How to Establish Standardized Protocols for PEMF Therapy?
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEMF Therapy Background and Objectives
Pulsed Electromagnetic Field (PEMF) therapy has emerged as a promising non-invasive treatment modality in recent years. This technology harnesses the power of electromagnetic fields to stimulate cellular repair and regeneration, offering potential benefits across a wide range of medical conditions. The evolution of PEMF therapy can be traced back to the mid-20th century, with significant advancements in understanding and application occurring over the past few decades.
The primary objective of establishing standardized protocols for PEMF therapy is to ensure consistent, safe, and effective treatment outcomes across various clinical settings. This standardization is crucial for several reasons. Firstly, it allows for better comparison of research results, facilitating more robust scientific validation of PEMF therapy's efficacy. Secondly, it provides healthcare practitioners with clear guidelines for treatment administration, reducing variability in patient care. Lastly, standardized protocols can enhance patient safety by establishing well-defined parameters for different conditions and patient populations.
Current trends in PEMF therapy development focus on optimizing treatment parameters such as frequency, intensity, and duration. Researchers are exploring the potential of personalized PEMF protocols tailored to specific medical conditions and individual patient characteristics. Additionally, there is growing interest in combining PEMF therapy with other treatment modalities to enhance overall therapeutic outcomes.
The technological landscape of PEMF devices is rapidly evolving, with advancements in miniaturization, portability, and user-friendly interfaces. These developments are making PEMF therapy more accessible for both clinical and home use. However, this proliferation of devices also underscores the need for standardized protocols to ensure consistent quality and effectiveness across different platforms.
As the field progresses, key objectives for PEMF therapy standardization include: establishing evidence-based guidelines for treatment parameters across various medical conditions; developing standardized methods for measuring and reporting PEMF device output; creating a unified classification system for PEMF devices based on their technical specifications and intended use; and implementing rigorous quality control measures for device manufacturing and clinical application.
Achieving these objectives will require collaborative efforts from researchers, clinicians, regulatory bodies, and industry stakeholders. By working towards standardized protocols, the PEMF therapy field can enhance its credibility, improve treatment outcomes, and potentially expand its application in mainstream healthcare.
The primary objective of establishing standardized protocols for PEMF therapy is to ensure consistent, safe, and effective treatment outcomes across various clinical settings. This standardization is crucial for several reasons. Firstly, it allows for better comparison of research results, facilitating more robust scientific validation of PEMF therapy's efficacy. Secondly, it provides healthcare practitioners with clear guidelines for treatment administration, reducing variability in patient care. Lastly, standardized protocols can enhance patient safety by establishing well-defined parameters for different conditions and patient populations.
Current trends in PEMF therapy development focus on optimizing treatment parameters such as frequency, intensity, and duration. Researchers are exploring the potential of personalized PEMF protocols tailored to specific medical conditions and individual patient characteristics. Additionally, there is growing interest in combining PEMF therapy with other treatment modalities to enhance overall therapeutic outcomes.
The technological landscape of PEMF devices is rapidly evolving, with advancements in miniaturization, portability, and user-friendly interfaces. These developments are making PEMF therapy more accessible for both clinical and home use. However, this proliferation of devices also underscores the need for standardized protocols to ensure consistent quality and effectiveness across different platforms.
As the field progresses, key objectives for PEMF therapy standardization include: establishing evidence-based guidelines for treatment parameters across various medical conditions; developing standardized methods for measuring and reporting PEMF device output; creating a unified classification system for PEMF devices based on their technical specifications and intended use; and implementing rigorous quality control measures for device manufacturing and clinical application.
Achieving these objectives will require collaborative efforts from researchers, clinicians, regulatory bodies, and industry stakeholders. By working towards standardized protocols, the PEMF therapy field can enhance its credibility, improve treatment outcomes, and potentially expand its application in mainstream healthcare.
Market Analysis for PEMF Therapy Standardization
The market for PEMF (Pulsed Electromagnetic Field) therapy standardization is experiencing significant growth and transformation. As the demand for non-invasive and drug-free treatment options continues to rise, PEMF therapy has gained considerable attention in both medical and wellness sectors. The global PEMF therapy devices market is projected to expand at a compound annual growth rate (CAGR) of over 7% from 2021 to 2028, driven by increasing awareness of its potential benefits in pain management, bone healing, and overall health improvement.
The primary market segments for PEMF therapy include hospitals, clinics, and home healthcare settings. Hospitals and specialized pain management clinics are adopting PEMF devices for post-operative care and chronic pain treatment, while the home healthcare segment is growing rapidly due to the increasing availability of portable and user-friendly PEMF devices. This trend has been further accelerated by the COVID-19 pandemic, which has led to a surge in demand for home-based healthcare solutions.
Geographically, North America currently dominates the PEMF therapy market, followed by Europe and Asia-Pacific. The United States, in particular, has seen a significant uptake in PEMF therapy, partly due to its aging population and the prevalence of chronic pain conditions. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth in the coming years, driven by improving healthcare infrastructure and rising disposable incomes.
The market for PEMF therapy standardization faces several challenges and opportunities. One of the primary challenges is the lack of uniform protocols and standardized treatment parameters across different PEMF devices and applications. This inconsistency has led to skepticism among some healthcare professionals and regulatory bodies, hindering wider adoption. However, this challenge also presents a significant opportunity for companies and organizations that can successfully establish and promote standardized protocols.
Another key market driver is the increasing focus on evidence-based medicine. As more clinical studies and research papers demonstrate the efficacy of PEMF therapy for various conditions, the demand for standardized, reliable protocols is expected to grow. This trend is likely to attract more investment in research and development, further fueling market growth and innovation in PEMF technology.
The competitive landscape of the PEMF therapy market is characterized by a mix of established medical device manufacturers and newer, specialized PEMF technology companies. Key players are investing in product development and clinical trials to differentiate their offerings and gain regulatory approvals. Collaborations between device manufacturers, healthcare providers, and research institutions are becoming more common, aimed at developing standardized protocols and expanding the range of applications for PEMF therapy.
In conclusion, the market for PEMF therapy standardization presents significant growth potential, driven by increasing demand for non-invasive pain management solutions and the growing body of clinical evidence supporting its efficacy. The establishment of standardized protocols is likely to play a crucial role in shaping the future of this market, potentially leading to wider acceptance in mainstream healthcare and opening up new opportunities for market players.
The primary market segments for PEMF therapy include hospitals, clinics, and home healthcare settings. Hospitals and specialized pain management clinics are adopting PEMF devices for post-operative care and chronic pain treatment, while the home healthcare segment is growing rapidly due to the increasing availability of portable and user-friendly PEMF devices. This trend has been further accelerated by the COVID-19 pandemic, which has led to a surge in demand for home-based healthcare solutions.
Geographically, North America currently dominates the PEMF therapy market, followed by Europe and Asia-Pacific. The United States, in particular, has seen a significant uptake in PEMF therapy, partly due to its aging population and the prevalence of chronic pain conditions. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth in the coming years, driven by improving healthcare infrastructure and rising disposable incomes.
The market for PEMF therapy standardization faces several challenges and opportunities. One of the primary challenges is the lack of uniform protocols and standardized treatment parameters across different PEMF devices and applications. This inconsistency has led to skepticism among some healthcare professionals and regulatory bodies, hindering wider adoption. However, this challenge also presents a significant opportunity for companies and organizations that can successfully establish and promote standardized protocols.
Another key market driver is the increasing focus on evidence-based medicine. As more clinical studies and research papers demonstrate the efficacy of PEMF therapy for various conditions, the demand for standardized, reliable protocols is expected to grow. This trend is likely to attract more investment in research and development, further fueling market growth and innovation in PEMF technology.
The competitive landscape of the PEMF therapy market is characterized by a mix of established medical device manufacturers and newer, specialized PEMF technology companies. Key players are investing in product development and clinical trials to differentiate their offerings and gain regulatory approvals. Collaborations between device manufacturers, healthcare providers, and research institutions are becoming more common, aimed at developing standardized protocols and expanding the range of applications for PEMF therapy.
In conclusion, the market for PEMF therapy standardization presents significant growth potential, driven by increasing demand for non-invasive pain management solutions and the growing body of clinical evidence supporting its efficacy. The establishment of standardized protocols is likely to play a crucial role in shaping the future of this market, potentially leading to wider acceptance in mainstream healthcare and opening up new opportunities for market players.
Current Challenges in PEMF Protocol Standardization
Despite the growing interest in Pulsed Electromagnetic Field (PEMF) therapy, the field faces significant challenges in establishing standardized protocols. One of the primary obstacles is the lack of consensus on optimal treatment parameters. Different studies and manufacturers employ varying frequencies, intensities, and durations, making it difficult to compare results and establish best practices.
The absence of a unified measurement system for PEMF devices further complicates standardization efforts. Various units and metrics are used to quantify the strength and characteristics of electromagnetic fields, leading to confusion and inconsistency in reporting and interpreting results. This disparity hampers the ability to replicate studies and validate therapeutic claims across different devices and research groups.
Another challenge lies in the diverse range of conditions treated with PEMF therapy. From pain management to bone healing and neurological disorders, each application may require specific protocols. The complexity of biological systems and individual patient variability add layers of difficulty in establishing universal guidelines that can be effectively applied across all therapeutic scenarios.
The regulatory landscape surrounding PEMF therapy also presents hurdles to standardization. Different countries have varying approval processes and classification systems for medical devices, leading to inconsistencies in how PEMF devices are evaluated and marketed. This regulatory divergence impedes the development of globally accepted standards and protocols.
Limited long-term studies and insufficient large-scale clinical trials pose additional challenges. While numerous small studies have shown promising results, the lack of comprehensive, multi-center trials makes it challenging to establish robust, evidence-based protocols that can be universally adopted.
The rapidly evolving nature of PEMF technology itself contributes to the standardization challenge. As new devices with advanced features and capabilities enter the market, existing protocols may become outdated, necessitating continuous revision and adaptation of standards.
Interdisciplinary collaboration is another area where challenges arise. Effective standardization requires input from various fields, including physics, biology, medicine, and engineering. Bridging the communication gap between these disciplines and aligning their perspectives is crucial for developing comprehensive and scientifically sound protocols.
Lastly, the commercial interests of device manufacturers can sometimes conflict with the goal of standardization. Proprietary technologies and competitive advantages may lead to resistance in sharing information or adopting universal standards that could potentially benefit the entire field but may not align with individual business strategies.
The absence of a unified measurement system for PEMF devices further complicates standardization efforts. Various units and metrics are used to quantify the strength and characteristics of electromagnetic fields, leading to confusion and inconsistency in reporting and interpreting results. This disparity hampers the ability to replicate studies and validate therapeutic claims across different devices and research groups.
Another challenge lies in the diverse range of conditions treated with PEMF therapy. From pain management to bone healing and neurological disorders, each application may require specific protocols. The complexity of biological systems and individual patient variability add layers of difficulty in establishing universal guidelines that can be effectively applied across all therapeutic scenarios.
The regulatory landscape surrounding PEMF therapy also presents hurdles to standardization. Different countries have varying approval processes and classification systems for medical devices, leading to inconsistencies in how PEMF devices are evaluated and marketed. This regulatory divergence impedes the development of globally accepted standards and protocols.
Limited long-term studies and insufficient large-scale clinical trials pose additional challenges. While numerous small studies have shown promising results, the lack of comprehensive, multi-center trials makes it challenging to establish robust, evidence-based protocols that can be universally adopted.
The rapidly evolving nature of PEMF technology itself contributes to the standardization challenge. As new devices with advanced features and capabilities enter the market, existing protocols may become outdated, necessitating continuous revision and adaptation of standards.
Interdisciplinary collaboration is another area where challenges arise. Effective standardization requires input from various fields, including physics, biology, medicine, and engineering. Bridging the communication gap between these disciplines and aligning their perspectives is crucial for developing comprehensive and scientifically sound protocols.
Lastly, the commercial interests of device manufacturers can sometimes conflict with the goal of standardization. Proprietary technologies and competitive advantages may lead to resistance in sharing information or adopting universal standards that could potentially benefit the entire field but may not align with individual business strategies.
Existing PEMF Standardization Approaches
01 Standardized PEMF treatment protocols
Development of standardized protocols for PEMF therapy, including specific frequency ranges, treatment durations, and application methods. These protocols aim to optimize therapeutic outcomes and ensure consistency across different treatment sessions and devices.- Standardized PEMF therapy protocols for specific conditions: Development of standardized protocols for PEMF therapy tailored to specific medical conditions. These protocols define optimal frequency, intensity, duration, and treatment schedules to maximize therapeutic benefits while ensuring safety and consistency across treatments.
- Customizable PEMF therapy systems: Creation of PEMF therapy systems with adjustable parameters, allowing healthcare providers to customize treatments based on individual patient needs. These systems often include pre-programmed protocols and the ability to create and save custom treatment plans.
- Integration of PEMF therapy with other treatment modalities: Combining PEMF therapy with other treatment modalities such as light therapy, heat therapy, or traditional medicine to enhance overall therapeutic outcomes. This approach aims to create synergistic effects and provide more comprehensive treatment options.
- PEMF therapy monitoring and feedback systems: Implementation of monitoring and feedback systems in PEMF therapy devices to track treatment progress, adjust protocols in real-time, and provide data for analysis. These systems may include sensors, mobile applications, and cloud-based data management for improved treatment efficacy and patient compliance.
- Portable and wearable PEMF therapy devices: Development of compact, portable, and wearable PEMF therapy devices for convenient at-home or on-the-go treatments. These devices often feature rechargeable batteries, wireless connectivity, and user-friendly interfaces to promote regular use and adherence to standardized protocols.
02 Customizable PEMF therapy systems
Advanced PEMF systems that allow for customization of treatment parameters based on individual patient needs. These systems may include adjustable frequency, intensity, and waveform settings, as well as pre-programmed protocols for specific conditions.Expand Specific Solutions03 Integration of PEMF with other therapies
Combining PEMF therapy with other treatment modalities, such as light therapy, heat therapy, or traditional medicine, to enhance overall therapeutic effects. This approach aims to create synergistic benefits and improve patient outcomes.Expand Specific Solutions04 PEMF therapy monitoring and feedback systems
Implementation of real-time monitoring and feedback systems in PEMF devices to track treatment progress, adjust parameters as needed, and provide data for analysis. These systems may include sensors, mobile applications, and cloud-based data storage for improved patient management.Expand Specific Solutions05 Portable and wearable PEMF devices
Development of compact, portable, and wearable PEMF devices for convenient at-home or on-the-go treatments. These devices aim to improve patient compliance and allow for more frequent, consistent therapy sessions outside of clinical settings.Expand Specific Solutions
Key Stakeholders in PEMF Industry
The competitive landscape for establishing standardized protocols in PEMF therapy is evolving rapidly, reflecting the industry's early growth stage. The market size is expanding as more companies enter the field, driven by increasing recognition of PEMF's potential therapeutic applications. Technologically, the sector is in a developmental phase, with companies like Galvanize Therapeutics, Regenesis Biomedical, and Venus Concept leading innovation. These firms are focusing on developing proprietary PEMF systems and protocols, while also working towards standardization. The involvement of established medical technology companies such as Medtronic indicates growing interest from larger industry players, potentially accelerating the path towards standardized protocols and wider market adoption.
Galvanize Therapeutics, Inc.
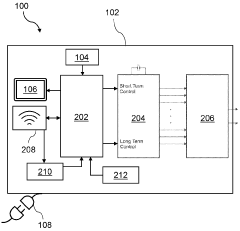
Technical Solution: Galvanize Therapeutics has developed a standardized PEMF therapy protocol using their AlphaStim technology. The protocol involves a specific waveform and frequency range, typically between 0.5 Hz and 100 Hz, which has been shown to be most effective for various conditions[2]. Treatment sessions are standardized to 20-60 minutes, with the exact duration determined by the condition being treated. The company has also implemented a standardized electrode placement guide for different body areas, ensuring consistent application across patients. To further standardize the protocol, Galvanize has developed a mobile app that guides practitioners through the treatment process, including electrode placement, treatment duration, and intensity settings[4].
Strengths: Non-invasive treatment, well-defined frequency range and session durations, standardized electrode placement guide. Weaknesses: May require multiple sessions for optimal results, effectiveness can vary between individuals.
Regenesis Biomedical, Inc.
Technical Solution: Regenesis Biomedical has established a standardized PEMF therapy protocol centered around their Provant Therapy System. This protocol utilizes a specific pulsed radiofrequency energy (PRFE) waveform, which has been FDA-cleared for several indications. The standardized treatment regimen involves 30-minute sessions, typically twice daily, with a fixed energy output of 27.12 MHz[5]. The company has developed a proprietary treatment algorithm that adjusts the pulse parameters based on the specific condition being treated, ensuring consistency across applications. To further standardize the protocol, Regenesis has implemented a smart device system that tracks treatment adherence and automatically adjusts the treatment schedule based on patient compliance[6].
Strengths: FDA-cleared for multiple indications, fixed energy output for consistency, smart device integration for treatment tracking. Weaknesses: Limited flexibility in treatment parameters, may not be suitable for all conditions.
Critical Research on PEMF Protocols
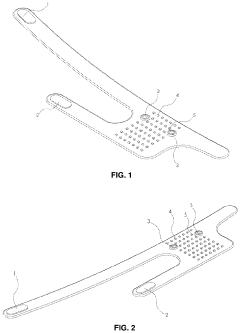
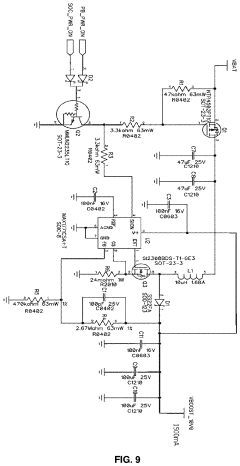
A pulsed electromagnetic field apparatus and method for generating frequencies
PatentWO2024127242A1
Innovation
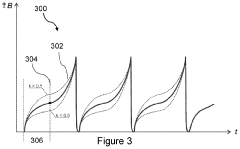
- A PEMF apparatus with a pulse generator and electromagnetic field generation means that uses modified sawtooth waveforms with pre-stress and relaxation periods, and quasi-sine signals with pulse width modulation, along with a feedback circuit for frequency stability and precision, and a bifilar antenna for scalar wave generation.
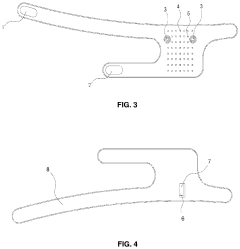
Flexible Photobiomodulation and Pulsed Electromagnetic Field Therapy Device
PatentPendingUS20230001222A1
Innovation
- A flexible wearable device that combines PEMF and PBM therapies, featuring a flexible substrate with electromagnetic coils and light-emitting diodes, controlled by a single module that can switch between pre-set frequency sequences, and is wirelessly enabled for remote control.
Regulatory Framework for PEMF Therapy
The regulatory framework for PEMF (Pulsed Electromagnetic Field) therapy is a critical aspect of establishing standardized protocols. Currently, the regulatory landscape for PEMF therapy varies significantly across different regions and countries, presenting challenges for widespread adoption and standardization.
In the United States, the Food and Drug Administration (FDA) has classified certain PEMF devices as Class II medical devices, requiring premarket notification (510(k)) clearance. These devices are primarily approved for specific indications, such as bone healing and pain management. However, the regulatory approach for broader applications of PEMF therapy remains less defined, creating a need for more comprehensive guidelines.
The European Union has implemented the Medical Device Regulation (MDR), which came into full effect in May 2021. Under this framework, PEMF devices are typically classified as Class IIa or IIb medical devices, depending on their intended use and risk profile. Manufacturers must demonstrate compliance with essential requirements, including clinical evaluation and risk management, to obtain CE marking for their devices.
In other regions, such as Asia and Australia, regulatory approaches to PEMF therapy vary widely. Some countries have adopted frameworks similar to those in the US or EU, while others have less developed regulatory systems for medical devices, potentially leading to inconsistencies in quality and safety standards.
To establish standardized protocols for PEMF therapy, it is essential to develop a harmonized regulatory framework that addresses key aspects such as device classification, safety standards, and efficacy requirements. This framework should consider the diverse applications of PEMF therapy and provide clear guidelines for manufacturers, healthcare providers, and researchers.
International organizations, such as the International Electrotechnical Commission (IEC) and the International Organization for Standardization (ISO), play crucial roles in developing technical standards for electromagnetic medical devices. These standards can serve as a foundation for regulatory bodies to establish consistent requirements across different jurisdictions.
Furthermore, regulatory agencies should collaborate to develop common guidelines for clinical trials and evidence generation specific to PEMF therapy. This would help ensure that safety and efficacy data are collected and evaluated consistently across different regions, facilitating the development of standardized protocols.
As the field of PEMF therapy continues to evolve, regulatory frameworks must remain adaptable to accommodate new technologies and applications. Regular review and updates of regulations, in consultation with experts and stakeholders, will be essential to maintain relevance and effectiveness in guiding the development and use of PEMF therapy protocols.
In the United States, the Food and Drug Administration (FDA) has classified certain PEMF devices as Class II medical devices, requiring premarket notification (510(k)) clearance. These devices are primarily approved for specific indications, such as bone healing and pain management. However, the regulatory approach for broader applications of PEMF therapy remains less defined, creating a need for more comprehensive guidelines.
The European Union has implemented the Medical Device Regulation (MDR), which came into full effect in May 2021. Under this framework, PEMF devices are typically classified as Class IIa or IIb medical devices, depending on their intended use and risk profile. Manufacturers must demonstrate compliance with essential requirements, including clinical evaluation and risk management, to obtain CE marking for their devices.
In other regions, such as Asia and Australia, regulatory approaches to PEMF therapy vary widely. Some countries have adopted frameworks similar to those in the US or EU, while others have less developed regulatory systems for medical devices, potentially leading to inconsistencies in quality and safety standards.
To establish standardized protocols for PEMF therapy, it is essential to develop a harmonized regulatory framework that addresses key aspects such as device classification, safety standards, and efficacy requirements. This framework should consider the diverse applications of PEMF therapy and provide clear guidelines for manufacturers, healthcare providers, and researchers.
International organizations, such as the International Electrotechnical Commission (IEC) and the International Organization for Standardization (ISO), play crucial roles in developing technical standards for electromagnetic medical devices. These standards can serve as a foundation for regulatory bodies to establish consistent requirements across different jurisdictions.
Furthermore, regulatory agencies should collaborate to develop common guidelines for clinical trials and evidence generation specific to PEMF therapy. This would help ensure that safety and efficacy data are collected and evaluated consistently across different regions, facilitating the development of standardized protocols.
As the field of PEMF therapy continues to evolve, regulatory frameworks must remain adaptable to accommodate new technologies and applications. Regular review and updates of regulations, in consultation with experts and stakeholders, will be essential to maintain relevance and effectiveness in guiding the development and use of PEMF therapy protocols.
Safety and Efficacy Considerations in PEMF Standardization
Safety and efficacy are paramount considerations in the standardization of Pulsed Electromagnetic Field (PEMF) therapy protocols. The establishment of standardized protocols requires a comprehensive approach that addresses both the potential risks and the desired therapeutic outcomes. One of the primary safety concerns in PEMF therapy is the exposure to electromagnetic fields, which necessitates careful regulation of field strength, frequency, and duration of exposure. Standardization efforts must define safe exposure limits based on extensive research and clinical trials, taking into account factors such as patient age, health status, and specific medical conditions.
Efficacy considerations in PEMF standardization involve determining optimal treatment parameters for various medical applications. This includes identifying the most effective frequencies, waveforms, and treatment durations for different conditions such as pain management, bone healing, and tissue regeneration. Standardized protocols should be developed through rigorous scientific studies that demonstrate reproducible therapeutic effects across diverse patient populations.
The development of standardized PEMF protocols also requires addressing the variability in device designs and specifications. Establishing uniform standards for PEMF devices, including output characteristics, calibration methods, and quality control measures, is essential for ensuring consistent and reliable treatment delivery. This standardization process should involve collaboration between medical professionals, researchers, regulatory bodies, and device manufacturers to create a consensus on technical specifications and performance criteria.
Safety monitoring and reporting mechanisms are crucial components of PEMF standardization. Protocols should include guidelines for patient screening, contraindications, and potential interactions with other medical devices or treatments. Additionally, a system for collecting and analyzing long-term safety data should be implemented to identify any rare or delayed adverse effects associated with PEMF therapy.
Efficacy evaluation in standardized PEMF protocols requires the development of validated outcome measures and assessment tools. These should encompass both objective physiological markers and subjective patient-reported outcomes to provide a comprehensive evaluation of treatment effectiveness. Standardized protocols should also specify appropriate follow-up periods and assessment intervals to capture both immediate and long-term therapeutic effects.
The integration of PEMF therapy into existing medical practices and treatment guidelines is another critical aspect of standardization. This involves developing clear indications for PEMF use, defining its role in combination with other therapies, and establishing protocols for patient education and informed consent. Standardized training programs for healthcare providers on the safe and effective application of PEMF therapy should also be developed to ensure consistent implementation of protocols across different clinical settings.
Efficacy considerations in PEMF standardization involve determining optimal treatment parameters for various medical applications. This includes identifying the most effective frequencies, waveforms, and treatment durations for different conditions such as pain management, bone healing, and tissue regeneration. Standardized protocols should be developed through rigorous scientific studies that demonstrate reproducible therapeutic effects across diverse patient populations.
The development of standardized PEMF protocols also requires addressing the variability in device designs and specifications. Establishing uniform standards for PEMF devices, including output characteristics, calibration methods, and quality control measures, is essential for ensuring consistent and reliable treatment delivery. This standardization process should involve collaboration between medical professionals, researchers, regulatory bodies, and device manufacturers to create a consensus on technical specifications and performance criteria.
Safety monitoring and reporting mechanisms are crucial components of PEMF standardization. Protocols should include guidelines for patient screening, contraindications, and potential interactions with other medical devices or treatments. Additionally, a system for collecting and analyzing long-term safety data should be implemented to identify any rare or delayed adverse effects associated with PEMF therapy.
Efficacy evaluation in standardized PEMF protocols requires the development of validated outcome measures and assessment tools. These should encompass both objective physiological markers and subjective patient-reported outcomes to provide a comprehensive evaluation of treatment effectiveness. Standardized protocols should also specify appropriate follow-up periods and assessment intervals to capture both immediate and long-term therapeutic effects.
The integration of PEMF therapy into existing medical practices and treatment guidelines is another critical aspect of standardization. This involves developing clear indications for PEMF use, defining its role in combination with other therapies, and establishing protocols for patient education and informed consent. Standardized training programs for healthcare providers on the safe and effective application of PEMF therapy should also be developed to ensure consistent implementation of protocols across different clinical settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!