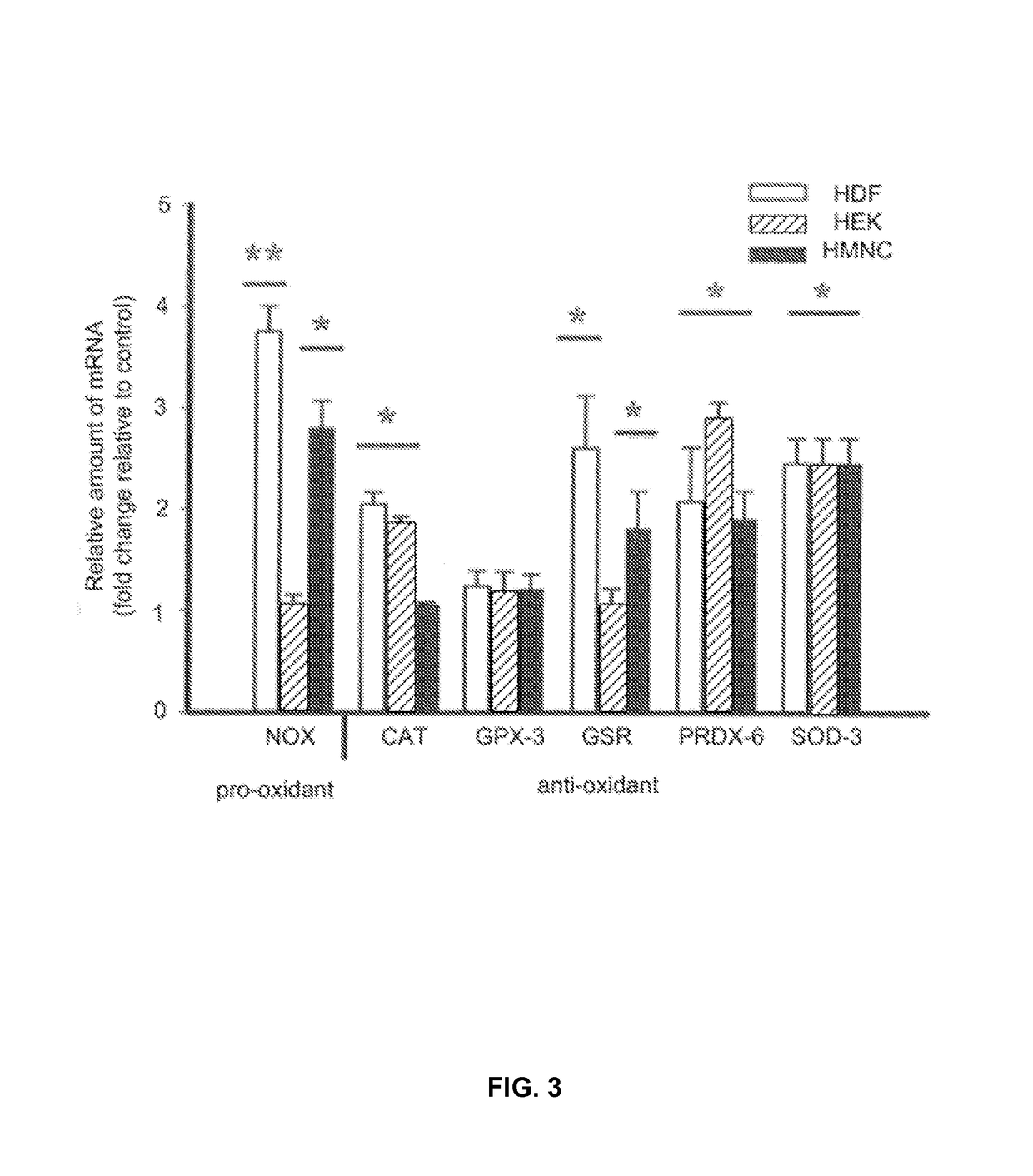
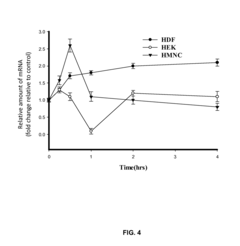
- PEMF therapy is administered to modulate gene expression associated with inflammation pathways, including heme oxygenase, antioxidant enzymes, lipid mediator biosynthesis, and cytokines, using specific parameters such as electric field strength, pulse rate, and duration to produce measurable clinical effects on pain, nerve function, and wound healing.
Method and apparatus for treatment of benign prostatic hyperplasia (BPH)
PatentInactiveUS20230398368A1
Innovation
- A non-invasive method utilizing pulsed electromagnetic field (PEMF) stimulation to increase the number of A2a receptors on cell membranes, enhancing the anti-inflammatory effects of adenosine and providing immunosuppressive action to reduce chronic inflammation and tissue damage in the prostate.
Regulatory Framework for PEMF Devices
The regulatory framework for PEMF (Pulsed Electromagnetic Field) devices plays a crucial role in bridging PEMF therapy with evidence-based practice. In the United States, the Food and Drug Administration (FDA) oversees the regulation of PEMF devices, classifying them as Class II medical devices. This classification requires manufacturers to submit a 510(k) premarket notification, demonstrating that their device is substantially equivalent to a legally marketed predicate device in terms of safety and effectiveness.
The FDA's regulatory process ensures that PEMF devices meet specific performance standards and safety requirements before they can be marketed. This includes evaluating the device's intended use, technical specifications, and potential risks. Manufacturers must provide clinical data supporting the device's efficacy and safety, which contributes to the growing body of evidence-based research in PEMF therapy.
In the European Union, PEMF devices fall under the Medical Device Regulation (MDR), which came into effect in May 2021. The MDR imposes stricter requirements for clinical evidence and post-market surveillance, further aligning PEMF therapy with evidence-based practice. Manufacturers must obtain CE marking, demonstrating compliance with all applicable EU regulations, before their devices can be sold in the European market.
The regulatory framework also extends to the claims that manufacturers can make about their PEMF devices. Both the FDA and the European Medicines Agency (EMA) require that any therapeutic claims be supported by robust clinical evidence. This requirement encourages manufacturers to invest in high-quality research, contributing to the scientific understanding of PEMF therapy and its applications.
International standards, such as those set by the International Electrotechnical Commission (IEC), provide guidelines for the safety and performance of PEMF devices. These standards help ensure consistency in device quality across different manufacturers and countries, facilitating the comparison of research results and the development of evidence-based protocols.
The regulatory landscape for PEMF devices is continually evolving, with agencies updating their requirements based on emerging scientific evidence and technological advancements. This dynamic process helps to maintain the balance between innovation and patient safety, while also promoting the integration of PEMF therapy into evidence-based medical practice.
The FDA's regulatory process ensures that PEMF devices meet specific performance standards and safety requirements before they can be marketed. This includes evaluating the device's intended use, technical specifications, and potential risks. Manufacturers must provide clinical data supporting the device's efficacy and safety, which contributes to the growing body of evidence-based research in PEMF therapy.
In the European Union, PEMF devices fall under the Medical Device Regulation (MDR), which came into effect in May 2021. The MDR imposes stricter requirements for clinical evidence and post-market surveillance, further aligning PEMF therapy with evidence-based practice. Manufacturers must obtain CE marking, demonstrating compliance with all applicable EU regulations, before their devices can be sold in the European market.
The regulatory framework also extends to the claims that manufacturers can make about their PEMF devices. Both the FDA and the European Medicines Agency (EMA) require that any therapeutic claims be supported by robust clinical evidence. This requirement encourages manufacturers to invest in high-quality research, contributing to the scientific understanding of PEMF therapy and its applications.
International standards, such as those set by the International Electrotechnical Commission (IEC), provide guidelines for the safety and performance of PEMF devices. These standards help ensure consistency in device quality across different manufacturers and countries, facilitating the comparison of research results and the development of evidence-based protocols.
The regulatory landscape for PEMF devices is continually evolving, with agencies updating their requirements based on emerging scientific evidence and technological advancements. This dynamic process helps to maintain the balance between innovation and patient safety, while also promoting the integration of PEMF therapy into evidence-based medical practice.
PEMF Clinical Trial Design
To bridge PEMF therapy with evidence-based practice, designing robust clinical trials is crucial. A well-structured PEMF clinical trial design should incorporate several key elements to ensure scientific rigor and validity. Firstly, the trial should clearly define the specific PEMF parameters to be tested, including frequency, intensity, duration, and waveform. These parameters must be precisely controlled and documented throughout the study to enable replication and comparison with other trials.
The selection of appropriate outcome measures is paramount. Primary endpoints should directly relate to the condition being treated and be clinically relevant. For instance, in pain management studies, validated pain scales and functional assessments should be utilized. Secondary endpoints may include quality of life measures, physiological markers, or imaging results to provide a comprehensive evaluation of PEMF effects.
Randomization and blinding are essential components of a high-quality PEMF trial. Participants should be randomly assigned to treatment and control groups to minimize bias. Double-blinding, where both participants and researchers are unaware of group assignments, is ideal but can be challenging with PEMF devices. Sham devices that mimic the appearance and sensations of active PEMF treatment without delivering the therapy should be employed for the control group.
The trial design should also consider the optimal treatment protocol, including the frequency and duration of PEMF sessions. Long-term follow-up is crucial to assess the durability of treatment effects and identify any delayed outcomes or safety concerns. Additionally, the sample size must be carefully calculated to ensure sufficient statistical power to detect clinically meaningful differences between groups.
Inclusion and exclusion criteria should be clearly defined to target the appropriate patient population and minimize confounding factors. Standardized protocols for concomitant treatments and medications must be established to isolate the effects of PEMF therapy. Furthermore, the trial should incorporate a run-in period to establish baseline measurements and exclude placebo responders.
To enhance the trial's scientific value, it should include mechanistic studies to elucidate the biological effects of PEMF therapy. This may involve biomarker analysis, imaging studies, or tissue sampling to provide insights into the therapy's mode of action. Lastly, the trial design should adhere to international standards such as the CONSORT guidelines for reporting randomized controlled trials, ensuring transparency and facilitating meta-analyses to build a robust evidence base for PEMF therapy.
The selection of appropriate outcome measures is paramount. Primary endpoints should directly relate to the condition being treated and be clinically relevant. For instance, in pain management studies, validated pain scales and functional assessments should be utilized. Secondary endpoints may include quality of life measures, physiological markers, or imaging results to provide a comprehensive evaluation of PEMF effects.
Randomization and blinding are essential components of a high-quality PEMF trial. Participants should be randomly assigned to treatment and control groups to minimize bias. Double-blinding, where both participants and researchers are unaware of group assignments, is ideal but can be challenging with PEMF devices. Sham devices that mimic the appearance and sensations of active PEMF treatment without delivering the therapy should be employed for the control group.
The trial design should also consider the optimal treatment protocol, including the frequency and duration of PEMF sessions. Long-term follow-up is crucial to assess the durability of treatment effects and identify any delayed outcomes or safety concerns. Additionally, the sample size must be carefully calculated to ensure sufficient statistical power to detect clinically meaningful differences between groups.
Inclusion and exclusion criteria should be clearly defined to target the appropriate patient population and minimize confounding factors. Standardized protocols for concomitant treatments and medications must be established to isolate the effects of PEMF therapy. Furthermore, the trial should incorporate a run-in period to establish baseline measurements and exclude placebo responders.
To enhance the trial's scientific value, it should include mechanistic studies to elucidate the biological effects of PEMF therapy. This may involve biomarker analysis, imaging studies, or tissue sampling to provide insights into the therapy's mode of action. Lastly, the trial design should adhere to international standards such as the CONSORT guidelines for reporting randomized controlled trials, ensuring transparency and facilitating meta-analyses to build a robust evidence base for PEMF therapy.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!